Figure 32–1 The hypothalamic-pituitary-thyroid axis. Thyroid hormone synthesis is initiated by the release of thyroid-releasing hormone (TRH) from the hypothalamus, which stimulates the pituitary to release thyroid-stimulating hormone (TSH). TSH acts on the thyroid gland to increase iodide uptake and the synthesis of thyroid hormones, T4 and T3. T4 is converted to T3 in liver and muscle. T3 activates thyroid receptors in target tissues. Both T4 and T3 exert feedback inhibition of TRH and TSH secretion.
Thyroid hormones are synthesized in a process that involves the uptake and organification of iodide and the subsequent coupling of iodotyrosine residues of thyroglobulin. These steps are depicted in Figure 32–2.
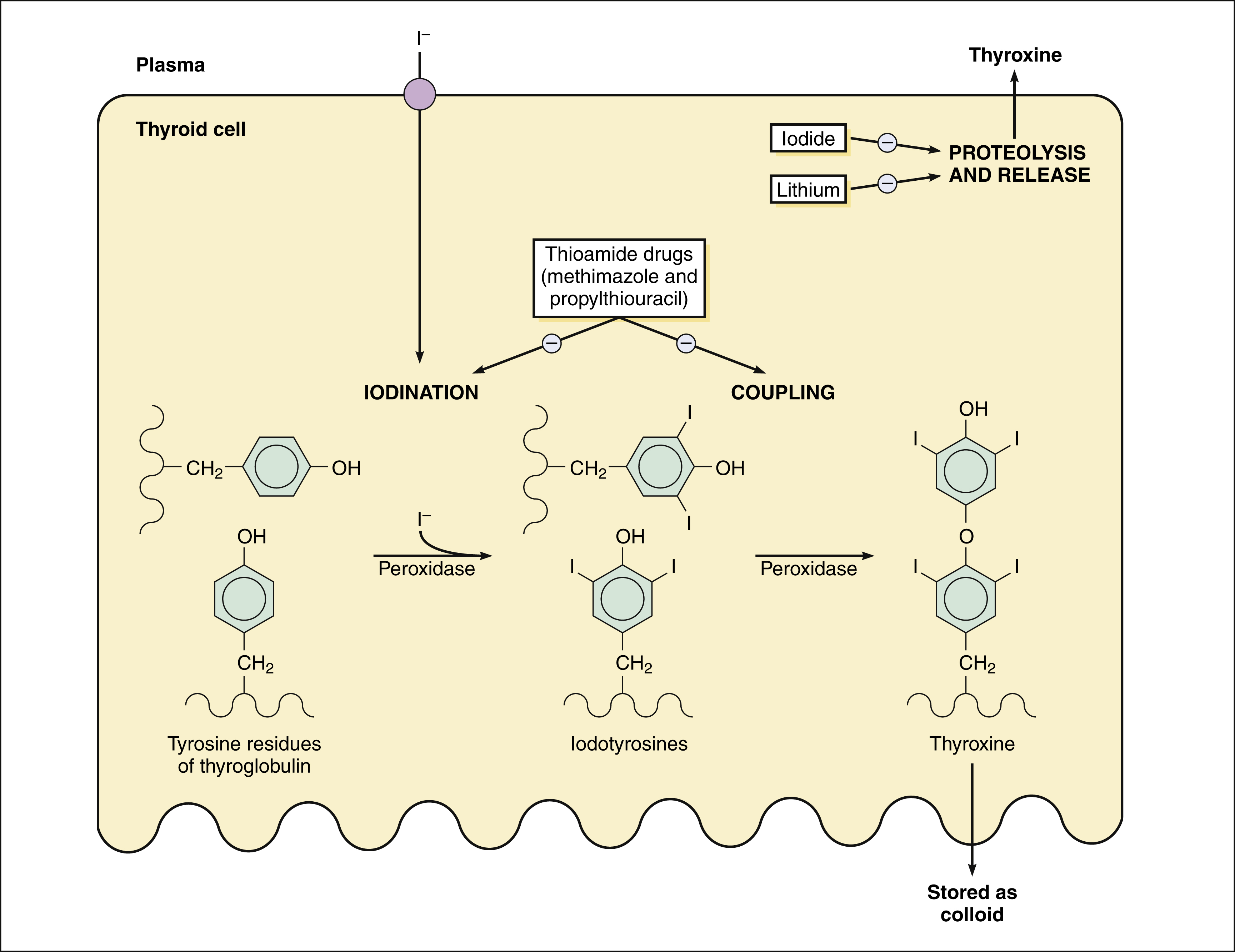
Figure 32–2 Thyroid hormone synthesis and sites of drug action. Iodide is accumulated by thyroid follicular cells by the sodium/iodide symporter. Thyroperoxidase catalyzes the iodination of tyrosine residues of thyroglobulin and the coupling of iodotyrosines to form triiodothyronine (T3) and tetraiodothyronine (T4, thyroxine). Thyroglobulin is stored as colloid in thyroid follicles and undergoes proteolysis to release T4 and T3 when stimulated by thyroid-stimulating hormone (TSH) or thyrotropin. Thioamide drugs inhibit the synthesis of thyroid hormones by inhibiting iodination and coupling of tyrosine residues. Elevated iodide concentrations and lithium inhibit the release of thyroid hormones.
After iodide is actively transported into thyroid follicle cells, it diffuses across the cells to the apical membrane, where it is oxidized and attached to tyrosine residues of thyroglobulin. This process is called iodide organification. The iodinated tyrosine residues, monoiodotyrosine and diiodotyrosine are then coupled to form T3 and T4. Iodide organification and the coupling reactions are catalyzed by thyroperoxidase.
Thyroglobulin is stored as colloid in the follicular lumen. During the release of thyroid hormones, thyroglobulin reenters the follicular cell by endocytosis and undergoes proteolysis. The release of T4 and T3 is stimulated by TSH via the formation of cyclic adenosine monophosphate in thyroid follicular cells.
T4 accounts for about 80% of the hormones secreted by the thyroid, and T3 accounts for the remainder. These hormones are transported to target organs by thyroid-binding globulin, thyroid-binding prealbumin, and albumin. In peripheral tissues, some of the T4 is converted to T3 and reverse T3 (rT3) by 5′-deiodinase and 5-deiodinase, respectively. T3 is about five times more active than T4, whereas rT3 in completely inactive. For this reason, the deiodinase enzymes have an important role in controlling the level of thyroid activity. The rate of conversion of T4 to T3 is also affected by a variety of other hormones, nutrients, and disease states. T3 and rT3 are eventually metabolized by deiodinase and sulfotransferase reactions to diiodothyronine sulfate.
When T3 enters the nucleus of target cell, it binds to specific receptors that activate gene transcription, leading to increased synthesis of proteins necessary for growth, development, and calorigenesis (heat production).
THYROID DISORDERS
Normal thyroid function, or euthyroidism, is maintained via feedback inhibition of TSH secretion so as to keep the plasma concentration of free (circulating or unbound) T4 within a narrow range. Abnormally low or high T4 and T3 levels result in clinical manifestations of hypothyroidism or hyperthyroidism, respectively.
Hypothyroidism is characterized by low T4 levels, and leads to impaired growth and development and decreased metabolic activity. In contrast, hyperthyroidism is due to high T4 levels, leading to hyperactivity of organ systems (particularly the nervous and cardiovascular systems) and an increased metabolic rate.
Thyroid disorders are relatively common. In many cases, patients seek medical attention because they notice a diffuse or nodular thyroid gland enlargement (goiter) or suffer from other manifestations of abnormal thyroid function. Thyroid disorders are diagnosed primarily on the basis of their clinical manifestations and plasma T4 and TSH levels. In most cases, TSH levels are abnormally high in persons with hypothyroidism and low in persons with hyperthyroidism.
Hypothyroidism
In infants and children, hypothyroidism causes irreversible mental retardation and impairs growth and development. In adults, hypothyroidism is associated with impairment of physical and mental activity and with slowing of cardiovascular, gastrointestinal, and neuromuscular functions. Hypothyroid patients may complain of lethargy, cold intolerance, weight gain, and constipation. The skin may become coarse, dry, and cold. Eventually, hypothyroidism causes myxedema, which is described as a dry, waxy swelling of the skin with nonpitting edema. Myxedema coma is characterized by hypothermia, hypoglycemia, weakness, stupor, and shock and is the end stage of long-standing, untreated hypothyroidism.
Many patients with mild hypothyroidism have a T4 level within the normal range. As the disease progresses, however, the T4 level usually falls below normal.
The most common cause of hypothyroidism in adults is autoimmune thyroiditis (Hashimoto’s disease). Other causes include thyroid surgery or radioactive iodine (RAI) treatment for hyperthyroidism; dietary iodine deficiency; and thyroid hypoplasia or enzymatic defects. Pituitary or hypothalamic dysfunction can cause secondary hypothyroidism.
Several types of drugs can induce thyroid disorders. Lithium inhibits the release of thyroid hormones by the thyroid gland (see Fig. 32–1) and can cause hypothyroidism by this mechanism. Amiodarone is an iodine-containing antiarrhythmic drug that can cause either hypothyroidism or hyperthyroidism through a variety of mechanisms that alter multiple thyroid functions.
The treatment for all forms of hypothyroidism is replacement therapy with a thyroid hormone preparation.
Hyperthyroidism
Manifestations of hyperthyroidism, or thyrotoxicosis, can include nervousness, emotional lability, weight loss despite an increased appetite, heat intolerance, palpitations, proximal muscle weakness, increased frequency of bowel movements, and irregular menses.
Most cases of hyperthyroidism are associated with overproduction of thyroid hormones by the thyroid gland, as indicated by the finding of increased RAI uptake. Excessive thyroid hormone production can result from excessive TSH, as occurs in patients with TSH-secreting pituitary adenomas, or it can result from gland stimulation by thyroid antibodies, as occurs in patients with Graves’ disease.
Graves’ disease results from the formation of antibodies directed against the TSH receptor on the surface of thyroid cells. These antibodies stimulate the receptor in the same manner as TSH, resulting in overproduction of thyroid hormones. Graves’ disease is characterized by hyperthyroidism, thyroid enlargement, and exophthalmus (abnormal protrusion of the eyeball). Exophthalmus results from stimulation of orbital muscles by thyroid antibodies.
Excessive thyroid hormone production also occurs in persons with thyroid nodules that are independent of pituitary gland control. Inflammatory thyroid disease (subacute thyroiditis) can cause a transient form of hyperthyroidism that is caused by the release of preformed thyroid hormone from thyroid follicles.
Three treatment modalities are used in hyperthyroidism: antithyroid agents, surgery, and RAI treatment. The goals of therapy are to eliminate excessive thyroid hormone production and to control the symptoms of hyperthyroidism. The choice of treatment depends on the type and severity of hyperthyroidism and on the individual characteristics of the patient. Antithyroid agents are primarily used for the short-term treatment of hyperthyroidism, either to induce remission of Graves’ disease or to control the symptoms of hyperthyroidism before thyroid surgery or RAI treatment. Surgery or RAI treatment can permanently cure hyperthyroidism. Either of these treatment modalities, however, often results in chronic hypothyroidism, which necessitates life-long thyroid hormone replacement therapy.
THYROID HORMONE PREPARATIONS
Synthetic levothyroxine is widely considered the drug of choice for thyroid hormone replacement in persons with hypothyroidism (Box 32-1). Thyroid hormones obtained from animal glands were used to treat thyroid disorders before the availability of synthetic hormones, but animal thyroid preparations are no longer recommended by endocrinologists because of their variable composition and stability, and their potential to cause allergic reactions to animal proteins contained in these preparations.
BOX 32–1 A CASE OF LETHARGY AND WEIGHT GAIN
CASE PRESENTATION
A 42-year-old woman complains to her health care provider of gaining 10 pounds over the last 6 months and of having a low energy level despite getting plenty of sleep. She has also had more trouble than usual with constipation and dry skin, and she has wanted to keep her home warmer than do other members of her family. On physical exam, her temperature is 97.3° F, her skin is dry, and she is found to have an enlarged thyroid gland (goiter). Laboratory tests are ordered and show that her TSH is 20 mU/L (normal is 0.4 to 5.5 mU/L), her free T4 is 0.6 ng/dL (normal is 0.8 to 2.7 ng/dL), and her thyroperoxidase antibody level is 150. She is started on a low dose of levothyroxine and instructed to have a TSH level obtained in 6 weeks.
CASE DISCUSSION
The patient’s enlarged thyroid and thyroid auto-antibodies are consistent with chronic autoimmune thyroiditis (Hashimoto’s thyroiditis). Her symptoms and lab values are indicative of mild hypothyroidism. Her treatment goals are to relieve her symptoms and normalize her TSH level. The serum TSH concentration is the primary test used to evaluate replacement therapy in persons with hypothyroidism. This test is very sensitive to minute changes in free T4 levels. A twofold change in the free T4 level can cause a 100-fold change in the TSH level. In patients who have had their dose or brand of levothyroxine changed, the TSH level should be measured after 2 to 3 months. When the optimum replacement dose has been attained, clinical and lab monitoring should be performed every 6 to 12 months or whenever there is a change in the patient’s status.
The synthetic thyroid hormone preparations include levothyroxine (T4), liothyronine (T3), and liotrix (a mixture containing T4 and T3 in a ratio of 4:1). A recent analysis of controlled trials concluded that combined T4 and T3 was not superior to T4 alone with respect to fatigue, depression, quality of life, or any other symptom of hypothyroidism, and liotrix is not currently recommended by most endocrinologists for treatment of hypothyroidism. The pharmacologic and pharmacokinetic properties of levothyroxine and liothyronine are shown in Table 32–1.
TABLE 32–1 Pharmacologic and Pharmacokinetic Properties of Levothyroxine (T4) and Liothyronine (T3)
| Property | Levothyroxine | Liothyronine |
|---|---|---|
| Relative potency | 1 | 4 |
| Oral bioavailability | 80% (variable) | 95% |
| Elimination half-life | 7 days | 1 day |
| Daily doses | 1 | 1–3 |
Levothyroxine
PHARMACOKINETICS
The oral bioavailability of levothyroxine is about 80%. Different brands and generic formulations of levothyroxine vary in hormone content and bioavailability, and formulations should not be substituted for one another without monitoring T4 and TSH levels. Food also affects the bioavailability of levothyroxine, and it is now recommended that levothyroxine be taken at the same mealtime each day in order to obtain consistent blood levels. Because the half-life of levothyroxine is about 7 days, once-daily administration of the drug produces little fluctuation in plasma hormone levels. Because about 35% of T4 is converted to T3 in peripheral tissues, levothyroxine administration produces physiologic levels of both T4 and T3.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


