Chapter 42 Thyroid and Antithyroid Drugs
| Abbreviations | |
|---|---|
| cAMP | Cyclic adenosine monophosphate |
| D1 | Iodothyronine deiodinase type 1 |
| D2 | Iodothyronine deiodinase type 2 |
| D3 | Iodothyronine deiodinase type 3 |
| DIT | Diiodotyrosine |
| MIT | Monoiodotyrosine |
| NADPH | Reduced nicotinamide adenine dinucleotide phosphate |
| PTU | Propylthiouracil |
| rT3 | Reverse T3 |
| T3 | L isomer of triiodothyronine |
| T4 | L isomer of thyroxine, tetraiodothyronine |
| TBPA | Transthyretin, thyroxine-binding prealbumin |
| TBG | Thyroxine-binding globulin |
| Tg | Thyroglobulin |
| TR | Thyroid hormone receptor |
| TRH | Thyrotropin-releasing hormone |
| TSH | Thyroid-stimulating hormone, thyrotropin |
Therapeutic Overview
| Therapeutic Overview |
|---|
| Hypothyroidism |
| Replacement therapy with synthetic thyroxine (T4) |
| Hyperthyroidism |
| Thioureylene drugs |
| β Adrenergic receptor antagonists |
| Glucocorticoids |
| Radioactive iodine |
| Surgery |
Mechanisms of Action
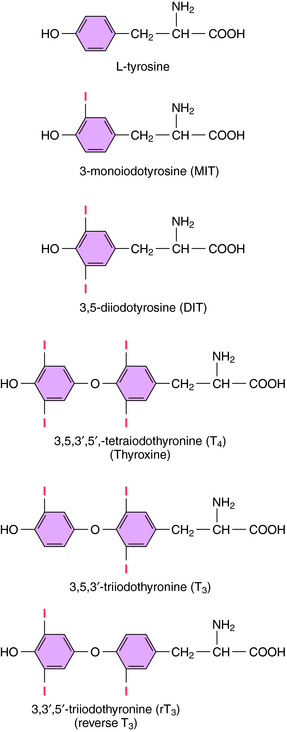
The iodinated compounds in thyroid follicular cells (thyrocytes) are derived by enzymatic condensation of the iodinated tyrosyl residues in thyroglobulin (Fig. 42-1). Among these are the two major thyroid hormones, L-isomers of T4 and T3. All of the T4 that is made is derived from the thyroid. In contrast, only a small fraction of the most biologically active form, T3, is produced and released from the thyroid. Most T3 is produced by peripheral tissues by removing an iodide from the outer ring of T4 through the action of 5’-deiodinase.
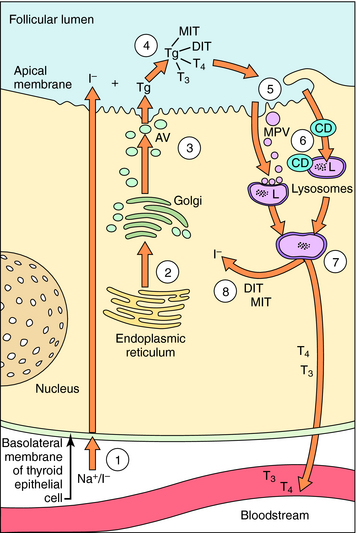
The synthesis and release of T3 and T4 are shown in Figure 42-2. Thyrocytes concentrate iodide from the circulation via a symporter present on their basolateral surface that admits Na+ down its electrochemical gradient. This sodium/iodide symporter, which is also present in salivary glands, breast, and stomach, can transport other anions, such as pertechnetate and perchlorate, which can competitively inhibit iodide transport. Once iodide enters the thyrocyte, it is transported to the follicular lumen by an anion transporter in the apical membrane termed pendrin. As iodide reaches the follicular lumen, it is oxidized by a mechanism involving thyroperoxidase, H2O2, and two reduced nicotinamide adenine dinucleotide phosphate (NADPH) oxidases. Activated iodide forms covalent links with specific tyrosyl residues on thyroglobulin (Tg), which is present in the follicular lumen, producing the thyroid hormone precursors monoiodotyrosine (MIT) and diiodotyrosine (DIT). This process is referred to as organification of iodide. MIT or DIT can donate their iodinated phenolic rings to acceptor iodotyrosyl residues on DIT in the Tg backbone and form an ether linkage with the acceptor phenolic ring to form T4 and T3 covalently bound to Tg. This process is called coupling. Iodinated Tg is transported to and stored associated with the apical membrane. When thyroid hormone is required, iodinated Tg is transported into follicular cells by endocytosis, where it undergoes proteolysis, releasing T4 and T3 into the circulation. MIT and DIT are deiodinated and reused.
Hormone synthesis in thyrocytes is regulated by TSH released from the pituitary. If the circulating level of thyroid hormone is abnormally elevated, pituitary sensitivity to thyrotropin-releasing hormone (TRH) is reduced, causing decreased production of TSH. Pituitary portal blood also carries counter-regulatory compounds that inhibit TSH release (i.e., dopamine and somatostatin) (see Chapter 38).
Thyroid hormones exert their major effects by binding to thyroid hormone receptors (TRs), members of the nuclear receptor superfamily (see Chapter 1). Two genes encode TRs, and each can be transcribed into alternatively spliced products. The relative proportions of each isoform expressed are developmentally dependent and tissue specific. TRs can homodimerize but are generally present as heterodimers, most commonly with the receptor for 9-cis-retinoic acid but also with other nuclear hormone receptors. TRs associate with DNA as part of a complex of transcription factors, even in the absence of thyroid hormone. When T3 binds to its receptor, the interactions of the receptor with transcription corepressors and coactivators change, and local chromatin structure is modified by changes in histone acetylation. Binding of T3 increases transcription of some genes and decreases the transcription of others. Thyroid hormones also regulate the processing of ribonucleic acid transcripts and the stability of specific messenger ribonucleic acids, and have other non-nuclear actions. Another potential regulatory process is the expression of the TR splice variant (TRα2), which interacts with thyroid hormone response elements but is not activated by T3. Consequently, expression of TRα2 can reduce sensitivity to thyroid hormone.
As shown in Table 42-1, antithyroid drugs can inhibit the synthesis, release, and metabolism of thyroid hormone and alter its peripheral effects.
| Compound | Mechanism of Action |
|---|---|
| Perchlorate | Inhibition of iodide transport |
| Thioureylenes | Inhibition of organification and coupling |
| Iodide, lithium | Inhibition of deiodination of T4 to T3 |
| Inhibition of hormone release | |
| β Adrenergic receptor blockers |