Chapter 9 Introduction to the Autonomic Nervous System
| Abbreviations | |
|---|---|
| ACh | Acetylcholine |
| AChE | Acetylcholinesterase |
| ANS | Autonomic nervous system |
| cAMP | Cyclic adenosine monophosphate |
| CNS | Central nervous system |
| COMT | Catechol-O-methyltransferase |
| DA | Dopamine |
| DOPA | Dihydroxyphenylalanine |
| Epi | Epinephrine |
| GI | Gastrointestinal |
| MAO | Monoamine oxidase |
| NE | Norepinephrine |
| PNS | Peripheral nervous system |
| SA | Sinoatrial |
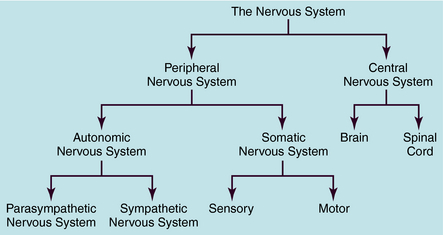
The human nervous system is the most complex of all systems in the body and is responsible for perceiving, processing, and transmitting information throughout the organism and generating responses to the information. The nervous system is divided into the peripheral nervous system (PNS) and the central nervous system (CNS) (Fig. 9-1). The PNS is subdivided into the autonomic nervous system (ANS), which controls automatic functioning, like breathing and heart rate, and the somatic nervous system, which receives sensory information and sends information to the CNS and from the CNS to skeletal muscles. The CNS is comprised of the brain and spinal cord and integrates and controls all bodily functions as well as thought processes. All these systems are interconnected and work together.
DIVISIONS OF THE AUTONOMIC NERVOUS SYSTEM
dilation of the pupil (mydriasis). In contrast, stimulation of the parasympathetic system conserves energy (“rest and digest”) and leads to responses characterized by decreased heart rate, blood pressure, and respiration; increased secretions; and constriction of the pupil (miosis).
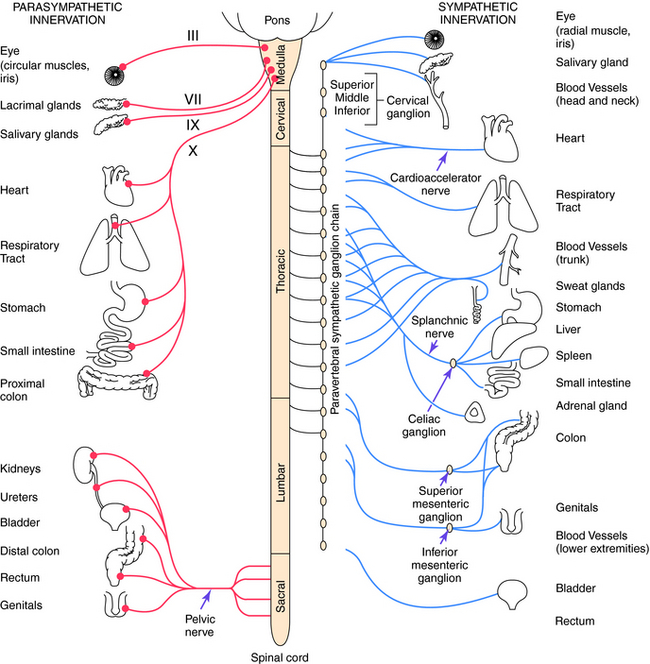
Although the parasympathetic and sympathetic systems differ both anatomically and functionally, they also share some features. The outflow of both divisions from the CNS consists of two neuron relays named after their anatomical location relative to the autonomic ganglia, or relay centers. Preganglionic neurons have their cell bodies in the spinal cord and the brainstem and their nerve terminals at autonomic ganglia, where they relay information to cell bodies of postganglionic neurons. Postganglionic neurons send their axons directly to effector organs (heart, blood vessels, visceral organs, and glands), where they relay information to these cells; these synapses are often referred to as neuroeffector junctions. Thus the preganglionic fibers of both the sympathetic and parasympathetic systems synapse with postganglionic fibers at autonomic ganglia. However, the location of the ganglia differs for the two systems, with parasympathetic ganglia located close to the organ innervated and most sympathetic ganglia located near the spinal cord. Due to the different ganglionic locations, the lengths of the preganglionic fibers relative to the postganglionic fibers also differ. Both sympathetic and parasympathetic preganglionic neurons release acetylcholine (ACh) as the neurotransmitter at ganglia. However, parasympathetic postganglionic neurons release ACh to relay their information at the neuroeffector junction, whereas most sympathetic postganglionic fibers release norepinephrine (NE, also called noradrenaline).
Parasympathetic and sympathetic neurons are defined anatomically, with parasympathetic neurons arising from the sacral region of the spinal cord and from the brainstem and sympathetic neurons arising from thoracic and lumbar regions of the spinal cord. They are not defined by the neurotransmitter released, and sympathetic fibers that innervate some sweat glands release ACh rather than NE. An anatomical representation of the sympathetic and parasympathetic systems is shown in Figure 9-2, and the commonalities and differences between the systems are summarized in Table 9-1.
TABLE 9–1 Comparison of the Parasympathetic and Sympathetic Divisions of the Autonomic Nervous System (ANS)

Parasympathetic Nervous System
Cell bodies giving rise to preganglionic parasympathetic nerves exit the CNS at cranial and sacral levels (see Fig. 9-2). The cranial portion of the parasympathetic outflow (cranial nerves III, VII, IX, and X) innervates structures in the head, neck, thorax, and abdomen. The sacral division of the parasympathetic nervous system forms the pelvic nerve and innervates the remainder of the GI tract and the pelvic viscera, including the bladder and reproductive organs.
Cell bodies for preganglionic sympathetic neurons originate in the intermediolateral cell column of the spinal cord at the thoracic and lumbar levels from T1 to L2 (see Fig. 9-2). Relatively short preganglionic, usually myelinated, neurons project to the sympathetic ganglia outside the spinal vertebrae. Most of these neurons synapse in the 22 segmentally arranged ganglia that form two chains located bilaterally adjacent to the spinal cord, and are often called the paravertebral chain. Postganglionic sympathetic neurons are generally unmyelinated and send long postganglionic fibers to their effector organs. Although most preganglionic sympathetic neurons synapse in the paravertebral sympathetic ganglia, several are prevertebral and lie near the bony vertebral column in the abdomen and pelvis (celiac, superior and inferior mesenteric, and aorticorenal), while a few (cervical ganglia and ganglia connected to urinary bladder and rectum) lie near the organs innervated.
Autonomic Regulation of Peripheral Organs
Both parasympathetic and sympathetic nerves innervate most organs of the body. Generally, these two branches of the ANS produce opposing responses in effector organs. There is generally a balance between sympathetic and parasympathetic effects on most organs, such that inhibition of one often leads to an increase in the response mediated by the other. However, there are some exceptions where the two systems cause similar responses. Some organs, such as the vasculature and spleen, receive only one type of innervation, which, in these cases, is sympathetic.
The importance of the dual innervation of most organs is evidenced by hypertension, which may involve an increase in sympathetic relative to parasympathetic control of the heart. Increased sympathetic effects can be produced by changes in neural firing rate, catecholamine concentrations at the neuroeffector junction or postjunctional receptors, or signal transduction pathways. Although there is support for each of these mechanisms, the first two are likely most important. Thus drugs that inhibit sympathetically mediated cardiovascular effects are useful for treating hypertension (see Chapters 11 and 12).
NEUROTRANSMISSION AND NEUROTRANSMITTERS IN THE AUTONOMIC NERVOUS SYSTEM
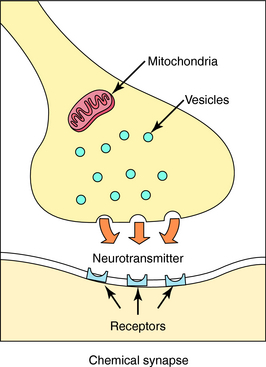
Neurotransmission is the process of effective transfer and integration of information in the nervous system. Neurotransmitters are endogenous substances released from nerve terminals, which act on receptors present on the membrane of postsynaptic cells. It is the interaction of the released neurotransmitter with the receptor that produces a functional change in the cell (Fig. 9-3).
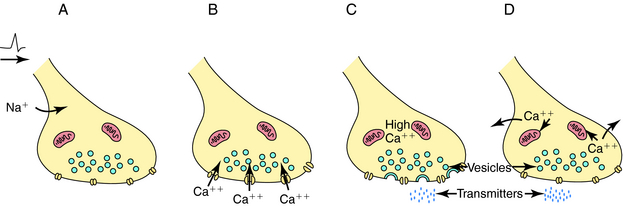
Depolarization of a presynaptic nerve terminal leads to release of a neurotransmitter into the extracellular fluid between the presynaptic and postsynaptic cells (the synaptic cleft). Calcium (Ca++) provides the essential link between depolarization and transmitter release. When a nerve terminal is depolarized, there is a large influx of Ca++ caused by opening of voltage-dependent Ca++ channels in the membrane. This influx promotes fusion of transmitter-containing synaptic vesicles with the plasma membrane resulting in exocytosis, which releases neurotransmitter into the synapse. After exocytosis, the voltage-dependent Ca++ channels inactivate rapidly, and the intracellular Ca++ concentration returns to normal by sequestration into intracellular compartments and active extrusion from the cell. The steps linking the arrival of an action potential to neurotransmitter release are summarized in Figure 9-4.
It is important to understand that voltage-dependent Ca++ channels in nerve terminals differ from those in other tissues. The Ca++ channel antagonists are an important class of drugs that block voltage-dependent Ca++ channels in cardiac and smooth muscle (see Chapter 20). However, there are distinct subtypes of these channels that can be distinguished by their electrical and pharmacological properties. The Ca++ channel antagonists block the channels most often found in cardiac and smooth muscle (L type) and have no effect on most of the voltage-dependent Ca++ channels found in nerve terminals (N type). This is fortunate, because if Ca++ channel antagonists also blocked neurotransmitter release, their toxicity would undoubtedly prevent them from being useful therapeutically.
After release, the neurotransmitter diffuses across the synaptic cleft to interact with specific receptors on the dendrites and cell body of the postganglionic neuron or on cells of the effector organ. The postsynaptic cell responds in an appropriate fashion to the received message. Thus the nerve terminal has mechanisms for storing and releasing neurotransmitters in response to depolarization, and the postsynaptic cell has receptors for detecting the presence and identity of different neurotransmitters and initiating appropriate changes in physiology or metabolism. Nerve terminals also have efficient mechanisms for the degradation and reutilization (reuptake
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree