Chapter 48 Bacterial Folate Antagonists, Fluoroquinolones, and Other Antibacterial Agents
| Abbreviations | |
|---|---|
| AIDS | Acquired immunodeficiency syndrome |
| CNS | Central nervous system |
| CSF | Cerebrospinal fluid |
| DNA | Deoxyribonucleic acid |
| GI | Gastrointestinal |
| IV | Intravenous |
| SMX | Sulfamethoxazole |
| TMP | Trimethoprim |
Therapeutic Overview
| Therapeutic Overview |
|---|
| Sulfonamides |
| Treatment of nocardiosis and toxoplasmosis |
| Topical agents for burn wounds |
| Trimethoprim-sulfamethoxazole Combination |
| No longer drugs of choice for upper respiratory tract infections |
| Urinary tract infections |
| Resistant bacteria |
| Treatment and prevention of Pneumocystic carinii infections and toxoplasma gondii encephalitis in AIDS patients (significant side effects) |
| Prevention of spontaneous bacterial peritonitis in patients with cirrhosis |
| Fluoroquinolones |
| Urinary tract infections |
| Prostatitis |
| Sexually transmitted diseases (increasing resistance in N. gonorrhea) |
| Bacterial diarrheal infections |
| Community-acquired pneumonia (third-generation agents only) |
| Osteomyelitis |
| Agents of biowarfare |
| Mycobacterial infections |
| Nitrofurans |
| Urinary tract infections |
| Polymyxins |
| Mainly topical uses |
| IV treatment only as therapeutic alternative for serious nosocomial infections caused by multi-resistant gram-negative organisms |
some frequency in the past few years for treatment of multidrug-resistant gram-negative infections.
Mechanisms of Action
Folic Acid Synthesis and Regeneration
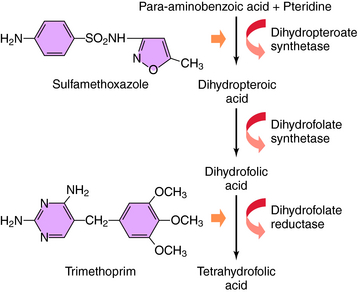
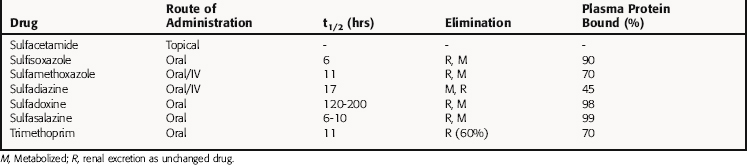
The bacterial synthesis of folic acid involves a multistep enzyme-catalyzed reaction sequence (Fig. 48-1). Tetrahydrofolic acid is the physiologically active form of folic acid and is required as a cofactor in synthesis of thymidine, purines, and bacterial DNA. Sulfonamides are structural analogs of p-aminobenzoic acid and competitively inhibit dihydropteroate synthase. TMP blocks the production of tetrahydrofolate from dihydrofolate by reversibly inhibiting the required enzyme, dihydrofolate reductase. Thus these two drugs block the synthesis of tetrahydrofolate at different steps in the synthetic pathway and result in a bactericidal synergistic effect.
TMP was used initially as an antimalarial drug but has been replaced by pyrimethamine, which acts by a similar mechanism. The antimalarial and antibacterial actions of TMP stem from its high affinity for bacterial dihydrofolate reductase. TMP binds competitively and inhibits this enzyme in bacterial and mammalian cells. Approximately 100,000 times higher concentrations of drug are needed to inhibit the human enzyme as compared with the bacterial enzyme. This enzyme is also inhibited by methotrexate, discussed in Chapter 54. TMP thus prevents conversion of dihydrofolate to tetrahydrofolate and blocks formation of thymidine, some purines, methionine, and glycine in bacteria, leading to rapid death of the microorganisms.
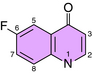
The fluoroquinolones include norfloxacin, ciprofloxacin, levofloxacin, moxifloxacin, and ofloxacin. Fluoroquinolones all have a fluorine at position 6 in the 2 ring structure (Fig. 48-2). Gatifloxacin was recently withdrawn from the market in the United States.
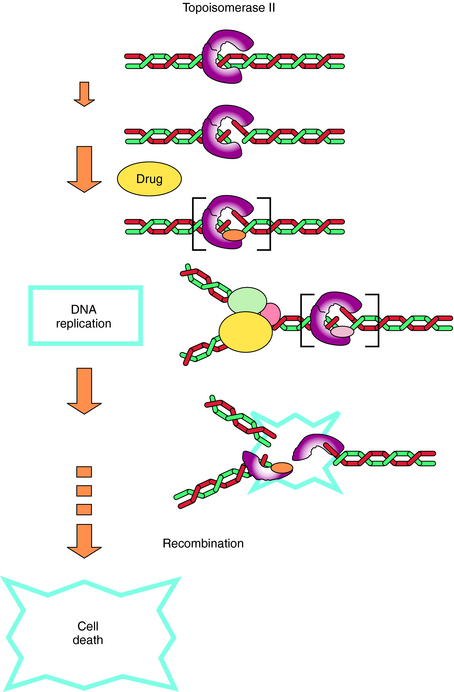
The fluoroquinolones act by inhibiting type 2 bacterial DNA topoisomerases, DNA gyrase, and topoisomerase IV. These topoisomerases are enzymes that consist of α- and β-subunits (encoded for by gyrA and gyrB or parC and parE, respectively) and catalyze the direction and extent of supercoiling and other topological reactions of DNA chains. Fluoroquinolones act by binding to and trapping the enzyme-DNA complex. This trapped complex blocks DNA synthesis and cell growth and ultimately has a lethal effect on the cell, possibly by releasing lethal double-strand DNA breaks from the complex. The primary target for the quinolones is determined by the differing sensitivities of DNA gyrase and topoisomerase IV to the particular quinolone in each organism (Fig. 48-3).
Nitrofurantoin is a member of a group of synthetic nitrofuran compounds that also includes nitrofurazone. The precise mechanism of action of the nitrofurans is not established. They inhibit many bacterial enzyme systems, most probably through DNA damage. A nitroreductase bacterial enzyme converts the compounds to short-lived intermediates, including oxygen free radicals, which interact with DNA to cause strand breakage and bacterial damage.
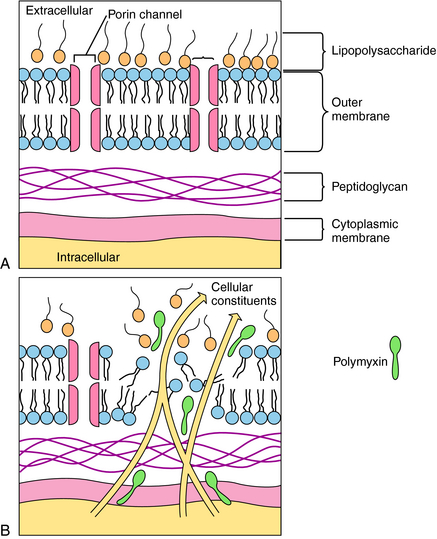
Polymyxins are detergents with both lipophilic and lipophobic groups that interact with phospholipids and disrupt bacterial cell membranes. The initial damage is to the cell wall, with a subsequent loss of periplasmic enzymes. The divalent cationic sites on the lipopolysaccharide component of the outer membrane of gram-negative organisms interact with the amino groups of the cyclic polymyxin peptide. The fatty acid tail portion of the drug molecule penetrates into the hydrophobic areas of the outer wall to produce holes in the membrane, through which intracellular constituents leak out of the bacteria (Fig. 48-4). A bacterium is rendered susceptible to the agent as a result of phospholipids in the bacteria cell wall interacting with the drug. The cell walls of resistant bacteria restrict the transport of polymyxin and prevent access of the drug to the cell membrane. Elevated concentrations of Ca++ or Mg++ reduce the activity of the polymyxins.