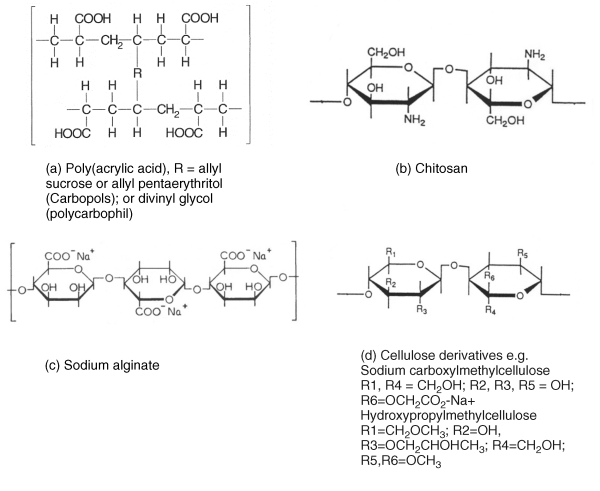
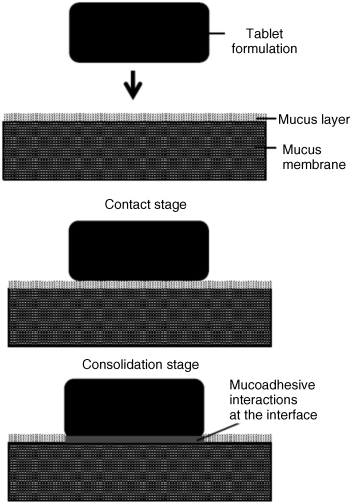
7 John D. Smart School of Pharmacy and Biomolecular Sciences University of Brighton UK In the pharmaceutical sciences the term mucoadhesion is used when a two surfaces, one of which is mucus or a mucous membrane and the other typically the surface of a drug delivery system, are held together for extended periods of time by interfacial forces [1]. Mucoadhesion has become of interest for its potential to optimise localised drug delivery, by retaining a dosage form at the site of action (e.g. the ocular surface or buccal mucosa), or systemic delivery, by retaining a formulation in intimate contact with the absorption site (e.g. within specific regions of the gastrointestinal tract). Mucoadhesives materials can also be used therapeutically to coat and protect damaged tissues (such as gastric ulcers) or to act as lubricating agents (in the eye and vagina). In this chapter, the mechanism by which mucoadhesives materials form adhesive bonds with a mucous membrane is considered in terms of the nature of the adhering surfaces and the forces that may be generated to secure them together. Mucous membranes (mucosae) are the moist surfaces lining the walls of various body cavities such as the gastrointestinal and respiratory tracts. The nature of the various mucous membranes is considered in detail in other chapters but basically their surface consists of an epithelial layer made moist usually by the presence of mucus. The epithelia may be either single layered (e.g. the intestines and bronchi) or multilayered/stratified (e.g. in the oral cavity and eye). The former contain goblet cells that secrete mucus directly onto the epithelial surfaces, the latter contain, or are adjacent to, tissues containing goblets cells (often within specialised glands) that secrete mucus, which is then deposited onto the epithelial surface. The major component of mucus gels is water (about 95% of its weight), dissolved into which are glycoproteins, proteins, lipids and inorganic salts [2]. The mucin glycoproteins are the most important structure-forming component of the mucus gel, resulting in its characteristic gel-like, cohesive and adhesive properties. The major functions of mucus are that of protection and lubrication. The thickness of this mucus layer varies from 50 to 450 μm in the stomach [3,4] to less than one μm in the oral cavity [5]. The most widely investigated group of mucoadhesives are hydrophilic macromolecules containing numerous hydrogen bond forming groups [6–10]. The presence of hydrogen bond forming groups (hydroxyl, carboxyl or amine) on the molecules favours adhesion. They require moisture to become activated and will adhere nonspecifically to many surfaces ([11]; indeed, they will show stronger adhesion to dry inert surfaces than those covered with mucus. In an aqueous environment (such as within the human body) they may overhydrate to form a slippery mucilage, and this can be responsible for adhesive joint failure. Like typical hydrocolloid glues, if the formed adhesive joint is allowed to dry then they can produce very strong adhesive bonds. Interestingly, these properties are similar to those of mucus itself. Typical examples of this type of mucoadhesive are carbomers, chitosan, alginates and the cellulose derivatives (Figure 7.1). These are available ‘off-the-shelf’ with regulatory approval but new enhanced materials have also now been developed. Figure 7.1 The structure of some common mucoadhesive polymers. In conventional chemistry, it is considered that for adhesion to occur molecules must bond across the interface. These bonds can arise in the following way [12]: There are six general theories of adhesion that have been adapted for the investigation of mucoadhesion [13–15]. The wetting theory This is primarily applied to liquid systems and considers surface and interfacial energies. It involves the ability of a liquid to spread spontaneously onto a surface as a prerequisite for the development of adhesion. The spreading coefficient (SAB) can be calculated from the surface energies of the solid and liquids using the equation: where γA is the surface tension (energy) of the liquid A, γB is the surface energy of the solid B and γAB is the interfacial energy between the solid and liquid. SAB should be positive for the liquid to spread spontaneously over the solid. The work of adhesion (WA) represents the energy required to separate the two phases, and is given by: The greater the individual surface energies of the solid and liquid relative to the interfacial energy, the greater the work of adhesion. The electronic theory This suggests that electron transfer occurs across contacting adhering surfaces due to differences in their electronic structure. This is proposed to result in the formation of an electrical double layer at the interface, with subsequent adhesion due to attractive forces. The adsorption theory This describes the attachment of adhesives on the basis of hydrogen bonding and van der Waals’ forces. It has been proposed that these forces are the main contributors to the adhesive interaction. A subsection of this, the chemisorption theory, assumes an interaction across the interface occurs as a result of strong covalent bonding. The diffusion theory This theory describes the interdiffusion of polymer chains across an adhesive interface. This process is driven by concentration gradients and is affected by the available molecular chain lengths, the compatibility of the two polymers and their mobilities. The depth of interpenetration depends on the diffusion coefficient and the time of contact. Sufficient depth of penetration creates a semi-permanent adhesive bond. The mechanical theory This assumes that adhesion arises from an interlocking of a liquid adhesive (on setting) into irregularities on a rough surface. However, rough surfaces also provide an increased surface area available for interaction along with an enhanced viscoelastic and plastic dissipation of energy during joint failure, which are thought to be more important in the adhesion process than a mechanical effect [15]. The fracture theory This differs a little from the other five in that it relates to the forces required for the detachment of the two involved surfaces after adhesion. This assumes that the failure of the adhesive bond occurs at the interface. However, failure normally occurs at the weakest component, which is typically a cohesive failure within one of the adhering surfaces. Mucoadhesion is a relatively complex process that is unlikely to be described fully by just one of the above theories. In considering how mucoadhesion arises, a whole range of ‘scenarios’ is possible depending, in particular, on whether the formulation is a solid (e.g. a tablet or patch), semi-solid (e.g. a vaginal gel) or liquid (e.g. an eye drop). The mucoadhesive process will differ in each case, so will be considered separately. Tablets, patches or microparticles are examples of solid formulations with the adhesive polymer forming the matrix into which the drug is dispersed, or the barrier through which the drug must diffuse [10,11]. It must be noted, however, that although initially dry, on exposure to biological fluids in vivo (or indeed on swallowing with water in the case of oral dosage forms), a degree of hydration may take place. In the study of solid adhesion generally, two steps in the adhesive process have been identified [16], which have been adapted to describe the interaction between mucoadhesive materials and a mucous membrane [1,17,18] (Figure 7.2): Figure 7.2 The two stages in solid mucoadhesion. Step 1 – Contact stage: An intimate contact (wetting) occurs between the mucoadhesive and mucous membrane. Step 2 – Consolidation stage: Various physicochemical interactions occur to consolidate and strengthen the adhesive joint, leading to prolonged adhesion. In the contact stage, the mucoadhesive and the mucous membrane have initially to come together to form an intimate contact. This may be facilitated by two surfaces being physically brought together, for example placing and holding a delivery system on the cornea or buccal mucosa. In others the contact of a particle may occur by deposition, such as in the nasal cavity or bronchi [19]. However, within the gastrointestinal tract other than at the two extremes (mouth and rectum) it is not possible to do this, and peristalsis and other gastrointestinal movement would be required to bring the dosage form into contact with the mucosa. Clearly this is much less easy to control and adhesion to luminal contents, or at an undesirable location, might easily occur, so the contact stage is a critical step in the adhesion process. For smaller particles and nanoparticles in suspension, adsorption onto the mucosa would be an essential prerequisite for the adhesion process, for example in locations such as the eye or mouth. The principles of the DLVO theory, described in the 1940s by Derjaguin and Landau, and separately by Verwey and Overbeek, to explain the stability of colloids [19] have been used to describe the physicochemical processes involved in the adsorption of bacteria onto surfaces [20,21]. It may, therefore, be applied when considering the adsorption of small particles onto a biological surface. In suspension a particle will be constantly moving due to Brownian motion and further movement will occur in vivo due to the flow of liquids within a body cavity and body movements such as peristalsis. When a particle approaches a surface it will experience both attractive and repulsive forces. Attractive forces arise from van der Waals’ interactions, surface energy effects and electrostatic interactions if the surface and particles carry opposite charges. Repulsive forces arise from osmotic pressure effects as a result of the interpenetration of the electrical double layers, steric effects and also electrostatic interactions when the surface and particle carry the same charge. The relative strength of these opposing forces will depend on the nature of the particle, the aqueous environment and the distance between the particle and surface. For example, the smaller the particle, the greater the surface-area-to-volume ratio and, therefore, the greater the attractive forces. Particles can be weakly held at a secondary minimum (about 10 nm separation), a region where the attractive forces are balanced by the repulsive forces allowing the particles to be easily dislodged. For stronger adsorption to occur, particles have to overcome a repulsive barrier (the potential energy barrier) to get closer to the surface (about 1 nm). If this barrier is sufficiently small or if the particle has sufficient energy, then adsorption into the primary minimum can occur. This type of adsorption would be required to allow a strong adhesive bond to form. This situation is complicated in vivo as the surface in question is usually a mucus gel rather than a solid, and the particles may become hydrated and/or coated with biomolecules, significantly altering their physicochemical properties [22–24]. The adhesive interaction necessary to retain a dosage form may only need to be weak if the forces promoting displacement are also small, such as for a small particle in the unstirred water layer at the surface of the gastrointestinal mucosae [25,26], or become lodged in these surface folds and crevasses of the gastrointestinal tract. This might explain how apparently inert materials have been reported to be ‘mucoadhesive’ [27–29]. For successful mucoadhesion to occur, strong or prolonged adhesion is usually required, for example with larger formulations exposed to stresses such as blinking or mouth movements. In these cases it has been proposed that a second ‘consolidation’ stage is required. Once activated by the presence of moisture, mucoadhesive materials adhere most strongly to solid dry surfaces [30]. Moisture will effectively plasticise the system, allowing mucoadhesive molecules to become free, conform to the shape of the surface and bond predominantly by weaker van der Waal and hydrogen bonding, although ionic interactions can also occur in some cases. The mucoadhesive bond is, by nature, very heterogeneous, making it extremely difficult to use spectroscopic techniques to identify the type of bonds and groups involved, although hydrogen bonds have been identified as being important [31,32]. Polymer/mucosae interactions have been investigated by evaluating surface energies [33–35]. Although of interest, these studies have met with varying degrees of success, which is unsurprising considering the heterogeneous nature of the adhering materials. When undertaking tensiometer studies of mucoadhesion, the high affinity of materials such as carbomers for water almost appears to have a ‘suction-like’ effect, which holds the formulation onto a solid surface [30]. For surfaces with only a thin mucus layer, a dry mucoadhesive polymer will almost certainly dehydrate and collapse this, by extracting the water component of the gel [17]. However, when a substantial mucus layer is present, its lubricant/anti-adherent properties will need to be overcome to allow strong adhesion. Here the adhesive joint can be considered to contain three regions (Figure 7.2
Theories of Mucoadhesion
7.1 Introduction
7.2 Mucous Membranes
7.3 Mucoadhesives
7.4 The Adhesive Interaction
7.4.1 Chemical Bonds
7.4.2 Theories of Adhesion
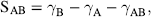
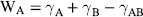
7.5 Mucoadhesion
7.6 Solid Mucoadhesion
7.6.1 Contact Stage
7.6.2 The Consolidation Stage
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


