The glands vary in the different mucosal zones. The cardiac mucosal zone, which extends for 1 to 15 mm (average 5 mm) distal to the lower end of the esophagus, has loosely packed mucus-secreting glands, some of which may be cystic. It appears that, in many adult individuals, the cardiac mucosal zone is irregular, so that biopsies apparently taken from “cardia” fail to reveal cardiac mucosa (2). This has led to speculation that cardiac mucosa is not a normal histologic feature but is the result of metaplasia caused by acid reflux (2). Autopsy studies on neonates and small babies appear to contradict this suggestion and have shown that cardiac mucosa is present in all instances (3). Studies of adult esophagogastrectomy specimens have also demonstrated that cardiac mucosa is present in all individuals, although in about 3% of cases, it consists of glands containing both mucous and oxyntic cells (oxyntocardiac mucosa) (4). Nevertheless, it remains a possibility that the cardiac mucosal region undergoes expansion in individuals who experience esophageal reflux.
The pyloric mucosal zone, which corresponds roughly to the gastric antrum, occupies the distal 3 to 4 cm of the stomach. This zone is also irregular, and pyloric mucosa is found more proximally along the lesser curvature than along the greater curvature. Pyloric glands are similar to cardiac glands, although cysts are not usually a feature. The glands are mucus-secreting, loosely packed, and occupy about half of the mucosal thickness. On H&E sections, the cytoplasm is lightly eosinophilic with a bubbly appearance.
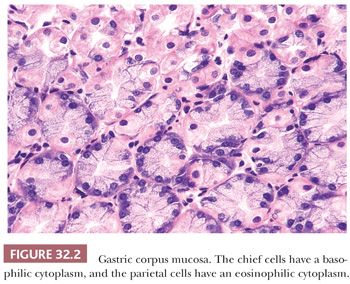
The remainder of the stomach is occupied by corpus (body or fundic) mucosa, which is thicker than other types because of the presence of glands that are specialized to produce acid and pepsin. These glands occupy 75% of the mucosal thickness and are tightly packed, with little intervening lamina propria. The parietal cells (acid-producing) have a light eosinophilic cytoplasm, and the chief cells (enzyme-producing) have a bluish or amphophilic cytoplasm (Fig. 32.2). Generally, chief cells occupy the basal half of the glands, whereas parietal cells occupy the superficial half. At the midpoint of the mucosa, the two types of cells gradually mingle. Throughout the stomach, the epithelial cell nuclei are basally located within the cell and have a rounded or oval contour with an even distribution of chromatin and small, fine nucleoli.
None of these mucosal zones has distinct boundaries; a gradual transition between them is seen, and isolated parietal cells are often found within mucous glands. Also, with age, there tends to be shrinkage of the corpus mucosa, which is replaced by pyloric mucosa, thus moving the corpus-pyloric border proximally (5). This change has been referred to as pseudopyloric metaplasia. A gradual merging of mucosal types is seen also at the gastroduodenal junction. In biopsy diagnosis, therefore, it is important to avoid overinterpreting patches of normal small-bowel mucosa in the pyloric canal as intestinal metaplasia.
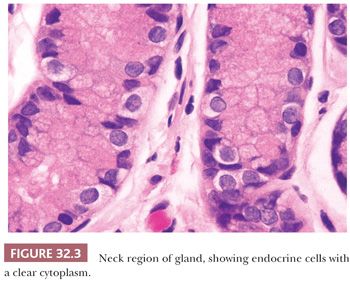
Endocrine cells are scattered throughout the glands of corpus mucosa, but within the pyloric glands, they are concentrated in the mucous neck region (the most superficial portion of the glands). On routine sections (Fig. 32.3), endocrine cells are inconspicuous, with clear rather than granular cytoplasm. In the antrum, 50% of endocrine cells are gastrin-producing G cells, 30% are serotonin-producing enterochromaffin (EC) cells, and 15% are the somatostatin-producing D cells. In the corpus mucosa, the majority of endocrine cells are enterochromaffin-like (ECL) and are histamine-producing (6), with smaller numbers of X cells (secretion product unknown) and EC cells (1).
Lymphoid tissue is sparse in the normal stomach. The superficial lamina propria between the gastric foveolae contains only small numbers of lymphocytes and mature plasma cells. These are predominantly of B-cell origin, although small numbers of T lymphocytes also may be found. Intraepithelial lymphocytes are either absent or very sparse. Lymphoid follicles with germinal centers are generally not found in the normal stomach. If present, they are often indicative of present or previous Helicobacter pylori infection. They may also be found in mucosa adjacent to gastric neoplasms. However, the normal gastric antrum and corpus may contain small aggregates of lymphocytes without germinal centers located immediately above or straddling the muscularis mucosae (7).
Detailed studies (8,9) disproved the previously held view that lymphatic channels extend to all levels of the gastric mucosa. By using careful techniques, it has been shown that lymphatics are found only in that portion of the lamina propria immediately superficial to the muscularis mucosae. This fact may well explain the observed low incidence of lymph node metastases in the early stages of gastric cancer.
BIOPSY SPECIMEN HANDLING
All gastrointestinal (GI) mucosal biopsies are delicate tissue that should be handled with care. The material should be removed gently from the biopsy forceps and fixed immediately. Ideally, the tissue should be oriented and attached to a supportive mesh, filter paper, or card before being placed into fixative. Formalin is an adequate fixative for most purposes, although some pathologists prefer Bouin fluid or Hollande fixative, both of which contain picric acid. Each biopsy specimen should be sectioned at 3 to 5 µm, and 20 to 30 serial sections should be prepared. These may be mounted on one to three slides, depending on the size of the biopsies. H&E staining is satisfactory for routine purposes; special stains are generally not required in the first instance, although some laboratories find it more convenient to routinely prepare special stains for Helicobacter organisms. The ideal number of biopsies to be taken will vary with the disease present, but two should be a minimum from each area of the stomach under study.
HETEROTOPIAS, DUPLICATIONS, AND CYSTS
Ectopic pancreas (pancreatic heterotopia) in the wall of the stomach is a relatively common finding, both at surgery and at autopsy. The exact prevalence is unknown but is of the order of 1% to 2% (10). It rarely gives rise to symptoms but occasionally has been associated with ulceration (11), obstruction (12), and intussusception (13). Typically, it appears as a firm intramural mass, 1 to 2 cm in diameter, located in the prepyloric region. The mass is spherical or hemispherical, with a nipple-like projection into the gastric mucosal surface, where the ectopic ducts enter the lumen. In 75% of cases, the mass is located in the submucosa, but it may be present also within the muscularis propria or subserosa. Histologically, it consists of well-differentiated ductlike structures, some of which may be dilated, intimately adjacent to muscle bundles. Acinar tissue is almost invariably present to a greater or lesser degree, although islets are found in only 30% of cases (Fig. 32.4).
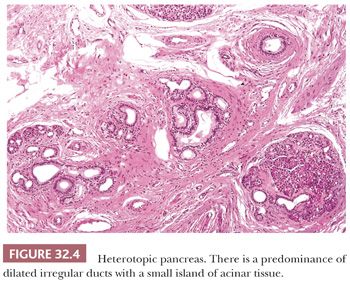
Rare histologic variants of ectopic pancreas may be encountered. Cases in which only ducts and ductular elements are present have been referred to as adenomyomas (14). Cases with mucus inspissation and duct rupture may give rise to an erroneous clinical and histologic impression of mucinous carcinoma (15). Examples in which the deep intramuscular and serosal portions of the lesion consist exclusively of islet cells may, on biopsy at the time of surgery, be difficult to distinguish from an endocrine tumor (16).
The presence of heterotopic mucous glands in the gastric submucosa has been described (17). This is relatively common and occurs in 1% to 2% of the general population. These collections, which resemble Brunner glands, may be focal or diffuse but probably have no clinical significance.
Duplications of the stomach are distinctly uncommon. They are seen mainly in the pediatric age group and are more common in girls. Usually, there is a cystic mass located within the abdomen but not necessarily attached or intimately related to the stomach (18–21). The lining consists of normal gastric mucosa with the usual muscle layers in the wall (19). Occasionally, the duplication can impinge on the gastric wall, producing overlying ulceration (20). Gastric diverticula (22) are probably an incomplete variant of duplication. They are usually solitary, although rare examples of multiple diverticula have been described (23). In contrast, pyloric duplication is most commonly acquired and is usually the result of extensive peptic ulceration with scarring and irregular healing (24). True congenital pyloric duplication accounts for only 10% of cases (25).
In neonates, an accessory juxtagastric lung may occasionally be present (26). This arises from a foregut bud in the corpus, with the main mass lying alongside the stomach. Histologically, it is made up of tubular structures lined by a ciliated epithelium. The arterial supply is generally directly from the aorta.
Acquired cysts of the stomach are an integral component of a variety of diseases, such as Ménétrier disease and gastritis cystica profunda. Most have an intramucosal location and are metaplastic in origin (27). Lesions of this type are discussed elsewhere. Primary cystic lesions appear to be of two types. Intramural cysts (28) are usually solitary masses, several centimeters in diameter, that are lined by respiratory epithelium. They are thought to be developmental in origin. Diffuse cystic disease of the mucosa and submucosa (29) is a rare condition that may be seen with a gross polypoid enlargement of the mucosa. These cysts are usually only a few millimeters in diameter and are lined by normal gastric epithelium. These, too, are considered congenital in origin, probably a form of heterotopia, but can be complicated by development of a carcinoma (30,31).
XANTHELASMA
These lesions, also called xanthomas or lipid islands, have been reported in up to 53% of autopsy stomachs and in up to 4% of endoscopy biopsies (32,33). Grossly, they appear as cream-colored plaques, less than 5 mm in diameter, mostly occurring along the lesser curvature in the region of the antrum, where they may be single or multiple. Histologically, they consist of loosely organized aggregates of foamy histiocytes in the upper lamina propria (Fig. 32.5). The nuclei are bland and the cytoplasm contains lipid, which is periodic acid-Schiff (PAS)–negative. These features serve to distinguish xanthelasma cells from a mucin-secreting adenocarcinoma, Whipple disease, and Mycobacterium avium-intracellulare infection.
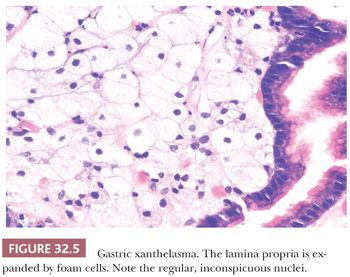
The pathogenesis of xanthelasmas is unknown, although they occur with increased frequency in patients with H. pylori infection and atrophic gastritis (34). They are commonly found in the gastric remnant after a partial gastrectomy, and it has been postulated that bile reflux is an etiologic factor (32). Xanthelasmas have also developed in patients with cholestasis and have regressed with relief of cholestasis (33). Biochemical analysis reveals that xanthelasmas consist of cholesterol, neutral fat, low-density lipoproteins, and oxidized low-density lipoproteins but are not linked to any type of inherited hypercholesterolemia (35).
HYPERTROPHIC PYLORIC STENOSIS
This is predominantly a disease of infants (36) but also may rarely affect adults (37). The infantile form is usually manifest at about 3 weeks of age. It has a predilection for first-born male infants and has an inheritance pattern that is polygenetically determined (38). The macroscopic appearance is of a fusiform mass 3 to 5 cm in diameter, involving the pyloric sphincter in a circular fashion. The overlying mucosa may contain secondary edema and ulceration. Microscopically, the thickening involves mainly the circular muscle and has a very abrupt termination distally. The lesion is thought now to be a primary muscle disorder, and any neuronal changes present are secondary.
Primary hypertrophic pyloric stenosis of adults is extremely rare. In addition to the circular form of hypertrophy seen in children, there are also focal and multinodular variants (37).
METAPLASIA
PYLORIC METAPLASIA
Pyloric metaplasia occurs when the corpus gland mass of acid and enzyme-secreting cells is replaced by mucus-secreting pyloric-type glands (2). In some individuals, pyloric metaplasia is an age-related change that starts distally and spreads proximally, producing a reduction in maximal gastric acid output. Pyloric metaplasia may also occur at the incisura in response to H. pylori and autoimmune gastritis–associated mucosal atrophy (39).
CILIATED METAPLASIA
This type of metaplasia was recently described in North American, European, and Japanese patients. Most examples have been found incidentally in gastrectomy specimens removed for neoplasms or peptic ulcers. Ciliated cells may be found lining dilated antral glands (40,41). The cause and significance of this change are unknown.
SUBNUCLEAR VACUOLATED CELLS
This change in the gastric mucosa is not strictly a form of metaplasia because it does not simulate the appearances of any normal cell and it probably represents a degenerative reaction to cell injury. Subnuclear vacuolation may affect the antral gastric glands, Brunner glands, and the antral foveolar epithelium (42). The vacuoles are clear with H&E stains and are PAS-negative. On ultrastructural examination, they consist of a membrane-lined space, derived from either endoplasmic reticulum or Golgi, that probably contains an abnormal accumulation of nonglycoconjugated mucous core protein (42). Subnuclear vacuolated cells have been found in stomachs showing chronic gastritis and in patients with probable duodenogastric reflux.
PANCREATIC ACINAR METAPLASIA
In this condition, single or multiple cell nests and lobules of acinar tissue are encountered among or at the base of gastric glands (43,44). These nests are surrounded by thin fibrous and smooth muscle septa. Islets of Langerhans are not present. Initially, pancreatic acinar differentiation of the gastric mucosa was considered to be a true metaplasia, resulting from the effects of chronic gastritis (43). More recently, however, it was identified in 24% of unselected biopsies from the gastric cardia. Its lack of correlation with other biopsy abnormalities suggests that it may, in fact, be congenital in origin (44).
INTESTINAL METAPLASIA
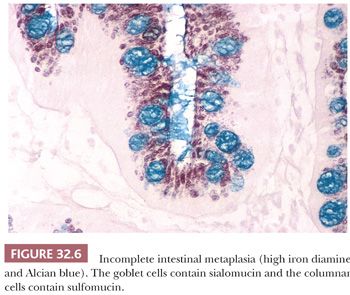
Stomachs with atrophic gastritis may also show intestinal metaplasia. This is a complex process in which the superficial and foveolar epithelium changes both morphologically and histochemically. There are two major types of metaplasia: small-bowel metaplasia and large-bowel metaplasia. Both may be either complete or incomplete, or there may be a mixture of types (Table 32.1). In incomplete metaplasia, the mucin content of the columnar cells is reduced and changes from the normal gastric neutral mucin to acidic mucin. In the case of small-bowel metaplasia, only sialomucin is produced; in large-bowel metaplasia, however, sialomucin and sulfomucin are found (Fig. 32.6). Because there is no change in cell structure, special staining is necessary to demonstrate these changes. Neutral mucin is PAS-positive and Alcian blue–negative at both pH 2.5 and pH 0.5. Sialomucin is PAS-positive, Alcian blue–positive at pH 2.5, but Alcian blue–negative at pH 0.5. Sulfomucin is weakly PAS-positive but is Alcian blue–positive at pH 2.5 and pH 0.5. It is also high iron diamine–positive (45).
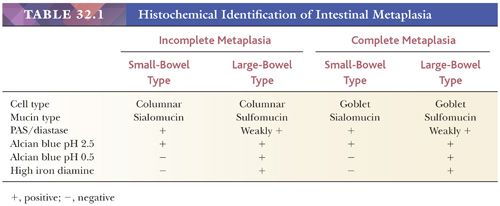
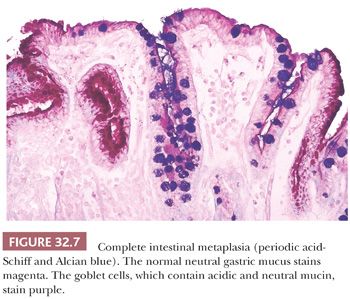
Complete intestinal metaplasia may be identified morphologically as loss of columnar mucus-secreting cells and their replacement by an epithelium that contains goblet cells and absorptive cells (Fig. 32.7). In the histochemical identification of intestinal metaplasia of all types, it is convenient to use a combined Alcian blue, pH 2.5, and high iron diamine stain. This is a relatively simple technical procedure and will stain sulfomucin brown-black and sialomucin aqua blue. In the most advanced forms of intestinal metaplasia, small villi may form, and there may be Paneth cells at the base of the glands. It should also be remembered that although intestinal metaplasia is defined by changes in mucin histochemistry, there are also profound molecular changes related to genes that are of importance in the development of gastric cancer (46). The clinical significance of finding intestinal metaplasia in gastric biopsies is discussed later.
INFLAMMATORY CONDITIONS
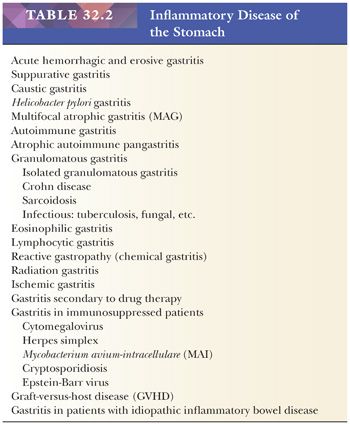
A classification of gastritis is given in Table 32.2. This is based on classifications originally proposed by Whitehead (47) and is consistent with more recent classifications (48,49). The scheme presented is not completely satisfactory because some entities are defined morphologically and some etiologically.
The term chronic nonspecific gastritis can no longer be regarded as a clinically useful diagnosis. This does not mean, however, that every gastric biopsy can be definitively assigned to one of the listed categories. Particularly, when the tissue supplied is insufficient or shows biopsy artifact, it is still reasonable to issue a report indicating that only nonspecific inflammation is present.
The earliest gastritis classifications divided chronic nonspecific gastritis into type A (associated with pernicious anemia) and type B (not associated with pernicious anemia) (50). Other authors later added a type C (chemical gastritis, related to drug therapy or bile reflux) (51). Although these concepts are still valid, the discovery of H. pylori as an important cause of gastritis means that type B gastritis is now too broad a category to be clinically useful.
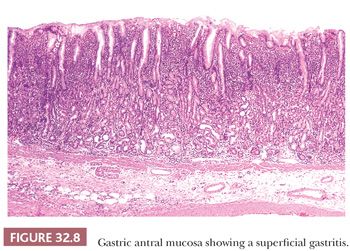
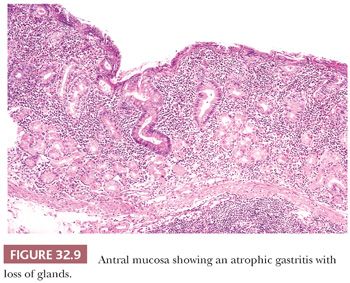
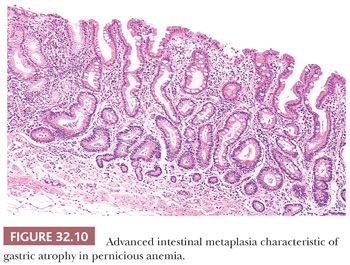
The terms superficial gastritis, atrophic gastritis, and gastric atrophy are still useful for descriptive purposes and may be retained. In superficial gastritis (Fig. 32.8), inflammation is largely confined to the portion of mucosa occupied by the gastric foveolae. In atrophic gastritis (Fig. 32.9), inflammation involves the whole thickness of the mucosa and is accompanied by a loss of gastric glands. In gastric atrophy (Fig. 32.10), there is widespread, if not complete, gland loss. Inflammation is often relatively scanty in advanced atrophy, and extensive complete intestinal metaplasia is frequently encountered.
A system of classification and description of gastritis, referred to as the Sydney system, has been developed (52). This is a moderately complicated scheme in which there are several components. Full application of the system requires that a minimum of five biopsies be taken: two from antral mucosa, two from fundic mucosa, and one from the incisura. Involvement of these areas by the inflammatory process is assessed and recorded separately. The morphologic features of inflammation are evaluated by a series of graded (mild, moderate, or marked) or ungraded variables. Graded variables include atrophy, chronic inflammation, activity, intestinal metaplasia, and H. pylori density. A diagrammatic visual scale for grading is provided (52). Ungraded variables include granulomas, eosinophils, and intraepithelial lymphocytes. The Sydney system has much to recommend it for academic studies, but for routine patient care, it is questionable whether grading has much clinical utility.
ACUTE HEMORRHAGIC AND EROSIVE GASTRITIS
A gastric erosion may be defined as an ulcer that has not penetrated the muscularis mucosae. Erosions are a relatively common finding at gastroscopy, but biopsies of them are performed less frequently because of the possibility of provoking bleeding.
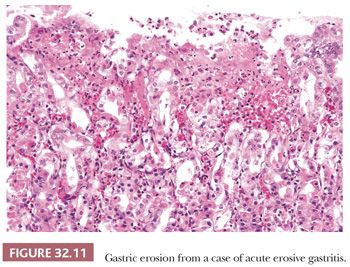
There is a rather wide spectrum of severity in hemorrhagic and erosive gastritis (ulcero-hemorrhagic gastritis) (49). At the upper end is acute hemorrhagic gastritis, in which multiple erosions are found in mucosa that bleeds profusely. This condition may be responsible for many hospital admissions with acute upper GI bleeding (53). Histologic examination of these specimens reveals erosions with a base consisting of slough and necrotic tissue, beneath which is viable basal mucosa (Fig. 32.11). Generally, neutrophilic infiltrates are not prominent. When healing occurs, there is regeneration of the normal mucosa with a minimum of architectural disturbance. At the edge of the erosions, the lamina propria shows intense capillary congestion, with extravasation of blood. It has been suggested that, in the early stages of hemorrhagic gastritis, subepithelial hemorrhage may exist without actual erosions.
At the lower end of the spectrum of acute hemorrhagic and erosive gastritis, the stomach contains only small numbers of localized erosions present in an otherwise normal mucosa. These may ooze blood, resulting in anemia, but rarely give rise to acute symptoms. The pathology of erosive gastritis in its various clinical forms is incompletely studied, although there is extensive literature on the etiology and pathogenesis. Recognized causes include alcohol (54), aspirin (55), other nonsteroidal anti-inflammatory drugs (NSAIDs) (53,55), antibiotics (56), and stress (shock, sepsis, and hypoxia) (53). Small erosions may also be encountered in patients with Helicobacter gastritis but these are rarely a source of clinically significant bleeding.
SUPPURATIVE GASTRITIS
Suppurative (phlegmonous) gastritis is a bacterial cellulitis, predominantly affecting the submucosal layer of the stomach (57,58). Streptococcus type A is the organism most commonly isolated, with occasional cases due to Staphylococci, Escherichia coli, or Haemophilus influenzae. Gas-forming organisms may produce submucosal bubbles (so-called emphysematous gastritis) (59). The route of infection is presumed to be via a breach in the mucosa; predisposing factors include alcoholism, chronic renal failure, immunodeficiency, hypochlorhydria, and endoscopic polypectomy. The clinical picture is one of rigors, fever, and rapid prostration, with a high mortality rate. Grossly, the stomach shows distention, with marked thickening of the wall due to submucosal edema. Histologically, there is submucosal edema, congestion, and hemorrhage, with a massive neutrophil exudation, among which bacterial organisms can be demonstrated. Typically, the overlying mucosa may be relatively unremarkable, making the diagnosis difficult on endoscopic biopsy.
CAUSTIC GASTRITIS
The type of injury will vary with the nature and concentration of the caustic substance ingested. Changes tend to be maximal in the antrum and consist of edema and hemorrhage. In severe examples, coagulative necrosis and deep ulceration are present (49,55). If the patient survives, fibrosis and stricture formation may develop.
HELICOBACTER GASTRITIS
There is an enormous volume of literature describing the epidemiology, clinical features, pathology, and significance of Helicobacter infection. Review articles concisely summarize the current knowledge (60,61). It is generally accepted that H. pylori is the cause of most cases of chronic gastritis. In North America and Western Europe, the majority of individuals with H. pylori infection have active superficial gastritis, most prominent in the antrum (diffuse antral gastritis, or DAG) but affecting all mucosal zones including the cardia. Superficial gastritis may result in duodenal ulcer formation, probably as a result of increased basal acid output following a heightened parietal cell response to stimulation (62). The effect is compounded by a concomitant reduction in duodenal bicarbonate secretion (62). In some patients, superficial gastritis may also be a cause of nonulcer dyspepsia. However, symptomatic relief following antibiotic therapy is obtained in only 9% of H. pylori–infected patients (63).
In contrast, H. pylori–infected individuals in underdeveloped countries typically have atrophic gastritis (multifocal atrophic gastritis), which is patchy in distribution and involves pyloric, body, and cardiac mucosa (64). Organisms may be sparse in this form of gastritis, which is associated with gastric peptic ulcer, occurring particularly at the antrum–corpus junction along the lesser curvature (62,65). Multifocal atrophic gastritis is also associated with the development of gastric adenocarcinoma, and some authors have emphasized a gastritis–atrophy–metaplasia–dysplasia–carcinoma sequence (66).
Why some patients develop gastric atrophy whereas others do not progress beyond superficial gastritis is unknown (67–69). In superficial gastritis, H. pylori organisms are in direct contact with surface and foveolar epithelial cells but not with cells in the glands. The bacteria are able to bind to class II major histocompatibility complex molecules on the cell surfaces, inducing apoptosis (60). Damage may be mediated by translocation of CagA protein into epithelial cells (70). In addition, enhanced mucosal levels of interleukin (IL) (especially IL-8) and tumor necrosis factor are mediated via CagA-producing strains of H. pylori (60). H. pylori also induces a vigorous systemic and mucosal humoral response (60). Up to 80% of patients infected by H. pylori may have autoantibodies directed against the canalicular membranes of parietal cells or the luminal membrane of foveolar epithelium (71). Moreover, the canalicular antibody has H+/K+ adenosine triphosphatase (ATPase) specificity (72). Damage to the epithelium may occur because of this antigenic mimicry where circulating antibodies or lamina propria T lymphocytes are the trigger for parietal cell destruction.
Acute gastritis due to H. pylori is rarely seen in biopsy material because the infection is usually either asymptomatic or accompanied only by minor GI discomfort. The histologic features include conspicuous “pit abscesses” and exudation of neutrophils into the surface epithelium. This is accompanied by marked epithelial degeneration and formation of small erosions with adjacent regeneration that may be syncytial in type (73). Initially, the gastritis affects all mucosal zones of the stomach equally, but later, the corpus inflammation partly regresses, leaving an antral predominant gastritis.
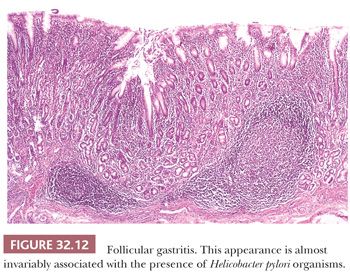
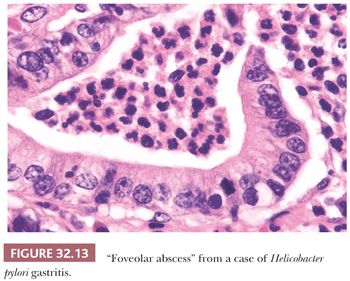
Diffuse chronic H. pylori gastritis predominantly affects the pyloric mucosa and, although inflammatory changes are generally scanty in the corpus, organisms may be found in the surface mucus in all areas, including the cardia. The mucosa shows a dense infiltrate of chronic inflammatory cells, in which plasma cells are especially prominent. In the corpus mucosa, any inflammation present is confined to the superficial zone. In the pyloric mucosa, inflammation is also predominantly superficial but may also involve the full thickness of the mucosa and may surround and separate the glands without causing atrophy. Lymphoid follicles with germinal centers are usually seen, particularly in the deeper portion of the mucosa straddling the muscularis mucosae (Fig. 32.12). This finding, which is easy to identify on low-power microscopic examination of biopsies, is highly suggestive of H. pylori infection (6). The surface and foveolar epithelium is infiltrated by neutrophils, which may be so prominent that pit abscesses are formed (Fig. 32.13). This neutrophil infiltration is termed active gastritis and is seen predominantly in areas where the Helicobacter organisms are most abundant (Fig. 32.14).
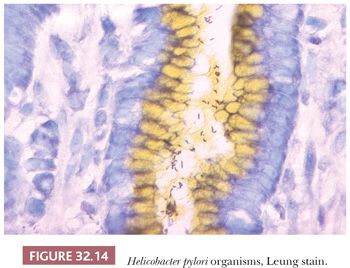
Often, the bacteria are easily seen on well-differentiated H&E sections, and it is not necessary to use Giemsa, modified Steiner, Leung (74), or immunohistochemical stains for routine diagnosis (75). The organisms are slender, curved spirals in the superficial mucous layer, where they tend to be attached to the epithelium at the site of intercellular junctions. Occasionally, following treatment with proton pump inhibitory drugs, H. pylori can be present in the stomach as coccoid forms (76). These are solid, round, basophilic, dotlike structures on routine histology. Ultrastructurally, they are U-shaped with the ends of the two arms joined by a membranous structure. Coccoid forms generally coexist with spiral forms but can be reliably identified only by specific IHC staining (77). It is important not to confuse coccoid forms of the organism with nonpathogenic cocci introduced into the stomach from the oropharynx during endoscopy.
Treatment of H. pylori gastritis with appropriate antibiotics will usually result in elimination of organisms and abolition of the neutrophilic infiltrate within 6 to 8 weeks (78). After this period, the finding of mucosal neutrophils usually indicates treatment failure (79). Chronic inflammation, however, is much slower to resolve. The corpus mucosa is usually normal 1 year after treatment, but the antral mucosa may be persistently abnormal for up to 2 years. Degenerate lymphoid follicles may persist in the antrum for up to 4 years (78). Some authors report that intestinal metaplasia remains unchanged following treatment (66), whereas others report a reduction (79,80).
Other intragastric organisms have been shown to produce gastritis histologically similar to H. pylori gastritis. Helicobacter heilmannii (formerly called Gastrospirillum hominis) is helical, measuring 3.5 to 7.5 µm in length and 0.9 µm in diameter, with truncated ends flattened at the tips and up to 12 sheathed flagella 28 nm in diameter (81). In North America and Western Europe, H. heilmannii is present in 0.3% to 1.1% of the general population (82). In parts of Southeast Asia, its prevalence may reach 6%. The organism is acquired by contact with household pets or farm animals (83). Most cases are symptomatic, producing dyspepsia, abdominal pain, and reflux. Biopsies from affected individuals show changes that are similar to, but usually less severe than, H. pylori gastritis. The same range of special stains that are used for H. pylori will also identify H. heilmannii organisms (82).
MULTIFOCAL ATROPHIC GASTRITIS
Multifocal atrophic gastritis (MAG) affects both the pyloric and corpus mucosa in a patchy fashion. The characteristic histologic feature is atrophy of the glands, which is accompanied by varying degrees of full-thickness chronic inflammation. Intestinal metaplasia is invariably present in the later stages but may not be found with minor degrees of atrophy. Early atrophy is difficult to identify in situations where there is an intense, full-thickness infiltrate in the lamina propria that pushes apart adjacent glands. Because minor degrees of atrophy do not seem to be clinically important, it is therefore recommended that in routine practice, MAG should be diagnosed only when there is intestinal metaplasia present. For scientific purposes, the diagnosis of atrophy without metaplasia should employ the principles described by Genta that require the use of trichrome stains to demonstrate both gland loss and lamina propria fibrosis (68).
Intestinal metaplasia is typically present as small mucosal patches of goblet cells and Paneth cells without the formation of villi. Adjacent to these patches, the mucosa may show active inflammation with pit abscess formation and Helicobacter infection. However, as intestinal metaplasia progresses, the gastric microenvironment changes so that inflammation lessens and H. pylori organisms are eliminated (84).
The generally held opinion is that most, but not necessarily all, examples of MAG represent the end stage of H. pylori gastritis (84,85). Factors promoting atrophy and metaplasia in H. pylori gastritis include the strain of the infecting organism (68,69) and host immunity factors (60,70,71).
AUTOIMMUNE GASTRITIS
This condition accounts for less than 5% of cases of chronic gastritis. There are usually no symptoms referable to the stomach, so biopsies are often obtained incidentally. Parietal cell antibodies are present in the serum of 60% of patients with autoimmune gastritis who have not yet developed pernicious anemia, and in 80% to 90% of patients with established pernicious anemia (64). In contrast, intrinsic factor antibodies are found in only 50% to 70% of patients with pernicious anemia but, when present, are diagnostically specific. It is becoming apparent that a causal link may exist between H. pylori and autoimmune gastritis, through the presence of cross-reacting canalicular antibodies (71,72,86). Epidemiologic evidence in support of this association comes from a study of first-degree relatives of patients with pernicious anemia (87). It has also been noted that patients with established pernicious anemia may experience an amelioration of anemia and elevation of vitamin B12 levels after treatment of H. pylori infection (88).
The histologic features of autoimmune gastritis include a diffuse inflammatory infiltrate in the lamina propria around the glands of the gastric corpus, inflammatory destruction of glands, intestinal or pyloric metaplasia, parietal cell pseudohypertrophy, and linear ECL hyperplasia, best demonstrated by IHC (89). The superficial mucosa is usually free of inflammation. Parietal cell pseudohypertrophy is thought to be secondary to hypergastrinemia and is recognized by “snouting” (luminal cytoplasmic projections).
In advanced disease, when pernicious anemia may be present, the histology is that of a severe atrophic gastritis or gastric atrophy, mainly affecting the corpus mucosa (49). There is a diffuse mucosal thinning, with partial or complete loss of acid and enzyme-secreting cells, and a variable increase of chronic inflammatory cells within the lamina propria. However, lymphoid follicles and an active gastritis are not usually present (Fig. 32.10). The surface and pit-lining epithelium generally shows patchy complete intestinal metaplasia with goblet cell formation. Less commonly, villi and Paneth cells may be encountered. Patients may also develop a variety of polyps including hyperplastic polyps, pseudopolyps, and adenomas (90).
Because patients with autoimmune gastritis have hypochlorhydria or achlorhydria, they also develop a compensatory hyperplasia of antral G cells with hypergastrinemia. Hypergastrinemia then causes ECL cell hyperplasia in the gastric corpus. In the early stages of hyperplasia, increased numbers of endocrine cells line the lower foveolar and glandular portion of the mucosa in a linear fashion. In the later stages, nodular hyperplasia of fundic ECL cells and microcarcinoid tumors can develop (see “Neuroendocrine Tumors”).
ATROPHIC AUTOIMMUNE PANGASTRITIS
This is a rare and recently described form of gastritis (49,91). Patients have a background of systemic autoimmune and connective tissue diseases such as lupus erythematosus, refractory sprue, or autoimmune enteropathy. Parietal cell and intrinsic factor antibodies may be present but, more typically, serum autoantibodies such as antinuclear antibodies are encountered. Biopsies show an intense pangastric mucosal chronic inflammatory infiltrate consisting predominantly of plasma cells and CD3+ lymphocytes. There is no association with H. pylori infection and no hypergastrinemia or endocrine cell hyperplasia.
CARDITIS
Inflammation of the cardia of the stomach (carditis) is of particular interest because of its possible relation to gastroesophageal reflux, short-segment Barrett esophagus, and gastric cardiac cancer. However, discussion of this topic is hampered by practical considerations revolving around the definition and delineation of the normal gastroesophageal junction. By strict anatomic criteria, this junction occurs where the tubular esophagus becomes the saccular stomach. The squamocolumnar junction may coincide with the anatomic junction but may also be found as much as 3 cm proximal to this point, with the intervening lower end of the esophagus being lined by cardiac or oxyntocardiac mucosa. By endoscopic examination, the squamocolumnar junction is generally easily recognized. The endoscopic esophagogastric junction (EGJ) is considered to be the point where the proximal gastric mucosal folds terminate and the mucosa becomes flattened. This corresponds to the distal margin of the lower esophageal sphincter. Without being told by the endoscopist where a biopsy was obtained, it may be impossible for a pathologist to determine whether it is from the cardiac gastric mucosa or from mucosa at the lower end of the esophagus.
It has been suggested that immunostains for cytokeratin subsets CK7 and CK20 will reliably distinguish short-segment Barrett esophagus from cardiac intestinal metaplasia (92,93). Characteristically, cardiac metaplasia has superficial and deep CK20 staining with weak or absent CK7 staining, whereas Barrett metaplasia has superficial CK20 staining with intermediate and deep CK7 staining. However, other authors have reported that these markers are unreliable (94,95). The variable results may reflect differences in fixation and staining methods, so at the present time, analysis of cytokeratin subsets cannot be recommended for use outside specialist centers.
The biopsy appearances of carditis may vary from a superficial gastritis resembling DAG, characterized by the presence of neutrophil infiltration of the foveolar and surface epithelium, to an atrophic gastritis resembling MAG, which shows foci of intestinal metaplasia (96). Carditis is present in 75% to 95% of unselected patients undergoing endoscopy (97,98). The prevalence is the same in men and women but increases with age (99). Intestinal metaplasia at the cardia is present in 10% to 22% of unselected patients (96,98).
The diagnosis of carditis is not controversial, but its significance is. Two schools of thought exist: one considers carditis the result of gastroesophageal reflux (100,101); the other, that it is caused by H. pylori infection (96,97,102–104). Data exist to justify both opinions. A study comparing the prevalence of carditis in individuals with and without symptomatic gastroesophageal reflux disease (GERD) concluded that it is similar in both groups (96). Active carditis is found in 89% of patients in which H. pylori is present within the stomach (105) and, furthermore, the intensity of carditis parallels that of distal gastritis (99). A similar association exists between cardiac intestinal metaplasia and metaplasia of the distal stomach (98). The activity of carditis lessens when H. pylori is treated (106). On the other hand, studies of individuals with clinical features of GERD have shown that carditis is present in 34% to 100% of cases (97,101,103). In contrast to H. pylori carditis, GERD carditis is not associated with inflammation elsewhere in the stomach (107). Intestinal metaplasia at the cardia is detected in 3% to 10% of patients with GERD but without H. pylori infection (108,109). Furthermore, there is a close connection between the severity of GERD as measured by 24-hour pH monitoring and the intensity of carditis (66). Patients with carditis secondary to GERD are likely to have fewer neutrophils in the epithelium than those individuals with carditis secondary to H. pylori (50% with GERD vs. 89% with H. pylori) (110).
Reconciling these two theories and sets of data may be achieved by assuming that both are correct and that there are two major causes of carditis (48). Therefore, at the present time, it is suggested that biopsies from the cardia be diagnosed descriptively, noting the type of epithelium and the presence or absence of inflammation, H. pylori, and metaplasia.
GRANULOMATOUS GASTRITIS
Although granulomatous gastritis is rare, a variety of infectious and noninfectious diseases must be considered in the differential diagnosis. The extent of gastric involvement is variable, although typically, the antrum is the major location. In the early stages, the only finding may be isolated granulomas in mucosal and submucosal tissue. In advanced disease, inflammation spreads to the muscularis propria, producing thickening, fibrosis, and often pyloric stenosis (111).
Tuberculosis is still an important consideration in the differential diagnosis, particularly if the granulomas are caseating. Especially in malnourished, alcoholic, and immunosuppressed individuals, the diagnosis may be unsuspected and there may be no obvious lung disease. When patients have obvious pulmonary tuberculosis, the frequency of gastric involvement has been estimated as 0.57% (112,113).
Fungal granulomas, occurring as a result of a fungemia in immunosuppressed patients, may also produce caseation and necrosis; however, in most cases, the cause will usually be obvious from the clinical picture. The organisms responsible include Candida, Histoplasma, and Phycomycetes (114–116). In nonimmunosuppressed individuals, fungi may be found growing in the craters of peptic ulcers among inflammatory debris. These are of little clinical significance, do not produce granulomas, and do not influence the rate of ulcer healing (117).
Rare cases of gastric syphilis have been described (118). In the tertiary stage of the disease, the stomach wall becomes fibrotic, with widespread endarteritis and poorly formed granulomas (gummas) present. Secondary syphilis of the stomach is characterized by nonspecific submucosal edema, early fibrosis, and large numbers of plasma cells in the lamina propria.
There are isolated case reports describing gastric granulomas in patients with Whipple disease (119). However, in most instances, the organisms of Whipple disease (Tropheryma whippeli) are present within histiocytes that are not organized into granulomas. Rarely, granulomas have been described in patients with H. pylori gastritis (111). These may result from foveolar injury with escape of mucin into the adjacent lamina propria.
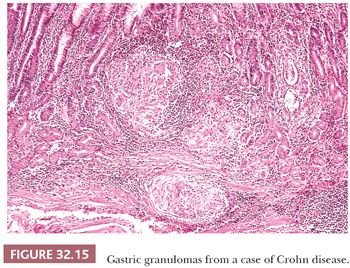
Noninfectious diseases are the usual causes of gastric noncaseating granulomas, and five major differentials should be considered: Crohn disease (120), foreign body reaction, tumor-associated granulomas (121), sarcoidosis (122), and isolated granulomatous gastritis (123,124). Crohn disease rarely produces gross clinical involvement of the stomach (Fig. 32.15). However, microscopic Crohn disease is present in about 35% to 40% of patients who also have terminal ileal involvement (113,120). In only 10% of these cases are granulomas present, although the likelihood of finding them increases with the number of biopsies examined. Active focal inflammation with a background of normal mucosa (focally enhanced gastritis) is the pattern usually encountered. Inflammation consisting of neutrophils and chronic inflammatory cells may be centered on foveolae, glands, or lamina propria. The lymphocytes are typically CD3+ and the histiocytes CD68+. Focally enhanced gastritis is more frequently encountered in the antral mucosa than the corpus mucosa (120). Small numbers of patients with Crohn disease may also have lymphocytic gastritis. Because gastric involvement is almost invariably synchronous with Crohn disease of the ileum or colon, exclusion of disease at these sites will eliminate Crohn disease as a cause of unexplained gastric granulomas.
Isolated granulomatous gastritis is the term applied to granulomatous inflammation confined to the stomach for which no obvious cause can be found (123,124). In some series, it accounts for about 25% of all gastric granulomas (123), but in others, it is rare (124). Before making a diagnosis of idiopathic granulomatous inflammation, it is important to exclude Crohn disease and other less common causes.
Gastric sarcoidosis (122) and isolated granulomatous gastritis are similar histologically: both have bland granulomas with little associated nonspecific inflammation. However, they tend to affect significantly different groups of patients. Sarcoidosis is more common in young black persons, whereas isolated granulomatous gastritis is more common in the older white male population. Sarcoid of the stomach is very frequently associated with granulomatous inflammation elsewhere, usually lungs, hilar lymph nodes, skin, or salivary glands. Exceptionally, gastric granulomas have been associated with Wegener disease (125) and xanthogranulomas extending from an adjacent chronic cholecystitis (126).
EOSINOPHILIC GASTRITIS
Eosinophils in small numbers are a normal component of the lamina propria of the stomach. Studies of control patients have found a mean density of 15.5 eosinophils per mm2 with a range of 0 to 110 per mm2 (127). It is also important to remember that a low-grade increase in eosinophils may be present in gastric mucosal biopsies in a variety of conditions, for example, Helicobacter gastritis (mean density, 25 per mm2; range, 0 to 219 per mm2) (127), MAG, allergic granulomatosis (Churg-Strauss syndrome) (128), Löffler syndrome (129), and Crohn disease (mean density, 31.4 per mm2; range, 0 to 203 per mm2) (127). In idiopathic granulomatous gastritis, sheets of eosinophils are present (mean density, 529 per mm2; range, 127 to 2108 per mm2) and the diagnosis may be relatively straightforward. In marginal cases, pathologists must use their subjective judgment to determine when a diagnosis of idiopathic eosinophilic gastritis is appropriate (130).
Large numbers of eosinophils may accompany invasion of the gastric wall by parasitic worms, such as Eustoma rotundatum (131) and Anisakis (132). In many instances, particularly in children, eosinophilic infiltration of the stomach is due to a food allergy, usually to milk or soy protein (133). Eosinophils in the gastric mucosa may also be encountered in some patients with connective tissue disorders (scleroderma, polymyositis, and dermatomyositis). These conditions are characterized by a bandlike eosinophil infiltrate in the lower lamina propria, just superficial to the muscularis mucosae (134).
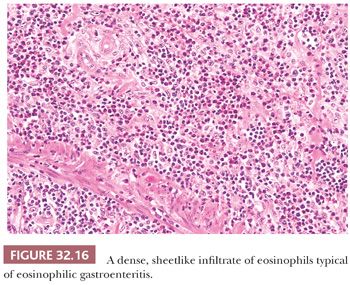
When all known causes of eosinophilic infiltration have been excluded, the diagnosis of idiopathic eosinophilic gastritis may be considered (135–137). Biopsies typically have more than 127 eosinophils per mm2 (>30 eosinophils per high-power field [hpf]) (127). This condition may involve any part of the GI tract but is most common in the antrum. In a particular patient, the inflammation tends to be segmental and maximally affect a single layer of the gut wall. Mucosal involvement will, therefore, result in nausea, vomiting, and abdominal pain. Involvement of the muscularis propria will produce thickening and scarring, often with pyloric stenosis. Serosal involvement, which is the rarest pattern, will produce a localized peritonitis with ascites. Specimens from either endoscopic biopsy or partial gastrectomy show an intense, but patchy, infiltration by sheets of eosinophils (Fig. 32.16), which displace and distort the mucosal pits, producing occasional pit abscesses. Mucosal edema and vascular congestion are present, sometimes with necrosis of the surface epithelium and small erosions. Deep ulcers are uncommon, however. A diagnosis of eosinophilic gastritis should not be made unless the eosinophilic infiltration is massive and sheetlike. A useful ancillary finding is the presence of a peripheral blood eosinophilia, noted in up to 75% of patients with idiopathic eosinophilic gastroenteritis (135).
LYMPHOCYTIC GASTRITIS
Lymphocytic gastritis is an uncommon condition, being present in 0.83% of unselected gastric biopsies and in 1.7% to 4.5% of inflamed gastric biopsies (138). Mild cases of lymphocytic gastritis appear normal endoscopically and are usually symptomless (138). More advanced cases may be characterized by mucosal nodules, surmounted by shallow aphthoid erosions (varioliform gastritis or chronic erosive gastritis) (139). However, it is not clear whether all examples of varioliform gastritis are the result of lymphocytic gastritis, or whether this endoscopic lesion can be caused by other diseases (139). Severe lymphocytic gastritis is a rare condition that may present with prominent mucosal folds, epithelial protein loss, and bleeding (140). This lesion has been termed hypertrophic lymphocytic gastritis and has some clinical similarities to Ménétrier disease (140,141).
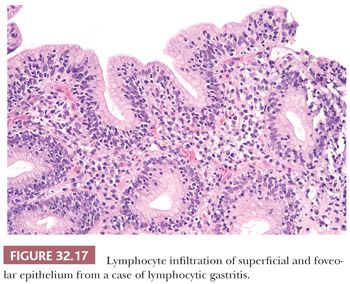
The essential diagnostic feature of lymphocytic gastritis is the presence of increased mucosal lymphocytes, both in the lamina propria as well as the surface and foveolar epithelium (Fig. 32.17). The glands are generally uninvolved. Twenty-five lymphocytes per 100 epithelial cells is considered the minimum for diagnosis, although in most cases, 35 to 65 lymphocytes per 100 epithelial cells are seen and formal counting is not required to establish the diagnosis (142). The lymphocytes are small and round with no nuclear atypia. Commonly, they are surrounded by a clear halo that is usually considered to be a shrinkage artifact. Rarely, small lymphoepithelial lesions containing up to three cells may be encountered. The overwhelming majority of lymphoid cells mark as T lymphocytes.
It is presumed that lymphocytic gastritis represents a T-cell response to an intraluminal antigen. The condition occurs most commonly in adults and children with celiac disease (38% of cases) (142–144) and in patients with H. pylori infection (29% of cases) (142,145,146). Therefore, a finding of lymphocytic gastritis should always prompt clinical consideration of occult or latent celiac disease. Between 10% and 45% of adult patients with celiac disease will have lymphocytic gastritis (143). Only 4% to 10% of H. pylori–infected patients will have lymphocytic gastritis, and these patients’ biopsies will also have epithelial neutrophil infiltration (142,146). Antral predominant lymphocytic gastritis is more likely to be celiac disease–associated, whereas corpus-predominant disease is more likely H. pylori–associated (142). Rare cases of lymphocytic gastritis have a subepithelial collagen band often 30 to 40 µm and sometimes 120 µm in thickness that entraps capillaries but does not extend down alongside foveolae. The band is generally separated from the surface basement membrane by a thin zone of edematous lamina propria. These cases have been labeled collagenous gastritis (147–149). In adults, collagenous gastritis occurs in association with lymphocytic colitis, collagenous colitis, or celiac disease. In children, however, the disease presents with anemia and is unassociated with colitis and celiac disease. Childhood collagenous gastritis may have a nodular mucosal pattern at endoscopy.
Other associations of lymphocytic gastritis include Crohn disease, malignant lymphoma, human immunodeficiency virus (HIV) infection (142), Epstein-Barr virus (EBV) infection (150), and common variable immunodeficiency (CVID) (151). In about 18% of cases, lymphocytic gastritis occurs as an isolated finding (142).
REACTIVE GASTROPATHY
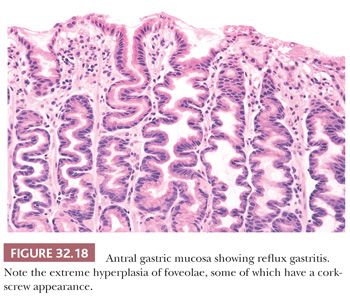
Reactive gastropathy, also known as reflux gastritis and chemical gastritis (50), is now the second most common diagnosis made on gastric biopsies performed in North America. As originally described in postgastrectomy stomachs (152), the mucosa shows edema, congestion, foveolar hyperplasia often producing a corkscrew appearance, and paucity of mucosal inflammatory cells (Fig. 32.18). Individual foveolar cells show mucin depletion with enlargement and diffuse hyperchromasia of nuclei. These are unlike the nuclei in active Helicobacter gastritis, which are vesicular with prominent nucleoli. The glandular portion of the mucosa is unaffected. Identical findings have also been described in the prepyloric region of intact stomachs with demonstrated bile reflux (153). These changes are considered to represent the results of increased surface cell exfoliation; thus, they are not specific for reflux and are encountered in 34% to 45% of patients taking NSAIDs (154). In a substantial proportion of cases, the cause is unexplained (155). Reactive gastropathy secondary to duodenal reflux is a diffuse abnormality that is maximal in the prepyloric mucosa. When the changes are the result of NSAIDs, they are patchy in distribution. The minimal criteria required for diagnosis of reactive gastropathy are foveolar cell mucin depletion and nuclear hyperchromasia occurring in an uninflamed biopsy. Dixon et al. (152) have developed a “reflux score” where five features (foveolar hyperplasia, edema and smooth muscle fibers in the lamina propria, vasodilatation and congestion of superficial mucosal capillaries, paucity of acute inflammatory cells, and paucity of chronic inflammatory cells) are graded on a 0 to 3+ scale. The maximum score is 15, and scores of 10 and higher are regarded as indicative of reflux gastropathy. Marginal changes may be reported as suggestive but nondiagnostic of reactive gastropathy. Of interest, not all authors have encountered these findings in duodenogastric reflux. Niemela et al. (156) observed that some cases of reflux were associated with mucosal infiltration by mononuclear cells, neutrophils, and eosinophils as well as with foveolar hyperplasia. This observation supports the suggestion that gastroduodenal reflux may be the cause of prepyloric ulcers in a mucosa already damaged by Helicobacter gastritis (157). In this clinical situation, it is hardly surprising that biopsies might show a mixed histologic picture.
RADIATION AND CHEMOTHERAPY GASTRITIS
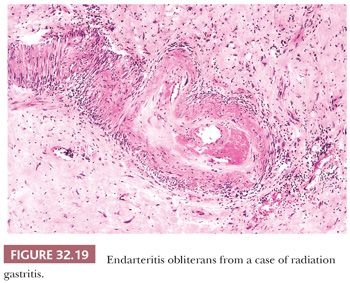
Assessment of radiation changes in the stomach is sometimes difficult because of the complicating effects of the underlying disease for which treatment was given. Generally speaking, the lesions will be similar to those produced by radiation elsewhere in the body. Small doses up to 13 Gy will cause degenerative changes in epithelial cells and a nonspecific chronic inflammatory infiltrate in the lamina propria. This phase is followed, after a period of about 4 months, by recovery with restoration of the normal structure. Intermediate doses of radiation (13 to 35 Gy) will produce permanent mucosal damage, usually in the form of atrophy of the corpus glands. Above 35 Gy, more serious damage is likely. Initially, there may be surface epithelial loss, resulting in mucosal erosions, which expose a lamina propria containing dilated, bleeding capillaries. Later, the erosions heal, producing a profound atrophic gastritis (158,159). At this stage, deep, nonhealing ulcers may form that are not responsive to peptic ulcer therapy and may require surgical excision. These are caused by localized mucosal ischemia resulting from a submucosal obliterative endarteritis (Fig. 32.19).
In the early stages of regeneration after radiotherapy, the gastric mucosa may show atypical changes that are not always easy to distinguish from true dysplasia and superficial carcinoma (Fig. 32.20). Criteria that favor a diagnosis of radiotherapy atypia include the following: patchy distribution of changes with a flat gross appearance, foveolar and glandular involvement with surface maturation, retention of nuclear polarity with vesicular nuclei containing prominent nucleoli, mitoses confined to foveolae with an absence of atypical mitoses, cytoplasmic degenerative changes characterized by eosinophilia and vacuolation, atypical changes within fibroblasts and endothelial cells, and absence of adjacent intestinal metaplasia (160).
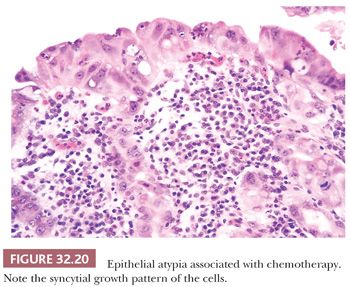
Distinguishing a peptic ulcer from a radiation-induced ulcer by mucosal biopsy is likely to be difficult, if not impossible. A similar diagnostic problem will also be encountered with gastrectomy specimens because peptic ulcers may also have extensive endarteritis at their base. However, examination of gastric vessels at some distance from the ulcer is likely to be more helpful in defining radiation as the cause, because these, too, will show radiation changes consisting of neovascularization, telangiectasia, and intimal obliterative lesions (158,159).
Gastric epithelial atypia has been described in individuals who have received chemotherapy for malignant disease (161). 5-Fluorouracil, administered by hepatic artery infusion for treatment of liver tumors, has been particularly incriminated. High concentrations of the drug may reach the stomach via collaterals or catheter displacement. Gastric ulceration may follow 10 to 45 days later. Grossly, the lesions resemble typical peptic ulcers, but biopsy reveals marked cytologic atypia of the adjoining epithelium. The features of atypia are essentially similar to those encountered after radiotherapy, although in some cases, epithelial cell nucleoli can be so prominent as to mimic cytomegalovirus inclusions (162).
ISCHEMIC GASTRITIS
Lesions of the stomach resulting from vascular disease are uncommon, probably because of the richness and diversity of the gastric blood supply and the presence of abundant vascular anastomoses. Minor ischemic damage may result in reactive gastropathy (163). However, in some circumstances, ischemia may produce superficial erosions (164) and even deep ulcers (165). Occasionally, biopsies may reveal the ghost outlines of glands and pits containing columns of necrotic epithelial cells with pyknotic nuclei (166). Ischemic ulcers are difficult to distinguish from peptic ulcers but are typically antral in location and irregular in shape, with sloping edges and a sclerotic white base. The adjacent mucosa frequently contains multiple erosions (165). In most cases, the underlying cause appears to be showers of atherosclerotic emboli, arising from the celiac and superior mesenteric arteries, although rarely, vasculitis of mural and mesenteric veins may be responsible (167).
It seems that a specific ischemic gastritis analogous to ischemic colitis or necrotizing enterocolitis does not occur in the stomach. Acute ischemia, due to shock or poor cardiac output, results in a hemorrhagic and erosive gastritis that may bleed profusely but heals rapidly when gastric perfusion is restored.
GASTRITIS RESULTING FROM DRUG THERAPY
Drug therapy is an extremely common cause of gastritis and may produce a variety of morphologic appearances. Alcohol (54), aspirin (54,55), and NSAIDs (55) are well-recognized causes of acute erosive gastritis and may produce a hemorrhagic or nonhemorrhagic lesion. Chronic use of these drugs may lead to more extensive ulceration. When compared with the usual type of gastric peptic ulcer, drug-induced ulcers are deeper and larger. They have little associated chronic gastritis and intestinal metaplasia and are not accompanied by H. pylori organisms.
NSAIDs may also result in reactive gastropathy (154). It seems probable that this arises from a direct irritative effect of the drugs on the mucosa rather than being the result of a drug-induced increase in bile reflux. Reactive gastropathy is not seen in all patients taking NSAIDs and it is particularly difficult to diagnose in biopsies where there is a concurrent H. pylori gastritis (168).
Long-term treatment by proton pump inhibitors (PPIs) can produce an exacerbation of H. pylori gastritis, which may spread to involve the corpus mucosa more severely (169). Some studies report an increase in the prevalence of gland atrophy (4.7% for PPI patients vs. 1% to 3% for non-PPI patients) (170). Other studies have been unable to confirm this finding (171). This may be due to differences in the way atrophy is diagnosed (167). PPIs do not produce gastritis in H. pylori–negative patients.
The hypochlorhydria associated with long-term PPI therapy stimulates linear hyperplasia of antral G cells and the resulting hypergastrinemia causes linear or nodular hyperplasia of the corpus ECL cells (172). Ultimately, small well-differentiated neuroendocrine tumors may develop, but to date, no fatal carcinoid neoplasms have been recorded (173). Within a few weeks of commencing PPI therapy, patients develop changes in corpus glands. These consist of gland dilatation with swelling and bulging of the corpus mucosa (so-called parietal cell protrusion or snouting). The gland lining becomes serrated rather than smooth (174,175). This effect is probably also related to hypergastrinemia and is independent of H. pylori infection (176). With prolonged high-dosage PPI therapy, the cytoplasm of the parietal cells expands and becomes bubbly secondary to dilatation of cytoplasmic canaliculi. In some (but not all) patients, long-term PPI therapy may result in the formation of multiple fundic gland polyps. These are usually multiple, sessile, and measure less than 10 mm in diameter (174). They are present in 17% of individuals after 3 months PPI treatment and in 35% of individuals after 5 months treatment (175). Anecdotal reports indicate that they may regress when PPI treatment is stopped, only to recur if treatment is resumed (174).
Other drugs may produce specific gastric lesions. Aluminum-containing antacids can precipitate in the stomach, resulting in mucosal calcinosis (177). This appears as small, deeply pink, partially calcified refractile crystals beneath the surface epithelium. The crystals contain aluminum, phosphorus, calcium, and phosphate (177). Patients with chronic renal failure or renal transplants are particularly vulnerable to this complication.
Iron-containing compounds can produce superficial mucosal erosions, deeper ulcers, or infarct-like necrosis. Similar lesions have also been described after doxycycline therapy (178). Following iron therapy, crystalline iron is deposited in the lamina propria, embedded within granulation tissue, and present within macrophages or in vessel walls (179). The appearances of oral iron toxicity must be distinguished from systemic iron overload (hemochromatosis) where iron is present in glandular epithelial cells (180).
Kayexalate in sorbitol is used to manage hyperkalemia in patients with chronic renal failure. It may produce ulcers covered by an inflammatory exudate. Angulated crystalline material may be present either within the surface epithelium or within ulcer slough (181).
Gastric mucosal alterations are observed in patients taking colchicine when the drug reaches toxic levels (49). This is typically seen in early renal or hepatic failure. The epithelium shows pseudostratification, loss of polarity, and numerous metaphase mitotic figures. Characteristic ring mitoses may also be identified.
GASTRITIS IN IMMUNOSUPPRESSED PATIENTS
Immunosuppression may occur in patients with the acquired immune deficiency syndrome (AIDS), in bone marrow and solid organ-transplant recipients, in patients receiving anticancer chemotherapy, or in patients with inherited immunologic defects.
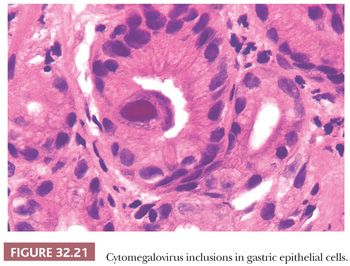
Cytomegalovirus (CMV) infection is one of the more common problems in immunosuppression. The disease is more frequent and more severe in the small bowel and colon but can affect the stomach. In the early stages, the endoscopic appearances may be normal, but on biopsy, typical eosinophilic intranuclear inclusions (Fig. 32.21) may be found, which may or may not be accompanied by smaller, purple-staining, granular cytoplasmic inclusions and a patchy, nonspecific infiltrate in the lamina propria. Initially, the inclusions are present in epithelial cells of the lower portion of the foveolae or in the upper part of the glands. In the more advanced disease, ulceration occurs and the inclusions become more frequent in mesenchymal cells, usually endothelium. Severe CMV gastritis is manifested by necrosis of epithelium with ulceration (182), leading to severe hemorrhage or even perforation. Mucosal thickening and polyps may also be encountered. In children with CMV infection, the hypertrophic mucosa may be similar to Ménétrier disease (183).
Gastritis due to herpes simplex virus (HSV) is rare (184). Basophilic, ground-glass intranuclear inclusions are found in epithelial cells, and in situ hybridization (ISH) with biotinylated DNA probes may be used to prove their identity (184). Other viral infections described in the stomach include varicella-zoster (185) and measles (186).
In AIDS patients, the protozoon organism Cryptosporidium produces persistent, watery diarrhea with vomiting and colicky abdominal pain (187). Organisms may be found in the stomach as well as in other parts of the GI tract, where they can be recognized in routine H&E-stained sections as rounded basophilic objects 2 to 4 µm in diameter, adherent to surface epithelial cells. Cryptosporidia stain positively with PAS, Giemsa, and Gram but stain negatively with Gomori methenamine silver. Other examples of protozoa that may rarely cause gastritis include Leishmania (188), Toxoplasma (189), and Giardia (190).
Mycobacterium avium-intracellulare (MAI) may be found in gastric biopsies from AIDS patients. The appearances often resemble Whipple disease with the lamina propria being filled by a monotonous infiltrate of foamy histiocytes. Granuloma formation and caseation are rare, although ulceration may be present (191). Both MAI and T. whippeli bacteria are PAS-positive, but only MAI is positive with a modified Ziehl-Neelsen stain.
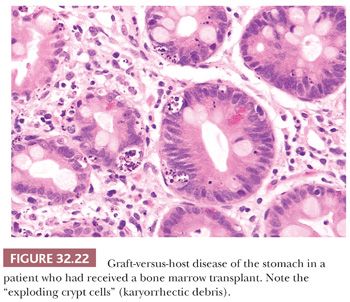
Gastric graft-versus-host disease (GVHD) may be encountered after allograft bone marrow transplantation. The appearances are similar to those encountered in GVHD at other locations in the GI tract, although the stomach is usually the least severely affected site. Early changes consist of isolated epithelial cell necrosis (Fig. 32.22), with associated karyorrhectic debris and pit or gland dilatation. This lesion, called an exploding crypt cell, may rarely be encountered in other diseases, for example, CMV infections. In severe disease, mucosal denudation will occur (192).
Chronic granulomatous disease involves the gastric antrum, where it may present with outlet obstruction (193). Distinct granulomas with necrosis may be present, or there may simply be a poorly organized mass of histiocytes, multinuclear giant cells, lymphocytes, and eosinophils. The presence of pigment containing macrophages is highly suggestive of the diagnosis.
The stomach in CVID may show lymphocytic gastritis and abundant lymphoid aggregates. Granulomas are rare (151).
GASTRITIS IN PATIENTS WITH IDIOPATHIC INFLAMMATORY BOWEL DISEASE
Clinically apparent gastritis is present in only 2% to 7% of patients with gastric Crohn disease. However, endoscopic and histologic evidence of disease may be encountered in 34% to 83% of cases (120). Generally, this occurs in individuals who already have obvious clinical disease in the terminal ileum (49). Gastritis is particularly common in children. The histologic lesions include granulomas and focally enhanced gastritis. The granulomas are often small and loosely aggregated. Giant cells may or may not be present. Focally enhanced gastritis consists of localized aggregates of lymphocytes and plasma cells in the lamina propria plus neutrophil or eosinophil infiltration of the foveolar or glandular epithelium (49,120,194).
Although focally enhanced gastritis was initially described in Crohn disease, it is now apparent that it may also be found in up to 21% of patients with ulcerative colitis (195). In addition, ulcerative colitis patients may have superficial plasmacytosis; basal mixed inflammation; multiple tiny, shallow erosions; and foveolar abscesses. The intensity of the gastric inflammation parallels the remissions and relapses seen in colonic disease.
PEPTIC ULCER DISEASE
By definition, a peptic ulcer results from the effects of acid on the mucosa; it may be acute or chronic. A chronic ulcer is defined clinically as one that does not heal over a reasonable period and is defined histologically as one that has fibrosis in the base and walls. An acute gastric ulcer is merely a large erosion that has penetrated through the muscularis mucosae into the wall of the stomach. Sectioning a chronic ulcer reveals three distinct zones: a superficial zone of necrotic slough and fibrin, infiltrated by neutrophils; a middle zone consisting of chronically inflamed granulation tissue; and a deep zone consisting of fibrous tissue with vessels showing endarteritis obliterans.
All benign gastric ulcers, except those that are the result of treatment by NSAIDs, are surrounded by areas of acute and chronic inflammation. The pattern of gastritis will differ depending on the location of the ulcer. Ulcers in the pyloric canal and in the immediate prepyloric area are accompanied by DAG and by the presence of Helicobacter. They have the same clinical and epidemiologic features as duodenal ulcers. More proximal ulcers, usually in the region of the incisura, are typically accompanied by MAG and intestinal metaplasia. Adjacent to these ulcers, H. pylori are either absent or present in smaller numbers.
At the edge of ulcers of all types, reactive epithelial changes can occasionally be confused with dysplasia or a carcinoma. It is important to diagnose these as accurately as possible. A vague report indicating the presence of “mild epithelial atypia” is not clinically useful. Examples of reactive changes are shown in Figures 32.9 and 32.13; examples of dysplasia are shown in Figures 32.34 and 32.35 in “Gastric Dysplasia.” Biopsies fixed in Bouin fluid or other nonformalin fixatives have very sharply defined basophilic nuclei in which the nucleoli are more prominent than those in biopsies fixed in formalin. Allowance should be made for this. Foci of intestinal metaplasia may show foveolae with basal nuclear crowding and increased mitotic activity similar to normal small bowel. However, toward the surface, epithelial maturation is identified. Inflammatory epithelial changes are typically of varying severity, with a gradual transition from normal to atypical. The transition between dysplastic and normal epithelium tends to be abrupt. Reactive nuclei are vesicular and rounded, with one or two enlarged nucleoli. Dysplastic nuclei are enlarged, crowded, and compressed and tend to have overall hyperchromicity (196). The appearances of gastric dysplasia are similar to those encountered in colonic adenomas. Adjacent to peptic ulcers, atypia is most severe in the areas of severe inflammation. Dysplasia may also show focal inflammation, but the less inflamed areas are equally atypical. When a clear-cut diagnosis is impossible, the term indefinite for dysplasia may be applied, and the mucosa should be rebiopsied after anti-inflammatory therapy.
Gastric hyalinization is a rare condition that may be a consequence of either a gastric ulcer or perimortem damage to the stomach mucosa (197). It is hypothesized that luminal acid leaks into the submucosa, producing thickening by an amorphous hyaline material.
THE POSTGASTRECTOMY STOMACH
A variety of histologic lesions have been described in the postgastrectomy stomach. MAG with intestinal metaplasia is commonly present (198), but it is not clear whether this develops after the gastrectomy or reflects a preexistent condition for which the gastrectomy was performed. Reactive gastropathy (152) commonly involves the gastric remnant after surgery, and in some stomachs, it may be more prominent than atrophic gastritis. Inflammatory polyps may occur in postgastrectomy stomachs, particularly in the region of the stoma.
The risk of developing cancer in patients who have had a subtotal gastrectomy is disputed. Some studies (199) have been unable to demonstrate any increased risk over a control population. Other investigators (200) have shown an excess of gastric cancer incidence that is not significant 20 years after gastrectomy but increases to 7% after 40 years. In Norway, 9.5% of all stomach cancer patients have had a previous partial gastrectomy (200). The cancer that develops is an intestinal-type adenocarcinoma located in the distal gastric remnant (201). In many instances, these carcinomas are preceded by dysplasia of the gastric mucosa (198).
POLYPS
A classification of gastric polyps is given in Table 32.3 (202,203). Enlarged mucosal folds are included because there is some clinical overlap between prominent folds and polyposis. Gastric polyps may be encountered in 6.35% of individuals undergoing esophagogastroduodenoscopy (202). Although histologic examination is the key to differential diagnosis, much practical diagnostic help can also be obtained by determining (a) if a biopsy comes from a discrete polyp or from a prominent mucosal fold, (b) whether a polyp is sessile or pedunculated, (c) whether polyps are present in other parts of the GI tract, and (d) whether the surrounding mucosa is normal.

PEUTZ-JEGHERS SYNDROME
Peutz-Jeghers polyps most commonly present in childhood or adolescence and are usually regarded as hamartomatous in type. Most are 1 to 3 cm in size, with a coarsely lobulated surface and a short, broad stalk. Histologically, the most useful diagnostic feature is the presence of a core of finely arborizing branches of smooth muscle from the muscularis mucosae (204). The core is covered by abundant mucosa, histologically identical with the normal stomach, but often disorganized. It consists predominantly of surface and foveolar epithelium, although occasional antral glands and small cysts may be found. Distinguishing gastric Peutz-Jeghers polyps from gastric juvenile polyps may be difficult as the histologic differences are not as clear-cut as with hamartomatous polyps occurring at other locations within the GI tract (205). Peutz-Jeghers polyps are considered to be at a low risk of evolving into a carcinoma. Most families with Peutz-Jeghers syndrome (PJS) have an autosomal dominant mutation of the serine/threonine kinase gene STK11 (206).
JUVENILE POLYPS
Juvenile polyps, sometimes called retention polyps, are rounded, smooth-surfaced lesions averaging 1 to 2 cm in diameter, with a short, narrow stalk. They consist principally of lamina propria that contains irregularly shaped cysts lined by normal gastric surface epithelium (207,208). Repeated episodes of torsion may produce stromal hemorrhage, surface ulceration, and secondary chronic inflammation. The appearances of juvenile polyps may mimic those of hyperplastic polyps; however, differential diagnosis is aided by consideration of the clinical setting. In rare instances (208), juvenile polyps may be confined to the stomach. There is a small but definite risk of colorectal carcinoma developing in patients with juvenile polyposis and also recent evidence of an increased risk for gastric cancer (208).
Juvenile polyps in the stomach almost invariably occur in the context of juvenile polyposis. This is a heterogeneous disorder and may or may not be familial. Familial juvenile polyposis may be due to mutation of the SMAD4 gene (chromosome 18) and the BMPR1A gene (part of the transforming growth factor [TGF]-β pathway).
COWDEN DISEASE
Cowden disease (included in the PTEN hamartoma syndrome) is an extremely rare syndrome in which there is an association between skin lesions on the face (typically tricholemmomas) and GI polyps. There is also an increased incidence of breast and thyroid carcinomas in these patients. The gastric lesions are generally sessile polyps, a few millimeters in diameter. The histology is poorly documented, and there is some diversity between different polyps. Generally, they contain splayed excess lamina propria and are dissected into lobules by disorganized fascicles of muscularis mucosae running upward from the base of the mucosa (204). Mutations in the PTEN gene have been described in Cowden disease and in a related condition, the Bannayan-Riley-Ruvalcaba syndrome (209).
HYPERPLASTIC POLYPS
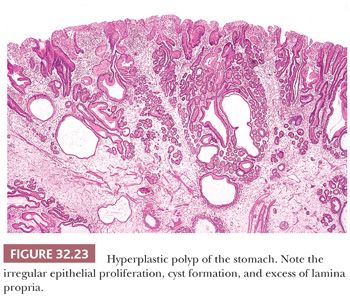
Hyperplastic polyps, formerly called regenerative polyps or hyperplaseogenous polyps, are common in both children and adults and account for 17% of polyps (202,203,210). Typically, they occur at the junction of pyloric and corpus mucosa but may also be encountered at the gastroesophageal junction (211,212). Hyperplastic polyps are often multiple and generally vary from 0.5 to 2.5 cm in diameter, with an average size of 1.4 cm (213), and have a coarsely lobulated surface. Small polyps tend to be sessile, but the larger ones may have a short, broad stalk (Fig. 32.23). The presumed histogenesis is an exaggerated regenerative response to mucosal damage; in many cases, the adjacent nonpolypoid mucosa shows chronic atrophic gastritis. Hyperplastic polyps of the stomach are structurally similar to inflammatory colonic polyps (pseudopolyps) but are not related to hyperplastic colonic polyps. In contrast to gastric adenomas, hyperplastic polyps rarely (2% to 3.5%) undergo malignant change (210,213), with the risk of malignancy largely being confined to polyps more than 2 cm in diameter. For this reason, and to avoid diagnostic confusion, it is strongly recommended that the older terminology of hyperplastic adenomatous polyp not be used.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


