SPECIMEN HANDLING
The gross examination of mass lesions in the liver in resection specimens should include number and size of nodules, macroscopic vascular invasion, distance of tumor from inked surgical margins, and an assessment of any macroscopic changes (such as cirrhosis or steatosis) in the nonneoplastic liver. Features such as tumor color, consistency, cystic and degenerative changes, necrosis, and presence of a fibrous capsule are helpful diagnostic clues and should be included but are not factors in tumor staging. Recommendations for reporting of primary hepatic tumors are available in checklist format from The Royal College of Pathologists (5a) and the College of American Pathologists (www.cap.org). One paraffin block per 1 to 2 cm of tumor diameter is adequate sampling for most hepatic mass lesion; small tumors (1 to 2 cm in diameter) should be submitted in toto. For known metastatic tumors, one or two sections per tumor deposit are generally sufficient, although additional sections may be needed if the tumor is largely necrotic. Sections should be selected to include tumor interface with nonneoplastic parenchyma, tumor relationship to hepatic capsule and any resection margins, any areas suggestive of biliary or vascular invasion, and nonneoplastic liver distant from the tumor. The surgical pathology report should include, in appropriate cases, comments on the presence and absence of hepatocellular dysplasia, ductal dysplasia, cirrhosis or fibrosis, iron overload, and hepatitis in the uninvolved liver.
The most widely applied ancillary technique to evaluate hepatic mass lesions is immunohistochemistry, discussed in greater detail later in this chapter. Electron microscopy is rarely indicated in the routine evaluation of liver tumors, but it may be helpful in carefully selected cases, such as the evaluation of poorly differentiated spindle cell lesions that fail to mark with cytokeratin (CK). Other techniques, such as flow cytometric DNA analysis, clonality studies, and chromosome or oncogene analysis, may be useful in selected cases.
INTRAOPERATIVE CONSULTATION
The primary purpose of frozen section of a liver mass lesion is to differentiate between benign and malignant lesions and often to document metastatic disease in a patient with intraabdominal carcinoma, such as colorectal or pancreatic carcinoma. Intraoperative consultation using frozen sections is generally accurate for assessment of hepatic lesions, with overall accuracy of 95% (6). Ideally, the pathologist should be informed of relevant clinical information, such as a history of chronic liver disease or prior malignancy, and should have reviewed any previous biopsy or resection material. The most common cause of diagnostic error is confusion of the biliary hamartoma (von Meyenburg complex) with adenocarcinoma (6). False-negative results are generally due to sampling, either from not cutting deeply enough into the frozen section block or not selecting the lesional tissue for frozen section. In addition to establishing the diagnosis, the pathologist may be called upon to evaluate the distance of the tumor from the surgical margins. For malignant lesions, primarily for colorectal carcinoma metastases, many surgeons have adopted a 1-cm margin as a target to minimize hepatic recurrence (7); however, some studies suggest that the width of a negative surgical margin does not affect survival or recurrence risk (8,9).
BIOPSY TYPES (FINE-NEEDLE ASPIRATION AND THICK-NEEDLE CORE BIOPSIES)
Most biopsies of hepatic mass lesions are performed under radiographic (ultrasound, endoscopic ultrasound, or CT) guidance or under direct visual observation during laparoscopy or open surgical procedures. Thick-needle biopsies provide optimal tissue morphology, allow for more accurate classification of benign lesions such as focal nodular hyperplasia, and provide tissue for molecular and other ancillary studies. However, FNA biopsy has several recognized advantages for diagnosis. Multiple aspirations with immediate assessment of biopsy material for adequacy can be safely performed, and biopsies can be obtained from patients with ascites, obstructive jaundice, or other contraindications to cutting needle biopsy. The sensitivity of guided FNA for a diagnosis of hepatic malignancy is roughly 92%, with a specificity close to 100% (10), in both adult and pediatric patients (11), similar to that reported for cutting needle biopsies (12). Combined use of FNA and cell block preparations can improve diagnostic yield and facilitate histologic subtyping (10). Reticulin staining of cell block preparations can help enhance diagnostic accuracy for well-differentiated HCC. Immunohistochemical studies to classify lesions more precisely and help pinpoint primary sites for metastatic tumors may also be performed on cell blocks.
HISTOLOGIC FINDINGS ADJACENT TO LIVER TUMORS
The hepatic parenchyma adjacent to a mass lesion may show a variety of nonspecific changes, some probably related to localized bile duct obstruction and alterations in blood flow. Portal tracts are expanded by edema and increased chronic inflammation, but confusion with chronic hepatitis can be avoided by noting that these changes diminish with increased distance from the mass. Bile ductular reaction with associated neutrophils is often due to local biliary obstruction. Distortion of hepatocellular plates, with alternating areas of sinusoidal dilation and compression, may be seen and, when well-developed, approaches the changes seen in focal nodular hyperplasia (13). When a mass lesion is suspected clinically but only portal edema, bile ductular reaction with neutrophils, and focal sinusoidal dilation are present in the biopsy, consideration should be given to sampling deeper levels of the biopsy tissue or obtaining an additional biopsy (14).
CYSTIC MASSES
This section includes discussion of true cysts, which have an epithelial lining, and nonepithelial-lined tissue-enclosed spaces that may contain liquids or solids, listed in Table 37.3. Cysts due to infectious agents (parasites and bacteria) are included in this section.
ABSCESSES
Amebic Abscesses
Hepatic abscess, the most common extraintestinal manifestation of Entamoeba histolytica infection (15), complicates amebiasis in 3% to 9% of cases. Amebiasis may be the most common cause of hepatic abscess in the cirrhotic liver in some areas (16) such as Thailand. In the United States, amebic liver abscess is rare, and the incidence appears to be decreasing (17). Travel to and origin in an endemic area are important risk factors, and development of amebic abscess may be a manifestation of immunodeficiency, including HIV infection (18). Patients with amebic abscesses tend to be younger men with a solitary right lobe abscess, compared to patients with pyogenic abscesses, who tend to be older than 50 years of age. Young Hispanic males living in southwestern states are most commonly affected in the United States (17). An amino acid substitution in the cytokine receptor homology domain of the leptin receptor may confer susceptibility to amebic intestinal infection and liver abscess (19).
The trophozoite reaches the liver through the portal system. The earliest amebic hepatic abscess lesions probably start as microabscesses that coalesce into a single mass lesion varying in size from less than 1 cm to huge lesions almost replacing the liver. The central cavity contains a homogenous thick, orange-brown liquid (often described as “anchovy paste”), which is bacteriologically sterile unless secondary bacterial infection has occurred. This material consists of necrotic hepatocytes and cellular debris surrounded by a shaggy necrotic fibrinous zone and a layer of granulation tissue in which the organisms are typically found (20), supported by a fibrotic rim of compressed liver. Extrahepatic extension of the abscess is not uncommon.
The trophozoites of E. histolytica are oval to round, measuring 10 to 60 µm in diameter. The small, single, round nucleus contains a distinctive central karyosome and has a thick, beaded nuclear membrane. The cytoplasm often contains phagocytosed red blood cells. Trophozoites may be confused with macrophages, which have a reniform rather than round nucleus and a more delicate nuclear membrane. The trophozoites can be identified on hematoxylin and eosin (H&E)–stained sections or with the periodic acid-Schiff (PAS) stain, iron hematoxylin preparations, or the Diff-Quick stain. Material from the center of the abscess yields fewer organisms than material obtained from the periphery. The aspirate fluid should be examined in both saline wet mounts and stained preparations. In diagnostically challenging cases, polymerase chain reaction (PCR)–based testing can be performed on aspirate fluid (21); E. histolytica DNA has also been detected by this technique in blood, urine, and saliva in patients with amebic liver abscesses (22).
Because serologic tests for the diagnosis of invasive amebiasis give positive results in more than 95% of cases (23), abscess aspiration is performed only in select cases. Metronidazole is the drug of choice, and surgical treatment is indicated only for complicated cases. Amebic liver abscesses usually resolve within 6 months, but mortality in complicated cases may be as high as 6% in developing countries (23).
Pyogenic Abscesses
Pyogenic abscesses remain uncommon surgical pathology specimens, although the incidence in the United States is increasing (24). The single most common etiology is from seeding of bacteria from a diseased biliary tract (43%) (25). Other causes include intra-abdominal infections such as appendicitis and diverticulitis, with seeding of the portal blood, generalized septicemia, and direct extension from a localized contiguous infection such as subphrenic abscess or perforated cholecystitis. The source of infection remains occult in up to 40% of cases (26). Diabetic patients are at greater risk than the general population, with a relative risk of 3.6 (27) to 11.1 times the general population (28), and constitute roughly 50% of patients with pyogenic liver abscesses (29). Higher rates of colorectal cancer are reported in patients with Klebsiella pneumoniae liver abscesses (30).
Similar to amebic abscesses, pyogenic liver abscess are usually found in the right lobe (31); they are usually single but are more likely to be multiple than amebic abscesses (32), and they vary in size. The pyogenic liver abscess, like its counterparts in other organs, contains creamy yellow, foul-smelling pus with abundant neutrophils admixed with necrotic liver tissue. A fibrous capsule containing a mixed inflammatory infiltrate may be present. Although Escherichia coli has historically represented the single most frequently recovered bacterium, the most common pathogen in many recent series is K. pneumonia, and a new hypervirulent strain has emerged as a cause of life-threatening infections in young immunocompetent hosts in Asia (33). Most cases of pyogenic abscess are treated with antibiotics, either alone or in combination with percutaneous drainage (34). Overall mortality, roughly 10% in some series (26,28), is related to the patient’s age and comorbidities such as diabetes mellitus (27).
ECHINOCOCCOSIS (HYDATIDOSIS)
Echinococcal infection, a zoonosis, is a common cause of hepatic cysts worldwide, particularly in the Middle East, Greece, Australia, North Africa, and some parts of South America. In the United States, it is principally seen in immigrants from these countries. It is caused most typically by Echinococcus granulosus, the agent of cystic hydatid disease; however, Echinococcus multilocularis, a tapeworm of red foxes that causes alveolar hydatid disease, is emerging as a significant pathogen in Europe (35) and is endemic in Canada and Alaska (36). Definitive diagnosis is based on imaging and serologic findings. Serum assays are about 90% sensitive for E. granulosus; however, false-positive results do occur. Serum assays for E. multilocularis have a high sensitivity and specificity. PCR-based assays are also available.
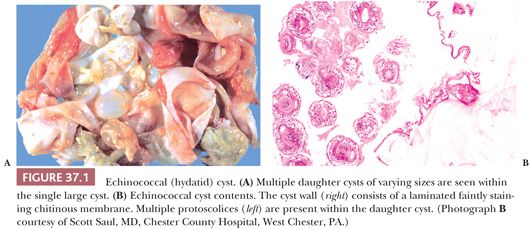
The large unilocular hepatic cysts of E. granulosus are rounded and fluid-filled. They are single in 75% of cases and are clinically silent in many cases but become symptomatic when they reach roughly 10 cm in diameter. The cyst wall contains three layers: an innermost germinal layer with nuclei; the laminated membrane, an avascular refractile layer; and an outer adventitial layer consisting of dense fibrovascular tissue with a varying number of chronic inflammatory infiltrate. Calcification of the outer layer in long-standing cysts is a radiologic clue to diagnosis. In fertile cysts, multiple daughter cysts representing detached brood capsules containing protoscolices are usually found within the cyst (Fig. 37.1). The daughter cysts give the appearance of internal septations seen on imaging studies in 25% to 50% of cases. The cyst fluid or hydatid sand obtained in aspirated specimens consists of daughter cysts, protoscolices, and loose hooklets but does not contain inflammatory cells.
E. multilocularis infection (alveolar echinococcosis) is limited to the Northern hemisphere; this organism is more tissue-invasive, with an infiltrative growth pattern that may mimic malignancy (37) on gross examination. Imaging techniques are the primary diagnostic tool, with serologic detection of specific antibodies used for confirmation of diagnosis and for epidemiologic studies (38). The hepatic lesions of alveolar echinococcosis consist of numerous small, irregular cysts (alveolar vesicles) ranging in diameter from less than 1 mm up to 20 mm. The lesions are poorly circumscribed and have an infiltrative appearance. The central part of the lesion is often necrotic, with dystrophic calcification. In contrast to hydatid cysts, brood capsules and protoscolices are rarely seen.
In the past, cystic hydatid disease has generally been treated with surgical resection, but the introduction of the PAIR (Puncture of cysts percutaneously, Aspiration of fluid, Introduction of protoscolicidal agent, and Reaspiration) method, in conjunction with treatment with albendazole, is often used as an alternative (36). Because FNA biopsy–derived hydatid cyst fluid may be submitted for cytologic analysis, a diligent search of all hepatic cyst fluid for hooklets, intact protoscolices, or laminated membrane is advisable. The treatment of choice for E. multilocularis infection remains complete surgical resection; debulking procedures offer no survival advantage (36).
NONPARASITIC NONNEOPLASTIC CYSTS
Solitary (Unilocular, Simple, Congenital) Cyst
Solitary hepatic cysts are unilocular biliary-type nonneoplastic cysts not associated with the presence of cysts in other organs, such as kidney; their occurrence antenatally and in newborns supports a congenital origin (39). They are quite common and are found in up to 14% of autopsies when a diligent search is performed, although in routinely performed postmortem examinations, they are found in less than 1% of cases (40); radiographic studies estimate the prevalence at roughly 11% (41). Solitary hepatic cysts likely originate from von Meyenburg complexes (bile duct hamartomas), small remnants of the ductal plate that fail to involute and may give rise to cysts when they become discontinuous from the biliary tree. Most solitary cysts are clinically silent, but larger cysts may be associated with abdominal pain, nausea, or jaundice. Rarely, torsion, rupture, or hemorrhage into a cyst causes an acute abdominal crisis.
Most solitary cysts are located superficially in the liver and measure a few centimeters in diameter, although clinically apparent ones may range up to 40 cm. Grossly, the lining is flat and glistening and the cyst contains clear or bile-tinged fluid in the absence of superimposed infection or hemorrhage. Microscopically, the cyst lining is composed of a single layer of columnar biliary-type epithelium or attenuated cuboidal or flattened epithelium. The underlying cyst wall is composed of fibrous tissue and lacks the dense mesenchymal “ovarian-type” stroma often found in biliary cystadenomas.
Ciliated hepatic foregut cysts are distinguished from simple biliary cysts by their ciliated lining and the presence of smooth muscle in the wall (42). They are postulated to arise from the embryonic foregut and show differentiation along bronchial lines. Unlike simple biliary cysts, which are more common in women and usually found in the right lobe of the liver, the ciliated foregut cyst is more common in men and in the left lobe (42).
Nonneoplastic hepatic cysts may show degenerative or metaplastic changes, such as squamous metaplasia, and the cyst contents may calcify. Complete surgical excision is often performed, and there is a small risk of malignant transformation, with adenocarcinoma or adenosquamous carcinomas the most frequently reported tumor types (43). Laparoscopic deroofing (44) and sclerotherapy are also effective therapies.
Polycystic Liver Disease and Other Hepatic Fibrocystic Disorders
Polycystic liver disease (PCLD) belongs to the family of fibrocystic diseases of the liver, a largely autosomal recessive group of disorders resulting from ductal plate malformation and often associated with renal cystic disease. Other disorders in this group, depending on the level of the biliary tract affected, are congenital hepatic fibrosis, Caroli disease, Caroli syndrome, and multiple von Meyenburg complexes. Although patients with autosomal dominant polycystic kidney disease (ADPKD) have hepatic cysts histologically identical to those of PCLD, the two disorders are now considered separate entities.
ADPKD is the most common hereditary renal disease, affecting from 1/400 to 1/1000 live births (45); hepatic cysts are rarely evident at birth but eventually develop in more than 90% of patients (46). Approximately 95% of ADPKD has been linked to mutations in one of two genes. PKD1, located on chromosome 16 and mutated in 85% of patients with ADPKD, encodes an integral membrane glycoprotein, polycystin-1. The second gene implicated in ADPKD, PKD2, located on chromosome 4, is responsible for 5% to 10% of cases and encodes an integral membrane protein known as polycystin-2. PKD1 and PKD2 form part of a mechanosensitive Ca2+ signaling pathway in renal tubule primary cilia; one function of this pathway is to inhibit renal tubular growth (47). Patients with PKD2 mutations are similar clinically to patients with PKD1 mutations, but they develop clinically apparent renal disease later in life (48). Although ADPKD is inherited in a dominant fashion, it is believed that the disease is recessive on a cellular level, in that loss of the wild-type allele in renal or biliary epithelial cells (the second-hit hypothesis) is necessary for cyst formation.
PCLD not associated with cystic kidney disease is much less common than multiple hepatic cysts arising in the setting of ADPKD. Two genes have been associated with the disorder: PRKCSH, which encodes the protein hepatocystin (the beta-subunit of glucosidase II), and SEC63, which encodes a component of the protein translocation apparatus in the endoplasmic reticulum (49). It remains unclear how aberrations in these proteins involved in protein trafficking in the endoplasmic reticulum lead to cyst formation (50).
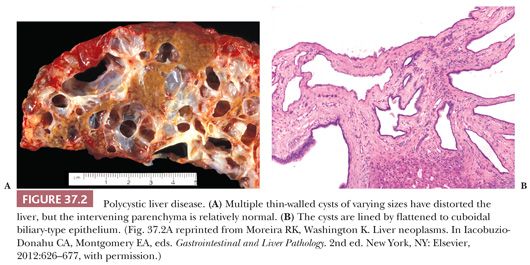
Liver cysts are not present at birth in patients with PCLD or ADPCKD, but they develop over time as fluid accumulates in the dilated biliary spaces of von Meyenburg complexes. Up to 30% of young adults will have liver cysts; this prevalence increases to 90% in older patients. Multiple unilocular cysts resembling simple biliary cysts and ranging in size from a few millimeters to more than 10 cm in diameter are scattered diffusely throughout the liver or, more rarely, are limited to one lobe (Fig. 37.2). The cysts usually do not compromise hepatic function but may produce hepatomegaly (sometimes massive) and abdominal discomfort. Women are more likely to be symptomatic from the cysts, and morbidity is related to the number of pregnancies and the use of oral contraceptive steroids (51). The severity of renal involvement is generally the more important determinant of patient outcome in ADPKD than the hepatic manifestations of the disease.
Hepatic complications of PCLD include cyst infection, intracystic hemorrhage, adenocarcinoma, Budd-Chiari syndrome, biliary obstruction, and portal hypertension. Treatment of hepatic cystic disease includes fenestration, radiologic cyst aspiration of sclerosis, liver resection, and in severe refractory cases, liver transplantation. Medical therapy aimed at slowing cyst growth includes somatostatin analogues and mammalian target of rapamycin (mTOR) inhibitors (51).
Congenital hepatic fibrosis (CHF), another of the fibropolycystic liver diseases, is also associated with renal cystic disease, most commonly autosomal recessive polycystic kidney (ARPKD), caused by mutations in the PKHD1 gene (52). The portal tracts are enlarged by fibrous tissue, often with portal–portal bridging, but the most distinctive feature is the presence of malformed biliary channels, often with inspissated bile, at the periphery of the portal tract. The portal vein branches are also affected and are generally decreased in number and smaller than usual. The hepatic lesions arise as the result of ductal plate malformation at the level of the interlobular bile duct. The changes of CHF may be mistaken for cirrhosis, but the characteristic patent biliary channels and lack of hepatocyte regeneration are features that help with the diagnosis of CHF. Four clinical forms of CHF are recognized depending on the clinical presentation: latent, cholangitic, portal hypertensive, and mixed cholangitic/portal hypertensive. Rarely, cholangiocarcinoma arises in the setting of CHF (43).
Caroli disease is characterized by saccular dilation of the intrahepatic bile ducts alternating with segments of normal caliber and is caused by ductal plate malformation affecting the larger intrahepatic bile ducts. When CHF is also present, the disorder is termed Caroli syndrome. Caroli disease and Caroli syndrome have been associated with ADPKD and ARPKD (53). Although the entire liver is usually involved, lobar or segmental disease, particularly with involvement of the left lobe, is not uncommon (54). The cysts are lined by biliary epithelium, which may be hyperplastic or attenuated or ulcerated in the setting of cholangitis. Inspissated bile and biliary stones are common in the dilated ducts, especially when cholangitis is present. The differential diagnosis includes primary sclerosing cholangitis; however, interlobular bile ducts are not obliterated in Caroli disease or Caroli syndrome, and prominent periductal fibrosis is generally not seen. As for most chronic inflammatory disorders involving the biliary tree, cholangiocarcinoma is a feared complication, arising in up to 14% of cases (55).
Multiple Hilar Cysts (Peribiliary Gland Cysts)
The walls of the large intrahepatic bile ducts and extrahepatic bile ducts contain peribiliary glands that are lined by mucinous or biliary type epithelium. Microscopic cystic dilation of these glands is not uncommon in autopsy or resection specimens but is generally an incidental finding. The cysts are usually multiple and range in size from 1 mm up to 2 cm or larger and occasionally result in obstructive jaundice (56). They are lined by a single layer of columnar to cuboidal or flattened epithelium and are typically associated with a mild, chronic inflammatory infiltrate in the pericystic fibrous tissue. Peribiliary gland cysts have been reported in patients with severe portal hypertension from cirrhosis and other causes (57), suggesting that altered blood flow in the liver may be related to the pathogenesis of the cysts in some cases. Some mucinous cystadenomas and hilar cholangiocarcinomas may arise from peribiliary glands.
Other Nonparasitic Cysts
Hepatic pseudocysts are similar to their pancreatic counterparts; they lack an epithelial lining and consist of a cystic space surrounded by fibrous tissue. They are generally the result of trauma (58) or hepatic involvement by a pancreatic pseudocyst (59). They may be quite large, often contain blood or bile, and like pancreatic pseudocysts are often secondarily infected. Other rare nonparasitic nonneoplastic cystic lesions of the liver include intrahepatic ileal and duodenal duplication cysts (60). Hepatic endometrioma is rarely reported (61).
NEOPLASTIC CYSTS
Biliary Cystadenoma and Cystadenocarcinoma
Mucinous Type. Mucinous cystadenomas of the liver are similar to their counterparts in the pancreas and account for approximately 5% of solitary hepatic cysts. These relatively uncommon tumors may be misdiagnosed as simple cysts, but it is important to make the correct diagnosis, given that the treatment of choice for biliary cystic neoplasms is complete excision to prevent recurrence. Up to 85% of biliary mucinous cystadenomas occur in women (62), and the distinctive mesenchymal ovarian-type stroma is found only in women. Mean age at diagnosis is roughly 45 years (63), although a wide age range, including childhood, is reported. Common presenting symptoms are probably due to mass effect and include abdominal discomfort or pain, abdominal distension, and less commonly, nausea and vomiting (64). Most mucinous cystadenomas are intrahepatic (84%), although location in biliary sites, such as the common bile duct (6%), hepatic ducts (4%), cystic duct (4%), and gallbladder (2%), is also reported (63).
Internal septations on radiographic imaging of a solitary hepatic cyst are highly suggestive of biliary cystadenoma (65), but calcifications, present in roughly 20% of cases, may lead to confusion with echinoccocal cysts. Elevated levels of CA19-9 and carcinoembryonic antigen (CEA) in cyst fluid may be helpful in distinguishing biliary cystadenomas from simple biliary cysts; reported CA19-9 levels in cyst fluid range from roughly 2200 to more than 1 million U/mL (normal, <33 U/mL) (66).
Intrahepatic mucinous biliary cystadenomas are solitary, sharply circumscribed lesions (Fig. 37.3). They range from 2.5 to 28 cm in diameter (mean, 15 cm) (63) and contain fluid which is usually clear and mucinous but is sometimes described as brown, gelatinous, or frankly hemorrhagic. Most cysts are multilocular. Although unilocular ones are occasionally encountered on gross examination, most will display microscopic locules in the cyst wall. Grossly, the internal surface of the cyst is smooth, with a delicate vasculature; a few trabeculations and papillary excrescences may be seen. Individual locules vary from a few millimeters to 18 cm in diameter. Solid areas are suggestive of malignancy and should be sampled thoroughly.
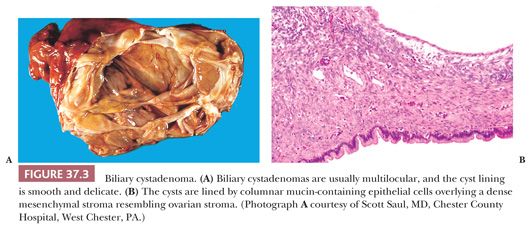
On microscopic examination, the cyst is lined by a single layer of biliary-type epithelium that contains basally oriented nuclei and apical mucin. The epithelium is usually columnar but may be focally flattened, denuded, or pseudostratified and may show areas of squamous metaplasia. Intestinal metaplasia is found in about 20% of cases, and neuroendocrine cells can be identified in about one-third of cases (63). The cyst lining may be ulcerated and the wall infiltrated by acute or chronic inflammation; occasionally, a xanthogranulomatous inflammatory response is seen (43). In one series (63), 10% of cases showed signs of dysplastic change (defined by cellular enlargement, stratification, hyperchromasia, and loss of polarity) and changes that in some classifications would be called borderline malignancy.
The dense subepithelial mesenchymal stroma found in 85% of cases resembles normal ovarian stroma and is usually found beneath a relatively acellular zone found just beneath the epithelial lining. This distinctive stroma is found only in tumors from women and is sometimes lacking in pediatric cases (43). The spindle cell stroma can be quite relatively inconspicuous but can be helpful on intraoperative consultation as a feature distinguishing biliary cystadenoma from simple biliary cyst. Other mesenchymal elements such as smooth muscle and mature adipose tissue are occasionally identified (63). The ovarian-type stroma expresses vimentin and is usually immunoreactive for muscle-specific actin and less often for desmin.
Cystadenocarcinomas are rare hepatic malignancies, accounting for 1% of primary hepatic carcinomas; one of the largest series of 18 cases was studied at the Armed Forces Institute of Pathology (AFIP) (63). They occur with nearly equal frequency in men and women, unlike biliary cystadenomas, and present roughly 15 years later than their benign counterparts (63). Most biliary cystadenocarcinomas arise in an underlying biliary cystadenoma; grossly, cystadenocarcinomas are multilocular, with exophytic or masses projecting into the cyst cavity (Fig. 37.4). These solid foci should be extensively sectioned (about one section per centimeter). In the AFIP series, roughly one-third of cases (all in women) displayed an ovarian-type stroma; in tumors from men, the underlying stroma is collagenous. Most cystadenocarcinomas have a papillary intracystic growth pattern, although cholangiocarcinoma-like tubular and solid patterns are also seen. Oncocytic as well as adenosquamous, squamous, hepatoid, and spindle cell variants (43) have been described. Stromal invasion is considered definitive evidence of cystadenocarcinoma, although some regard high-grade dysplasia with prominent cytologic atypia and complex architecture as sufficient to warrant a diagnosis of malignancy.
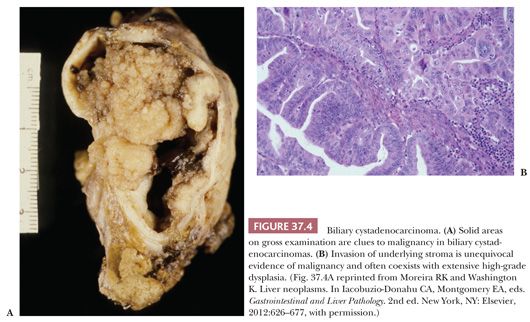
Treatment appropriate for simple hepatic cysts, such as aspiration, drainage, or fenestration, results in near-universal recurrence of biliary cystic neoplasms, and complete excision is recommended (65). Although some regard all hepatic mucinous cystic neoplasms to be of uncertain malignant potential, the prognosis for noninvasive and low-grade tumors that are completely excised is excellent. Frankly malignant tumors have a worse outcome, with roughly 50% of patients surviving for up to 4 years. The prognosis may be worse for men (63).
Serous (Microcystic or Glycogen-Rich) Type. Serous cystadenoma is very rare in the liver but is histologically identical to its pancreatic counterpart (43). The multiple small cystic spaces are lined by attenuated cuboidal epithelial cells with clear, glycogen-rich, mucin-negative cytoplasm. These lesions lack the dense ovarian-type stroma seen in most biliary mucinous cystadenomas.
Teratoma and Other Germ Cell Tumors
Hepatic teratomas are rare benign neoplasms composed of tissues derived from mesoderm, ectoderm, and endoderm (43). Most reported cases have occurred in children, usually within the first year of life. Occurrence of tumors in older patients should suggest spread to the liver and maturation of a malignant germ cell tumor originating elsewhere. True hepatic teratomas are described as large, cystic, and calcified. Commonly found tissues include squamous epithelium, gastrointestinal mucosa and smooth muscle, renal cortical elements, fat, and primitive neural elements. Outcomes are variable, with large tumor size associated with a high morbidity and mortality rate. Malignant germ cell tumors such as primary yolk sac tumor and choriocarcinoma as well as cases of combined hepatoblastoma or HCC with germ cell tumors have been reported (67,68).
Mesenchymal Hamartoma
Clinical Features. Mesenchymal hamartoma, a benign hepatic mass lesion typically composed of large, fluid-filled cysts and loose mesenchymal stroma, makes up about 6% of all pediatric liver tumors, as reported in a review of more than 1200 pediatric hepatic neoplasms (69), and is the second most common benign pediatric tumor (70). The tumor is more common in boys; 85% occur in children under the age of 3 years, with the majority diagnosed in the first year of life (71), and a few cases have been detected by prenatal ultrasound in the last trimester of pregnancy. Rarely, cases in adults are reported (72). Most pediatric patients present with abdominal distension or an upper abdominal mass; older patients and adults may present with abdominal pain or jaundice. Although liver tests are generally normal, the serum alpha-fetoprotein (AFP) level can be moderately to markedly increased (71,73), which could lead to an erroneous diagnosis of hepatoblastoma if biopsy or resection is not performed.
Although several hypotheses, such as a developmental malformation, localized vascular insult, or toxic injury (71), have been proposed to explain the pathogenesis of mesenchymal hamartoma, growing evidence suggests that the lesion should be considered a true neoplasm. Multiple studies have reported a balanced translocation involving the same breakpoint on chromosome 19q13.4 (71), and other chromosomal abnormalities have been reported, including the presence of aneuploidy. Undifferentiated sarcoma has been reported in association with mesenchymal hamartoma (71,74) and in some cases has been demonstrated to share the same cytogenetic abnormality (74).
Pathologic Findings. Mesenchymal hamartomas are typically solitary, unencapsulated, and well circumscribed, although small satellite lesions at the margin have been described (71). The tumors vary from 5 to 30 cm, with roughly 75% occurring in the right lobe. About 20% of cases arise from the inferior surface of the liver and are pedunculated. On cut section, multiple cysts, ranging in size from a few millimeters to 14 cm, are present in more than 90% of cases. The tumors in young infants may be solid (Fig. 37.5A), but in older patients, the lesion becomes fibrotic with age and large multilocular cysts may simulate a biliary cystadenoma. The cysts, which contain clear or mucoid fluid, alternate with solid, pink-white areas. Necrosis, calcification, and hemorrhage are rare.
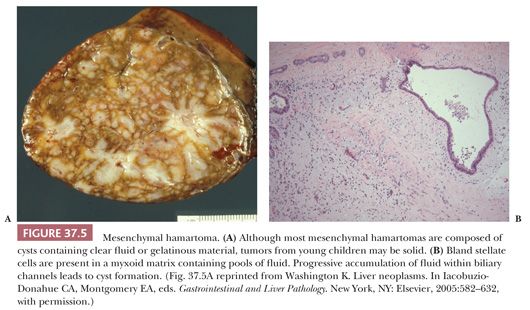
On microscopic examination, the mesenchymal hamartoma contains both mesenchymal and epithelial components. The stroma consists of relatively primitive mesenchymal tissue composed of bland stellate and spindle cells with features of myofibroblasts embedded in an edematous or myxoid matrix with a variable amount of collagen; origin of these spindle cells from activated hepatic stellate cells has been postulated (75). Fluid accumulation in areas of degenerated mesenchyme may lead to an alveolar pattern, simulating a lymphangioma (Fig. 37.5B). Proliferation of thick-walled veins may focally resemble a vascular malformation, and angiomatous elements have been described, including cases with features of hemangioendothelioma (71). Extramedullary hematopoiesis is present in the stroma adjacent to small bile ducts or vessels at the periphery of the lesion in more than 85% of cases (43). The smaller cysts are often lined by attenuated biliary epithelium; larger cysts may lack an epithelial lining and consist of pools of fluid accumulated in the stroma.
Hepatocytes are most commonly found in small plates at the periphery of the lesion; less commonly, they are interspersed as small nodules or single cells within the tumor mass. Small bile ducts, often with unusual contours and a branching pattern reminiscent of ductal plate malformation, are found within the stroma. The bile ductal elements may contribute to cyst formation by progressive dilation due to fluid accumulation. A periductal neutrophilic infiltrate may be seen; accumulation of bile within the ducts is rare. Frequently, the bile duct structures have a mesenchymal “collar” when they are located at the tumor periphery. Extension of the vascular stroma into adjacent hepatic parenchyma along portal tracts may contribute to the growth of the lesion (43).
The differential diagnosis for mesenchymal hamartoma includes infantile hemangioma, which may contain abundant myxoid foci, leading to confusion on biopsy. However, infantile hemangioma is composed predominantly of small vessels; bile ducts are small and do not demonstrate cystic dilation or irregular contours as in mesenchymal hamartoma. Features helpful in differentiating mesenchymal hamartoma from embryonal (undifferentiated) sarcoma are summarized in Table 37.23.
Although mesenchymal hamartoma is a benign lesion, progressive abdominal distention may be fatal in infants, and the treatment of choice is complete excision. Rarely, enucleation may be performed for otherwise unresectable massive tumors. Incomplete resection is not advisable because of the risk of symptomatic tumor recurrence and the potential risk of transformation to undifferentiated sarcoma.
EPITHELIAL TUMORS
BENIGN AND BORDERLINE HEPATOCELLULAR TUMORS
The three most important benign hepatocellular proliferations resulting in mass lesions are hepatocellular adenoma (HCA), focal nodular hyperplasia (FNH), and nodular regenerative hyperplasia (NRH). These masses, which arise in the normal or near-normal liver, are best separated from those that are associated with cirrhosis or other chronic liver diseases because of the recognition of the latter types of nodules as putative precancerous lesions. The benign hepatocellular lesions present formidable diagnostic challenges on needle biopsies or FNA, as they may simply demonstrate bland hepatocytes indistinguishable from normal liver. In the normal liver, FNH and HCA are most common; NRH is encountered less frequently as a mass in clinical practice but rather may mimic cirrhosis. In the cirrhotic liver, macroregenerative nodules (MRNs) and those nodules that display some, but not all, of the architectural and cytologic features of HCC (borderline nodules or dysplastic nodules [DNs]) are the important diagnostic considerations. In biopsy specimens, diagnosis of DNs and distinction from well-differentiated (WD)-HCC can be challenging. Clinical history and consideration of the possibility of clinically occult chronic liver disease are essential for arriving at a precise diagnosis for the benign and premalignant hepatocellular lesions. Major points of differential diagnosis are summarized in Table 37.4.
Other benign hepatocellular proliferations that should be considered are compensatory segmental or lobar hyperplasia (43), manifested as enlargement of one hepatic lobe following atrophy of another lobe secondary to ischemic injury or prolonged biliary obstruction; accessory lobes, a developmental anomaly; and regenerative nodules occurring in livers with massive necrosis.
HEPATOCELLULAR ADENOMA
HCA (Table 37.4) is a rare, benign hepatocellular neoplasm that arises in a normal or nearly normal liver. Four subtypes, based on molecular and immunohistochemical findings, are described—HNF1A-mutated, beta-catenin–activated, inflammatory (telangiectatic), and unclassified adenomas (76) (Table 37.5).
Clinical Features
The HNF1A-mutated HCA occurs in women using oral contraceptive pills (OCPs); other subtypes are seen in men in the setting of anabolic or androgenic steroid use and in the setting of fatty liver disease and metabolic syndrome (77). Up to 80% of young female patients have typically used OCPs. HCAs are rarely encountered in children, in whom they account up to 4% of all hepatic tumors (78), but are found more often in children with glycogen storage disease (most commonly type 1), where they may have features of inflammatory adenomas (79). Other associated metabolic disorders include familial diabetes mellitus and Hurler disease (80). In addition to the association with anabolic or androgenic steroids used to treat hypogonadism and chronic anemia and also taken by body builders, antiestrogen agents such as danazol and clomiphene are also linked to development of HCA (77).
Patients with HCA most often present with an abdominal mass (25% to 35%), chronic or intermittent abdominal pain (20 to 25%), or acute abdominal pain due to hemorrhage into the tumor or into the peritoneal cavity (43). Intraperitoneal rupture occurs in less than one-third of patients but is a medical emergency and is frequently associated with shock. Occasionally (<10% to 20%), HCA is an incidental finding discovered on imaging studies. Serum AFP levels are in the normal range.
Pathologic Findings
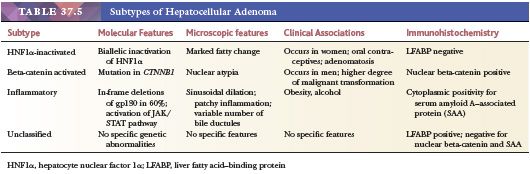
Macroscopic Features. HCA is usually a single, sharply circumscribed nodule (70% to 80%), often subcapsular and usually unencapsulated, in the right lobe of an otherwise normal liver (Fig. 37.6A). In 10% of cases, the tumor is pedunculated. HCAs range in size from less than 1 cm to 38 cm, with most lesions larger than 10 cm in diameter. Those arising in association with androgen use or storage disorders are more frequently multiple (43). Ruptured HCA may be obscured by blood clot and difficult to recognize, but the tumor is usually paler or more yellow than the adjacent liver. The cut surface has a variegated appearance, generally due to the presence of blood clot and yellow necrotic areas. Fibrous septa are usually a result of previous infarct. Some HCA appear slate-gray or black because of excessive lipofuscin accumulation; green discoloration due to bile staining is uncommon. Rare cases of coexistent FNH are well documented.
HISTOPATHOLOGY. On first glance, most HCAs resemble normal liver microscopically, being composed of virtually normal hepatocytes in cords that are one to two cells thick and separated by sinusoids lined by inconspicuous Kupffer cells. However, no normal portal tracts are present and there is a notable lack of any biliary epithelium in many lesions, although a variable ductular reaction is present in many inflammatory adenomas. Normal bile ducts are not identified. The third key feature of HCA is the presence of haphazardly distributed arteries and thin-walled veins (Fig. 37.6B). Hepatocellular rosettes (pseudoglands) should not be confused with bile ducts. The reticulin framework is largely intact. Extramedullary hematopoiesis and noncaseating epithelioid granulomas are rarely found. Chronic inflammatory infiltrates are often found in the portal tracts and lobules in the inflammatory subtype, which typically displays dilated sinusoids (Fig. 37.6C).
The hepatocytes of HCA frequently display clear or vacuolated cytoplasm due to accumulation of glycogen or fat. Cytoplasmic inclusions representing alpha1-antitrypsin (A1AT), megamitochondria, and Mallory hyaline are occasionally found. Focal cellular pleomorphism and prominent nucleoli may occasionally be seen, especially in tumors associated with androgenic steroid use. The presence of mitoses and vascular invasion is not seen in HCA and is suggestive of malignancy. Sinusoidal dilation is common in the inflammatory subtype; larger blood-filled (pelioid) spaces, a myxoid stroma, and areas of necrosis, infarct, and hematoma may also be seen. Organization of these foci may lead to the accumulation of hemosiderin-laden macrophages and the formation of fibrous scars with a lamellar pattern.
Four distinct subsets of HCA, based on genotypic analysis and morphology, have recently been described. Tumors exhibiting biallelic mutation of the HNF1A gene inactivating the hepatocyte nuclear factor-1α (HNF1α) account for up to 40% of HCAs (81); these tumors are characterized by marked steatosis, lack of cytologic atypia, lack of inflammatory infiltrate, and lack of expression of liver fatty acid–binding protein on immunohistochemical studies (76). A second subtype due to beta-catenin–activating mutations at hotspots in exon 3 comprises 10% to 15% of HCAs (76). These tumors display cytologic atypia, are more frequently found in men, and are more likely to undergo transformation to overt HCC. Although these lesions typically lack a classic trabecular pattern and vascular invasion, and display a relatively low nuclear-to-cytoplasmic ratio, cytogenetic changes typically observed in HCC have been demonstrated in them, suggesting that they represent low-grade HCC (82). The inflammatory (telangiectatic) subtype comprises up to 50% of adenoma and is characterized by activation of the JAK/STAT pathway (76). This subtype appears to be associated with excessive alcohol consumption, metabolic syndrome, and obesity (83). Many cases previously classified as the telangiectatic subtype of FNH now appear to be better classified as inflammatory HCA. Immunohistochemistry for serum amyloid A–associated protein shows granular cytoplasmic expression in inflammatory HCAs (Fig. 37.6D). Unclassified adenomas that lack the previously described molecular changes comprise approximately 10% of HCAs.
The term adenomatosis is sometimes used for those cases with multiple (usually more than 10) adenomas (84). Germline mutations of CYP1B1 and TCF1 have been reported in patients with familial hepatic adenomatosis (85); additional studies are needed to clarify the role of mutations in these genes in sporadic cases or adenomatosis.
Differential Diagnosis, Treatment, and Outcome
The detection rate for HCA using multiphase CT ranges from 86% to 100%, depending on whether enhancement techniques are used (86); MRI may be helpful in subclassification of adenomas, with good correlation with resection findings (87). Radiographic features of HCA include decreased or absent uptake on scintigraphy, centripetal hypervascularity with a central hypovascular region on angiography, and the absence of a central scar. Small needle biopsies and FNA material may be insufficient to allow distinction of HCA (Table 37.4) from other benign hepatocellular tumors such as FNH, from well-differentiated HCC, and from normal liver, although in many instances, HCAs and their subtypes can be diagnosed with confidence on core biopsy (88,89). In children, clinical features helping to distinguish HCA from the fetal pattern of hepatoblastoma are the lack of elevated serum AFP, older age, and association with metabolic disease in HCA. Histologic features favoring HCA over hepatoblastoma include larger cell size and the absence of an alternating light and dark cytoplasmic pattern.
Histologic overlap of the inflammatory variant of HCA with FNH is a source of diagnostic difficulty. Although the presence of bile ductules has traditionally been interpreted as favoring a diagnosis of FNH and excluding HCA, recent evidence has emerged indicating that the so-called telangiectatic variant of FNH is better classified as the inflammatory subtype of HCA (90). In addition, large HCAs may produce compression of the adjacent liver at the interface with the mass, with bile ductular reaction and the formation of short, fibrous septa in the adjacent nonneoplastic liver, which may be confused with FNH. Immunohistochemical studies demonstrating maplike expression of glutamine synthetase in FNH and cytoplasmic positivity for serum amyloid A–associated protein in inflammatory HCA may aid in making the distinction (81,89).
Distinction of HCA from well-differentiated HCC also causes diagnostic difficulties in some cases, particularly in tumors from men and in the setting of glycogenosis or androgenic steroid administration. It should be remembered that HCA arises in the normal or near-normal liver in the majority of patients. Most HCAs lack significant cytologic atypia and have an intact reticulin framework; a trabecular growth pattern indicates HCC. Demonstration of unequivocal vascular invasion is indicative of malignancy but may require large numbers of sections. The absence of large oncocytic hepatocytes with prominent nucleoli aids in differentiating HCA from fibrolamellar hepatocellular carcinoma (FL-HCC), and fibrosis is distinctly uncommon in HCA. Because HCA generally lacks the numerous chromosomal abnormalities seen in HCC, fluorescent in situ hybridization or comparative genomic hybridization may be useful in selected cases (91).
Although HCA may regress after withdrawal of OCPs, the lesions often persist. The likelihood of rupture and bleeding cannot be predicted from size of the tumor alone, although risk is higher with tumors greater than 5 cm. Malignant transformation of HCA occurs only in tumors larger than 4 cm and is primarily seen in men with tumors with beta-catenin mutation (76). The risk of HCC developing in the context of HCA related to glycogen storage disease type 1a also generally affects males (43).
Because of the risk of bleeding, complete surgical excision of HCAs, if technically feasible, has been the gold standard because of the risk of bleeding and malignant transformation. However, more conservative approaches have been proposed for small lesions without beta-catenin activation in women (76). Close follow-up of patients with HCAs is recommended for tumors that are incompletely excised, demonstrate cellular pleomorphism, or arise in the absence of exogenous hormones.
FOCAL NODULAR HYPERPLASIA
Clinical Features
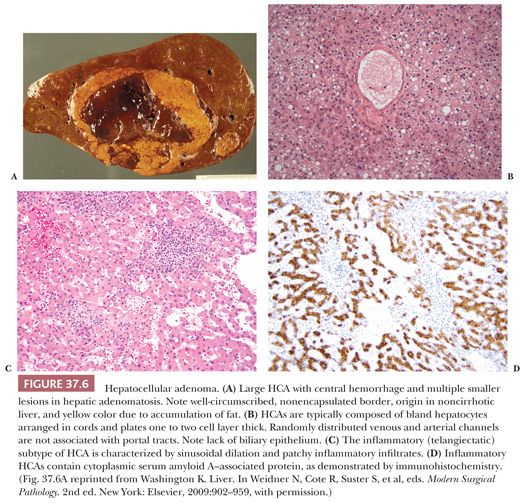
FNH (Table 37.4) is a mass lesion composed of hyperplastic hepatocytes arranged around an anomalous arterial supply. It usually arises in an otherwise normal liver, although it may also occur in livers with vascular alterations. FNH is found in both sexes and in patients of all ages; although older studies report a striking female preponderance (43), other more recent studies indicate that a sizeable percentage occur in men (92). Peak age of diagnosis is in the third and fourth decades, with mean age of 37 years (92). Up to 15% of cases occur in the pediatric age group, in which FNH represents 2% to 10% of all pediatric hepatic tumors (93). Most cases of FNH are asymptomatic and the lesion is discovered incidentally (80%) at surgery or autopsy (43). An abdominal mass (10% to 15%) is the most common complaint in symptomatic patients. The putative association of FNH with OCPs remains unresolved, although recent evidence suggesting that the telangiectatic variant of FNH should be reclassified as HCA may explain the apparent association (90). OCP use may promote FNH growth but does not appear to induce FNH formation. Generally, FNH remains static over time. Serum AFP levels are within the normal range.
FNH arises as a result of local overgrowth of nonneoplastic hepatocytes around a vascular anomaly, particularly an arterial malformation, although associations with other aberrant blood flow states such as portal vein atresia and portosystemic shunt have been reported, and hyperplastic nodules with features of FNH have been reported in hepatic venous outflow obstruction (94). FNH occasionally occurs with coexisting hepatic cavernous hemangiomas, but multiple FNHs more frequently have this association, and FNH is much more common in the setting of hereditary hemorrhagic telangiectasia than in the general population (95). Patients with multiple FNHs who have one or more other lesions, including hepatic hemangioma, systemic arterial structural defects (arterial dysplasia, Klippel-Trenaunay-Weber syndrome, brain telangiectasia, and berry aneurysm), or brain tumors (astrocytoma and meningioma), are considered to have the multiple FNH syndrome. FNH-like nodules also occur in cirrhotic livers and may cause confusion with HCC on imaging studies (96). FNH may occur after treatment for childhood cancer, with hematopoietic stem cell transplantation identified as the most important risk factor in these cases (97).
Pathologic Findings
Macroscopic Features. FNH is usually solitary (70% to 80% of cases) and subcapsular, ranging in size from 1 cm to over 10 cm, although most nodules measure 5 cm or less (Fig. 37.7A). The lesion is sharply circumscribed but unencapsulated and may bulge about the surface of the liver but is rarely pedunculated. On cut surface, FNH is tan and lighter in color than the adjacent liver, which is essentially normal. A central stellate scar with radiating fibrous septa dividing the mass into multiple smaller nodules is a characteristic finding. Lesions smaller than 1 cm often lack a central scar, whereas multiple scars are more common in large tumors. FNHs rarely demonstrate intratumoral hemorrhage or necrosis. Green discoloration from bile accumulation is distinctly uncommon, and this lack of bile staining aids in making the distinction between FNH and FL-HCC, which is frequently bile-stained. The telangiectatic type of FNH, now considered a form of HCA, is distinguished in macroscopic features from the much more common solid type of FNH described earlier by the presence of multiple dilated vascular channels in the center of the mass, which may resemble hemangioma or peliosis (98). Other variant types are described, such as a mixed hyperplastic adenomatous form and FNH with cytologic atypia (98).
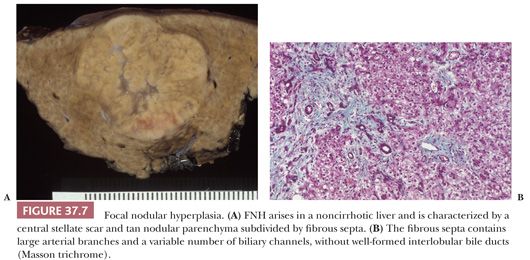
Macroscopic Features. FNH is composed of nodules of bland hepatocytes surrounded by fibrous septa that contain artery branches, bile ductules (a variable but key feature) (43), a variable chronic and/or acute inflammatory infiltrate, and decreased or absent interlobular bile ducts and portal vein branches (Fig. 37.7B). The degree of bile ductular reaction is variable, ranging from a few inconspicuous biliary channels to numerous proliferating structures resembling a bile duct adenoma. Normal portal tracts and central veins are lacking in the nodule. The central scar is composed of dense collagen and contains numerous thick-walled arteries and bile ductules. The arteries may display fibromuscular hyperplasia. In the telangiectatic variant, numerous small, dilated vessels (resembling hemangioma) are seen centrally, and the adjacent sinusoids are markedly dilated (98,99). The histologic features of typical FNH overall resemble a biliary type of cirrhosis with ductopenia.
The hepatocytes in FNH lesions are similar to those in the surrounding liver, although they may be somewhat larger and paler and may incorporate variable quantities of fat or glycogen. Mallory hyaline and other features of chronic cholestasis such as feathery degeneration and accumulation of excess copper may be found adjacent to the fibrous septa. Nuclear pleomorphism, prominent nucleoli, and mitotic figures are not found in classic FNH, but cytologic atypia may be seen in variant forms (98). The hepatocyte plates are one to two cells thick and are supported by an intact reticulin framework. In the periseptal region, the sinusoids may become capillarized (43).
Differential Diagnosis, Treatment, and Outcome
FNH is a nonneoplastic lesion that has little risk of hemorrhage, and if the diagnosis can be established with certainty by radiology, asymptomatic lesions are often not resected; most are stable or even regress upon follow-up (100). Features suggestive of FNH on CT or MRI are a mass with a central scar. Centrifugal hypervascularity is seen on Doppler ultrasound, and technetium-99 sulfur colloid scan shows normal or increased uptake. Sensitivity of 77% for a preoperative diagnosis of FNH by MRI with rare false-positive results have been reported (92). However, atypical forms of FNH may be confused with HCA, and a central stellate scar in FL-HCC may lead to diagnostic difficulties. For cases with nondiagnostic imaging studies and persistent symptoms, resection is generally recommended. Frozen section of needle or wedge biopsies may help avoid technically difficult resections.
In resection specimens, the diagnosis of FNH is usually straightforward because of the characteristic features of the lesion (Table 37.4), but small biopsies are more difficult to interpret and FNA biopsies are not useful to support a diagnosis of FNH. However, the diagnosis of FNH can be established with confidence on core biopsy when the biopsy is known to come from the mass and the lesion contains the characteristic pattern of radiating fibrous bands with ductular reaction, prominent arteries, and bland hepatocytes with features of chronic cholestasis. If the tissue specimen demonstrates only normal-appearing hepatocytes without the fibrous scars and ductular reaction, distinguishing FNH from normal liver, HCA, well-differentiated HCC, or an MRN may be impossible. The fibrous septa and bile ductules make the distinction from cirrhosis problematic when it is unclear whether the biopsy has come from a mass lesion in a noncirrhotic liver. Arteries without stroma adjacent to hepatocytes (intranodular or nontriadal arteries) are a rare finding in FNH but are typical of HCA and DNs in cirrhosis. A maplike distribution of glutamine synthetase expression by immunohistochemistry may aid in the diagnosis (101).
NODULAR REGENERATIVE HYPERPLASIA
Clinical Features
NRH (Tables 37.4 and 37.6) is characterized by small regenerative hepatocyte nodules, usually distributed diffusely throughout the liver, with little or no associated fibrosis. Although NRH often resembles cirrhosis grossly and clinically may cause noncirrhotic portal hypertension, it is distinguished microscopically from cirrhosis by the lack of fibrous septa. Prevalence of NRH at autopsy, where it is usually an incidental finding, ranges from 0.6% to 2.6% overall (102). In an unselected autopsy series, roughly 5% of patients had portal hypertension; in other reports from research centers focusing on symptomatic patients with NRH, as many as 57% of reported cases were associated with portal hypertension.

NRH occurs in patients of all ages and, although it is more common in older individuals, it can also be seen in childhood; there is no sex predilection. Most patients are asymptomatic for many years. Symptoms when present are usually due to portal hypertension or to an underlying disorder; rarely, NRH is associated with hepatic failure. Ascites is uncommon because patients usually have normal hepatic synthetic function and normal albumin levels. NRH has been associated with a wide variety of autoimmune disorders (most commonly rheumatoid arthritis or other collagen vascular diseases associated with a vasculitic process), myeloproliferative or lymphoproliferative disorders, drugs (including chemotherapeutic agents and HIV treatment) (103–105), and toxins (Table 37.6) (106), but occasionally no associated disorder is identified. The underlying etiology appears to be disturbed hepatic circulation, leading to irregularly distributed blood flow within the liver. The regenerative nodules appear to be a hypertrophic or hyperplastic response to normal or slightly increased blood flow, with atrophic areas between the nodules associated with obliterated portal vein branches (107).
Pathologic Findings
Macroscopic Features. The macroscopic appearance of NRH may be mistaken for cirrhosis or metastatic tumor. The liver is generally normal or small in size and may appear shrunken. The capsular surface is finely granular, resembling micronodular cirrhosis, and the parenchyma is diffusely transformed into white-tan nodules 1 to 3 mm in size, separated by congested internodular parenchyma (Fig. 37.8A). Nodules up to 1.5 cm have been described but are generally composed of multiple smaller nodules when examined microscopically (102). In partial nodular transformation, a variant of NRH, large nodules up to 8 cm may be present, often clustered at the hepatic hilum (43). Central hemorrhage may be seen in larger nodules.
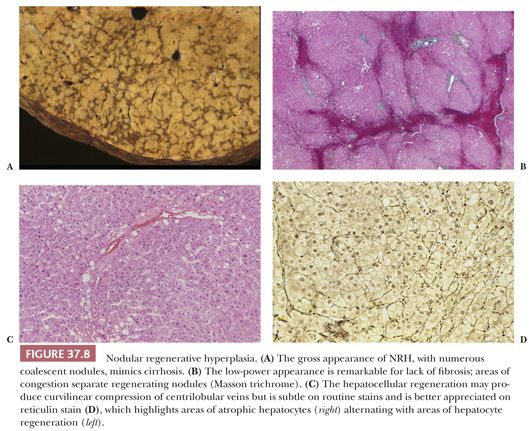
Microscopic Features. The nodules of NRH are often difficult to recognize microscopically, but correlation of gross findings with the histologic appearance helps in making the diagnosis. Key histopathologic features (Fig. 37.8B) are the presence of regenerating nodules of hepatocytes separated by zones of atrophic hepatocytes (usually centrilobular), often associated with sinusoidal congestion or curvilinear dilation and compression of central veins (Fig. 37.8C), and lack of significant fibrosis, which distinguishes NRH from cirrhosis or FNH. The hepatocyte plates within the nodules resemble those of the surrounding parenchyma, are typically one to two or more cells thick, and show little variation in nuclear size. The hepatocytes are often paler than the surrounding atrophic zones, due to accumulation of glycogen or fat within the cytoplasm, and contain less lipofuscin. In contrast, the hepatocytes between the individual nodules are often small and atrophic, with widened sinusoids and increased cytoplasmic lipofuscin. Increased accumulation of A1AT in periportal hepatocytes has been described in NRH (107). Reticulin stain is helpful in demonstrating alternating areas of regeneration and atrophy (Fig. 37.8D) and should be used on apparently normal liver biopsies from patients with portal hypertension.
Portal vein branches in smaller portal tracts may show occlusive or obliterative changes in NRH (102), but hepatic artery branches are typically not affected. Minor degrees of periportal or pericentral fibrosis may be seen, with occasional delicate fibrous septa, but established fibrous scarring typical of chronic liver disease is not present. Bile ducts are normal.
Differential Diagnosis
NRH must be distinguished from other nodular hepatic disorders related to hepatocellular proliferation, such as hepatic adenoma, FNH, and cirrhosis. The diagnosis may be difficult to establish on biopsy, and morphologic findings must be correlated with clinical features to avoid misdiagnosis. At a minimum, the diagnosis of NRH requires demonstration of hyperplastic nodules separated by atrophic zones of hepatocytes, without significant fibrosis. NRH may be superimposed on another primary hepatic disorder, such as early-stage primary biliary cirrhosis, and may contribute to portal hypertension at precirrhotic stages in that disorder. An incidental focus of nodular hyperplasia (hyperplastic focus), without diffuse involvement of the liver, should not be classified as NRH (102).
MACROREGENERATIVE AND DYSPLASTIC NODULES IN CIRRHOSIS
With advances in ultrasound detection and implementation of screening and surveillance programs for HCC, small nodular lesions, some of which represent precursor lesions for HCC, are now identified frequently in cirrhotic livers and often pose diagnostic challenges for the pathologist. These atypical hepatic nodules that do not meet histologic criteria for malignancy are also found in explanted livers, most commonly in the setting of chronic viral hepatitis or alcoholic liver disease. Although the natural history of these space-occupying nodules in cirrhosis is still not fully understood, it is now clear that DNs are grossly recognizable precursor lesions for HCC. Studies in which such nodules have been sampled and followed to determine their outcome have contributed to our knowledge of hepatocarcinogenesis (108,109).
Nodular lesions arising in the setting of cirrhosis show a wide spectrum of histologic changes, from large MRNs without atypical features to nodules falling just short of the morphologic criteria considered diagnostic of HCC. In particular, morphologic differentiation between early HCC (defined as measuring less than 2 cm) and high-grade DNs remains problematic. Grossly, these nodular lesions are usually similar in color and texture to the background cirrhotic nodules, although they may be paler than the rest of the liver or bile stained (43). Generally, they are well circumscribed by a rim of fibrous tissue, similar to other cirrhotic nodules. On histologic evaluation, there are two types of nodules: macroregenerative (considered nonneoplastic) and dysplastic (those with atypical features). Although terminology applied to these nodular lesions has been quite varied, the most widely accepted classification scheme is that proposed by the International Working Party in 1995 (110). Because imaging studies and serum AFP levels (normal or within the range seen in chronic liver disease) cannot readily distinguish these nodules from early HCC, microscopic evaluation of these lesions is necessary for definitive classification.
Regenerative Nodules
Regenerative nodules are divided into monoacinar and multiacinar, based on the number of portal tracts within the nodule. Monoacinar nodules containing only one portal tract are generally multiple, small, and diffusely involve the liver, as in NRH (110). Multiacinar regenerative nodules distinctly larger than the surrounding cirrhotic nodules but lacking cytologic and architectural atypia are termed macrogenerative nodules or large regenerative nodules (Fig. 37.9A). A minimum size criterion has not been established but is based on the size suitable to distinguish the large nodules from background cirrhotic nodules; generally, MRNs measure at least 0.5 cm, but size criteria of 0.8 to 1.0 cm have also been used (111). These MRNs are usually multiple and measure 0.5 to 1.5 cm but can be 5 cm or more in diameter (Fig. 37.9A). They are found in 15% to nearly 50% of cirrhotic livers and are three to four times more common than DNs.
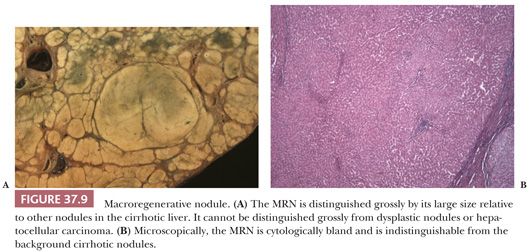
Microscopically, hepatocytes within multiacinar regenerative nodules, including MRNs, are identical to those in the surrounding extranodular liver and may demonstrate histologic abnormalities characteristic of the underlying disease (Fig. 37.9B). The hepatocellular plates are one or two cells thick, as typically seen in cirrhotic nodules, and the reticulin framework is intact or minimally disrupted. Portal tracts scattered throughout the nodule may be relatively normal in morphology or show obliteration of portal vein branches and bile ductular reaction (110); they are frequently fewer in number compared with the background liver. Solitary or unpaired intranodular arteries are absent or very rare, and there is little or no evidence of sinusoidal capillarization (conversion of fenestrated sinusoidal channels to continuous capillary vessels, characterized by the acquisition of sinusoidal immunoreactivity for factor CD34). Nonspecific changes such as steatosis, feathery degeneration, hemosiderin deposition, or accumulation of Mallory hyaline are not sufficient to classify the nodule as dysplastic.
Dysplastic Nodules
DNs are nodular lesions displaying some degree of cytologic or architectural atypia but lacking definitive histologic features of malignancy. Although they generally occur in the setting of cirrhosis, DNs may be found in livers with only mild scarring. On macroscopic examination, they cannot be distinguished from MRNs; although they may be slightly larger than MRNs, DNs are rarely larger than 2 cm. The International Working Party has defined a DN as being 1 mm or more in diameter and having either histologic features indicative of presumed genetic alteration (characterized by “the presence of nuclear or cytoplasmic alterations and the topographic clustering of such variations to form recognizable subpopulations of cells”) or proof of genetic alteration (e.g., clonality) without definitive histologic features of malignancy (110). If the population of cells is less than 1 mm, the term dysplastic focus is used. DNs are further subdivided into low and high grades, depending on the degree of histologic abnormality, for purposes of risk stratification and clinical utility.
Two categories of atypical cytologic features are recognized: small cell change and large cell change. Dysplastic foci or nodules often consist of hepatocytes with small cell change, characterized by smaller cell size, a greater nuclear-to-cytoplasmic ratio, cytoplasmic basophilia, and denser cellularity in comparison with the surrounding extranodular hepatocytes (Fig. 37.10A). Small cell change has been shown to have a higher proliferative rate and a lower apoptotic rate compared to the surrounding liver and is considered a precursor lesion for HCC. Whether large cell change, characterized by cellular enlargement, nuclear pleomorphism, and hyperchromasia (Fig. 37.10B), is a precursor lesion for HCC remains more controversial. Although large cell change has been associated with HCC development, it may also be seen in the setting of chronic cholestasis (112) and may not be directly related to hepatocarcinogenesis.
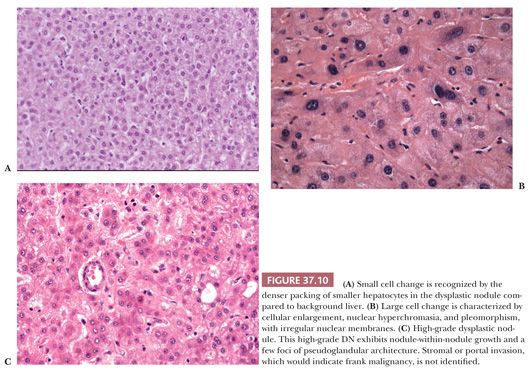
In low-grade DNs, the hepatocytes show minimal nuclear atypia or may appear normal, and there is no mitotic activity (113). Areas suggestive of a clonal population, such as a uniform population of hepatocytes with a maplike growth pattern, may be seen and are useful in making the distinction between low-grade DN and MRN, which may otherwise be impossible. Liver cell plates are one to two cells wide and may show nuclear deviation toward the sinusoids. In high-grade DNs, the architectural and cytologic abnormalities are more pronounced, and bulging nodule-within-nodule formations (Fig. 37.10C) suggestive of subclone evolution are often seen. Nuclear hyperchromasia and pleomorphism, rare mitotic figures, focal pseudogland formation, and focal areas of liver plates that are more than two cells wide may be identified. Stromal invasion is not seen. Unpaired (nontriadal) arteries are often conspicuous and are a useful clue for diagnosis. Capillarization of the sinusoids is more common in DNs than in MRNs.
Association with Hepatocellular Carcinoma
DNs are generally considered an important precursor to HCC (108,109). Longitudinal studies of MRNs suggest that these lesions have a low risk of transformation to HCC and are usually stable in size and can even regress by imaging studies (108). At the molecular level, MRNs are similar to ordinary cirrhotic nodules (114). In contrast, DNs do not regress and often increase in size (109). In one study of cirrhotic patients followed longitudinally, roughly 80% of high-grade DNs, 36% of low-grade DNs, and 12% of MRNs diagnosed on needle biopsy progressed to HCC within 5 years (109). Therapeutic intervention (ablation or resection) must be seriously considered after a histologic diagnosis of high-grade DN because of the high risk of development of HCC or the possibility of undersampling leading to a false-negative diagnosis of HCC. Patients with apparent MRNs should be followed-up with an appropriate imaging study at more frequent intervals than the usual patient with cirrhosis.
Differential Diagnosis and Reporting
The differential diagnosis of MRNs and benign hepatocellular nodules such as FNH or hepatic adenoma that develop in the noncirrhotic liver is usually straightforward, especially when dealing with a resection specimen in which the background liver is available for assessment. Distinguishing MRNs from other benign lesions may be impossible in biopsy specimens, in particular those in which limited clinical information is available. Features useful in differential diagnosis are summarized in Table 37.4. Separation of low-grade DNs from MRNs, and of high-grade DNs from HCC, may be difficult on histologic grounds, but features useful in differential diagnosis are summarized in Table 37.7.
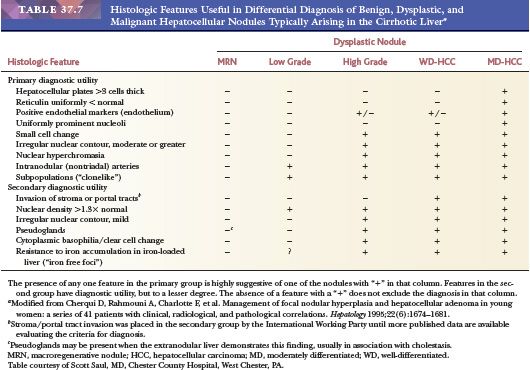
The most reliable diagnostic criteria for distinction of HCC from high-grade DN are vascular, capsular, or stromal invasion, although these features are rarely present on needle biopsies. Stromal invasion is considered the most helpful feature indicating HCC in such cases (113). In addition, marked cellular atypia, diffuse trabecular or pseudoglandular architecture, and numerous unpaired arteries favor a diagnosis of HCC. Up to 40% of early HCCs show fatty change, which declines with increasing tumor size (113). Presence of residual portal tracts within the nodule does not rule out early HCC (<2 cm). Demonstration of loss of the reticulin framework mass may be particularly helpful in diagnosis of well-differentiated HCC (115), and immunoreactivity for glypican-3 favors malignancy (116). Because of the importance of architectural abnormalities, core biopsy of nodular lesions in cirrhosis is favored over FNA. A core or FNA biopsy of a hepatocellular nodule in the setting of chronic liver disease that is not diagnostic for HCC should be regarded with caution because of the potential for undersampling. Close correlation with the size of the lesion, serum AFP levels, and imaging characteristics of the lesion are necessary for optimal interpretation.
MALIGNANT HEPATOCELLULAR TUMORS
Hepatocellular Carcinoma
Epidemiologic Factors. Malignant epithelial tumors account for about 98% of all primary hepatic malignancies, with HCC representing by far (roughly 90%) the single most common histologic type (43). Although it is relatively uncommon in North America and Western Europe (incidence 4.1 to 5.8 per 100,000 male population), HCC is one of the most prevalent malignant tumors worldwide, accounting for roughly 5% of all human cancers and is the third most common cause of cancer death globally (117). The highest incidence of HCC is seen in Eastern Asia, including China, where is it primarily attributed to chronic hepatitis B infection, but the rising global incidence of this malignancy is attributed in part to chronic hepatitis C. Men are at higher risk than women, with the incidence ratio varying from 1.4 to 3.3 in different parts of the world; sex ratios tend to be higher in high-risk countries (117). The incidence of HCC is age-related but with a different distribution pattern in low- and high-risk areas, reflecting differing age of exposure to risk factors. In developed countries, patients usually develop HCC after age 50, whereas it is not uncommon to find HCC in patients aged 20 to 35 in high-incidence areas. Studies of migrant populations have shown that although incidence remains high in the first generation immigrants from high-risk areas, subsequent generations show a decrease in HCC rates.
Etiology and Pathogenesis. Factors important in the pathogenesis of HCC are summarized in Table 37.8. HCC is highly linked to chronic liver disease, in particular chronic hepatitis B (HBV) and C (HCV) virus infection and alcoholic liver disease. Overall, 50% to 55% of cases of HCC are attributed to HBV and 25% to 30% to HCV (118), but virtually any condition associated with chronic hepatic injury and especially with cirrhosis may predispose to HCC. The annual risk of HCC developing in a cirrhotic liver is estimated at 1% to 6%, with the risk generally highest in the context of viral sources and hereditary hemochromatosis. Obesity-related liver disease is increasingly recognized as a risk factor for HCC (119,120). Other risk factors include aflatoxin contamination of food, most commonly seen in sub-Saharan Africa, and co-infection with hepatitis D virus in patients with chronic hepatitis B (121).
Although the strong etiologic association of HCC with cirrhosis is well known, the pathogenetic sequence leading to malignancy is less well elucidated. Liver cell proliferation is increased during chronic hepatitis but is often decreased in cirrhosis. Activation of stellate cells in cirrhosis leads to increased production of growth factors and other substances that alter hepatocyte proliferation. Limitation of the regenerative reserve of the liver has been attributed to accelerated telomere shortening in hepatocytes in chronic liver disease, leading to telomere dysfunction and susceptibility to chromosomal alterations (122), which would correlate with the observation of allelic gains and losses of numerous chromosome in HCC (123), reviewed by Yam and colleagues (124). Specific cell cycle checkpoint abnormalities in HCC include frequent mutations in the third nucleotide of codon 249 of TP53 in HCCs linked to exposure to aflatoxin B1 (122), associated with high levels of chromosomal instability; other non–aflatoxin-associated TP53 mutations are more frequently found in large, high-grade HCCs (124). An alternative pathway of carcinogenesis, with extensive methylation of CpG dinucleotides in the promoters of cancer-related genes, is associated with beta-catenin mutations but not with high levels of chromosomal instability (125) and is frequently seen in non–HBV-related tumors. HBV infection promotes carcinogenesis by at least three mechanisms: integration of viral DNA into the host genome, leading to chromosomal instability; insertional mutations at specific sites, leading to activation of genes involved in cell proliferation (implicated in 20 to 40% of HCCs arising in HBV); and ability of expression of HBX viral protein to modulate cell proliferation and inactivate p53 activities (126). HCV does not integrate into the hepatocyte genome and has not been found to be directly oncogenic.
Other molecular events in HCC include epigenetic alterations, including DNA hypermethylation in tumor suppressor genes. The most commonly altered signaling pathway in HCC is the Wnt/beta-catenin pathway; others affected include p53, Rb1, PI3K/mTOR, MAPK, and TGF-β pathways (121,124). Whole genome and exon analyses of HCC reveal a complex landscape of genetic alterations, with significant intratumoral heterogeneity (127).
Clinical Findings. Surveillance of patients with chronic liver disease and cirrhosis has led to detection of HCC at asymptomatic stages in many cases, but many still present at advanced stages. Common presenting symptoms are abdominal pain, fullness or a mass, or worsening of symptoms attributed to cirrhosis. HCC may invade hepatic veins and spread to the inferior vena cava or even into the right atrium, producing right heart failure as the initial manifestation. HCC rarely presents with initial symptoms attributable to metastatic spread. The most common metastatic sites are hilar and other abdominal lymph nodes, bone, and lung, although spread to less common sites such as gastrointestinal tract, skin, heart, kidney, spleen, and pancreas may be seen on occasion. Although most HCC patients die from cancer progression, an appreciable percentage succumb to complications from cirrhosis.
HCC accounts for about 70% of all malignant neoplasms found in the cirrhotic liver in regions of low incidence. Although up to 30% of HCCs in North America may arise in a normal liver, it is much more likely that a malignant tumor in the normal liver represents a metastasis (2% HCC vs. 98% hepatic metastasis) (128). HCC in the noncirrhotic liver generally appears at a later stage and with a larger mass than HCC arising in patients with cirrhosis.
Screening and early detection programs for HCC rely on a combination of ultrasonography and serum levels of AFP and have led to the diagnosis of many early (<2 cm), asymptomatic HCCs. Although it is difficult to demonstrate a decrease in disease-specific or all-cause mortality due to the need for large cohorts and randomization to screening or no screening, screening for HCC is considered appropriate because whereas the cure rate for symptomatic cancers is very low (0% to 10% 5-year survival), early-stage tumors amenable to resection or liver transplantation are associated with 5-year survival rates of up to 50% (129).
AFP, a glycoprotein produced primarily by fetal liver, remains the most useful serologic marker for HCC, although its limitations are well recognized; in particular, AFP is not a good marker for early HCCs (117) and probably has a limited role in surveillance. Sensitivity ranges from 40% to 65% and specificity from 76% to 96% (130). A cutoff of 20 ng/mL is commonly used, and values of about 400 ng/mL are considered diagnostic for HCC. Because patients with chronic viral hepatitis may have elevated serum levels in the absence of HCC, AFP appears to be more useful in the patient with nonviral liver disease. Progressive increase in serum AFP is also suggestive of HCC and warrants further investigation. A variant of AFP differing in its sugar chains, AFP-L3, appears to be more specific for HCC than total AFP. Serum level of serum des-γ-carboxy prothrombin (DCP) has been suggested as a useful marker, with sensitivities ranging from 28% to 89% and specificities from 87% to 96%; DCP does not appear to be nonspecifically elevated in chronic liver disease. Because DCP and AFP levels do not correlate, combination of both markers may improve accuracy in HCC diagnosis (130): DCP seropositivity using a sensitive assay correlates with large tumor size and inversely with tumor differentiation (131). Other promising markers being evaluated include glypican-3, insulin-like growth factor-1 (IGF-1), and hepatocyte growth factor (HGF) (130).
Although serum AFP levels may be elevated in nonneoplastic conditions associated with hepatocyte regeneration such as acute and chronic viral hepatitis and cirrhosis, levels higher than 400 to 500 ng/mL are rarely seen. In chronic viral liver disease, increases in serum AFP are often episodic and correlate with increased transaminases. Other malignant neoplasms often associated with very high levels (>1000 ng/mL) of serum AFP include hepatoblastoma, germ cell tumors containing a yolk sac component, and hepatoid adenocarcinomas arising in various sites, such as stomach or ovary.
Pathologic Features of Early Hepatocellular Carcinoma
MACROSCOPIC FEATURES. Early HCC is defined as measuring less than 2 cm in diameter (110). It may be indistinguishable from an MRN on gross inspection and may be recognized only at the microscopic level, often arising within a DN. Two types are recognized: the distinctly nodular type and the indistinctly (vaguely) nodular type. The latter is ill-defined on gross examination, with a vaguely nodular appearance with indistinct borders and may be difficult to distinguish from the surrounding cirrhotic liver (113). A fibrous capsule is commonly present in the distinctly nodular type of early HCC greater than 1.5 cm. The nodules often bulge above the cut surface, are rarely necrotic, and may be variegated, with green areas corresponding to bile staining and yellow areas reflecting fat accumulation in tumor cells (Fig. 37.11A). Morphologic characteristics of some early HCCs change when the nodule attains a size of 2 to 3 cm, due to development of a well-defined capsule, dedifferentiation, and clonal expansion.
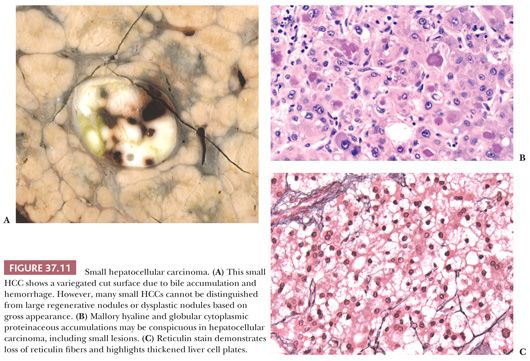
MICROSCOPIC FEATURES. Early HCCs are nearly all well differentiated, consisting of relatively thin, irregular trabeculae (up to three cells thick) of small, crowded hepatocytes with fatty or clear cell change; increased eosinophilia or basophilia may also be seen. Mallory hyaline may be prominent (Fig. 37.11B). Reticulin stain showing loss of reticulin fibers may be helpful in establishing the diagnosis (Fig. 37.11C). Acinar and pseudoglandular structures may also be seen but are typically smaller than those seen in moderately differentiated HCC. Early well-differentiated HCC is distinguished from high-grade DNs by a nuclear density greater than twice normal and by mild but definite nuclear atypia (hyperchromasia, irregular nuclear contours) (Table 37.7). Nucleoli are often inconspicuous. As the nodule of HCC enlarges to greater than 1 cm, there is clonal dedifferentiation, often in a nodule-in-nodule fashion. The moderate or poorly differentiated foci are always found toward the center of the nodule, with a peripheral component of well-differentiated HCC that diminishes with increasing tumor size. Prominent fatty change, present in 36% of cases, often declines when the tumor attains a size of 3 cm or more and may be related to the inadequate development of arterial blood vessels at early stages of tumor growth. Stromal and portal tract invasion may occur, but vascular invasion is rarely identified.
Advanced Hepatocellular Carcinoma
MACROSCOPIC FEATURES. The macroscopic appearance of advanced HCC varies depending on the presence or absence of cirrhosis and the size of the tumor. Tumors arising in a noncirrhotic liver usually grow as a single large mass, occasionally with satellite nodules (massive or expanding type), whereas those associated with cirrhosis often grow as multiple discrete nodules (nodular type) or numerous minute indistinct nodules (diffuse type) that may be indistinguishable from cirrhosis (cirrhotomimetic) (Fig. 37.12). The tumors are occasionally pedunculated. Staging criteria for HCC are listed in Table 37.9; they principally depend on the size and number of the tumor nodules and the presence or absence of vascular invasion (132).
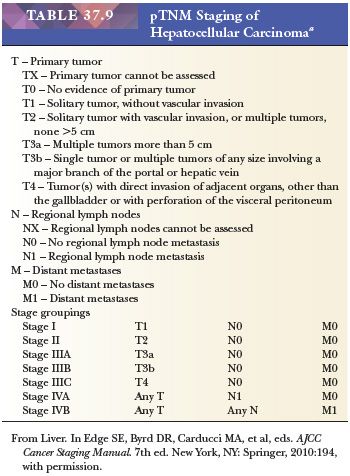
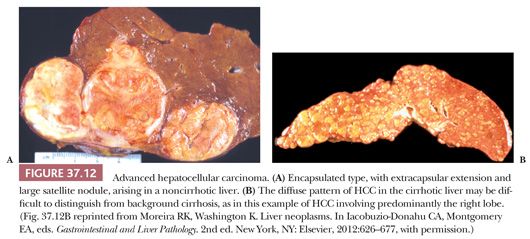
The liver is enlarged by one or more tumor nodules that are fleshy, variegated, with green bile-stained, pale yellow, and white areas, and areas of hemorrhage and necrosis. Except for the rarely encountered fibrolamellar and scirrhous variants, HCC is soft, with little fibrosis. Invasion of the portal vein branches or hepatic veins is common in larger tumors. Involvement of major bile ducts, with intrabiliary growth, can lead to obstructive jaundice. Multiple tumor nodules may be due to synchronous primaries (multicentric growth) or may represent intrahepatic metastases from tumor spreading through portal vein branches. Although genetic analysis may be needed to confirm multicentricity, features favoring synchronous primary HCCs include multiple HCCs of different histology, presence of peripheral areas of well-differentiated HCC in multiple nodules, and multiple early HCCs or concurrent early HCC with a classic larger HCC (121).
MICROSCOPIC FEATURES. The neoplastic cells and microarchitecture of HCC resemble normal liver to a greater or lesser extent depending on the degree of differentiation, which ranges from tumors so well differentiated that the distinction from hepatic adenoma is problematic to tumors that are highly anaplastic and show little evidence of hepatocellular differentiation (Fig. 37.13). In 1954, Edmondson and Steiner (133) devised a four-tiered grading system based on autopsy data and subsequently modified in a large series reported from the AFIP (134), and other similar systems have been proposed (43) (Table 37.10). The World Health Organization (WHO) classification divides tumors into well, moderately, poorly, and undifferentiated grades (121). Most tumors are moderately differentiated (grades 2 to 3), and more than one histologic grade is often present within a given tumor. Although tumor grade has not universally been shown to have a significant impact on outcome, higher nuclear grade has been reported to predict poorer survival in HCCs resected with curative intent (135), and higher tumor grade corresponds to positivity on positron emission tomography imaging.
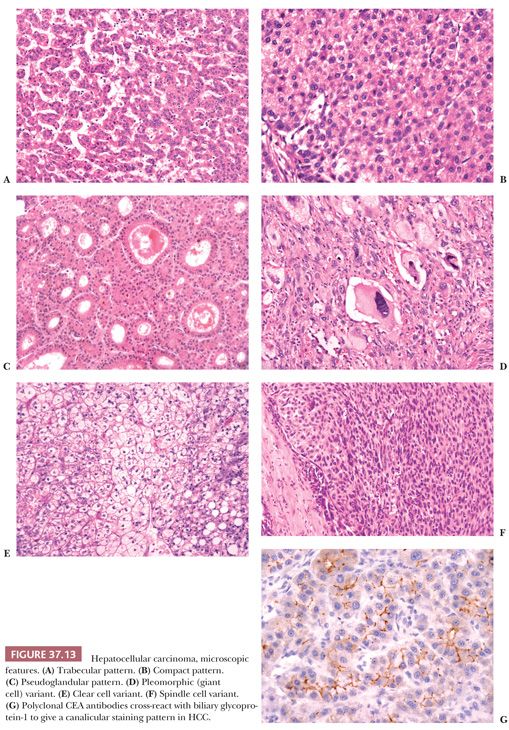
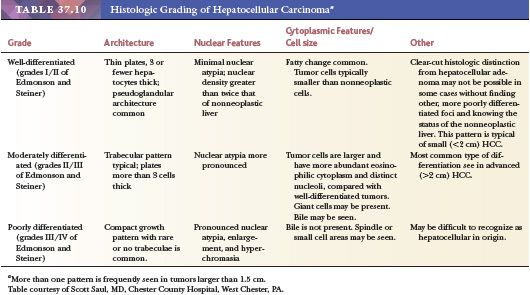
Bile located within neoplastic cells or tubular lumina is pathognomonic of HCC, but it is found in less than one-third of cases and is not evident in poorly differentiated tumors. The presence of bile canaliculi is also diagnostic. Other than in HCC, bile and bile canaliculi have been identified only in hepatoid adenocarcinomas, most commonly arising in the stomach (136). As a rule, typical HCCs do not contain abundant stroma, which explains why HCCs tend to be soft, in contrast to many other carcinomas which induce desmoplastic stromal response. Fibrolamellar carcinoma and the scirrhous pattern of HCC are rare variants that are exceptions to the rule that HCC lacks significant fibrosis; many early HCCs also contain prominent fibrotic stroma, as does the “steatohepatitic variant,” characterized by prominent steatosis, sinusoidal fibrosis, and ballooning degeneration (137). Large blood-filled cystic spaces or vascular lakes within the tumor may mimic peliosis hepatis (pelioid pattern). The WHO (121) recognizes four architectural patterns in addition to the special types of fibrolamellar carcinoma (discussed later): scirrhous HCC, undifferentiated carcinoma, lymphoepithelioma-like carcinoma, and sarcomatoid HCC.
Histologic Patterns. Multiple histologic patterns are often found in the same tumor. Only the fibrolamellar variant appears to have prognostic significance, but familiarity with these architectural patterns may be helpful in arriving at a diagnosis of HCC.
• Trabecular (sinusoidal, plate-like). This commonly found pattern resembles normal hepatic architecture in that the tumor cells grow in cords or plates separated by vascular channels lined by endothelial cells and Kupffer cells (Fig. 37.13A), with little or no supporting stroma. The trabeculae vary in thickness, from only a few cells thick (microtrabecular) to broad structures 20 or so cells thick (macrotrabecular). The reticulin framework is generally reduced or absent.
• Compact (solid). This variant of the trabecular pattern is seen in 5% to 15% of HCCs. Confluent growth or compression of adjacent trabeculae results in a solid growth pattern, with inconspicuous or obliterated sinusoids (Fig. 37.13B).
• Pseudoglandular (acinar). This pattern is usually admixed with the trabecular pattern and is rarely seen as the dominant pattern in HCC. It is present at least focally in many HCCs and may be mistaken for metastatic adenocarcinoma, cholangiocarcinoma, or HCC combined with cholangiocarcinoma (Fig. 37.13C). The spaces represent greatly dilated bile canaliculi or degenerated macrotrabeculae and are lined by a single layer of tumor cells. The pseudoglands may appear to be freely floating and are not embedded in fibrous stroma, a feature which helps distinguish this pattern from adenocarcinoma. The pseudoglandular spaces may contain bile plugs and/or proteinaceous eosinophilic material that is positive for PAS but negative for mucicarmine and Alcian blue and does not represent mucin secretion.
• Scirrhous. This variant accounts for less than 1% to 2% of all HCCs. It is discussed later under “Scirrhous Hepatocellular Carcinoma.”
CYTOLOGIC APPEARANCE AND VARIANTS. The tumor cells of HCC are usually polygonal but may be cuboidal or even columnar. They have a moderate amount of finely granular eosinophilic cytoplasm that may be more basophilic than normal liver and usually have distinct cell borders. Bile canaliculi are readily identified in most well to moderately differentiated tumors but may be inconspicuous by light microscopy in high-grade HCCs. Compared to normal hepatocytes, the tumor cells display a higher nuclear-to-cytoplasmic ratio. The nucleus is usually round to oval, with coarse chromatin, a single prominent nucleolus, and a thickened or irregular nuclear membrane. Intranuclear cytoplasmic invaginations are a common, albeit nonspecific, finding in HCC.
A variety of cytoplasmic inclusions may be identified in HCC cells (Table 37.11). Fat droplets can be seen in up to two-thirds of tumors (43). Diffuse accumulation of fat or glycogen results in a clear appearance to the cytoplasm, a variant termed clear cell carcinoma, which must be distinguished from metastatic renal cell carcinoma and other clear cell neoplasms. Mallory hyaline, representing clumps of intermediate filaments, is found in approximately 20% of cases and may be very prominent, especially in early HCC and the steatohepatitic variant. Globular proteinaceous eosinophilic inclusions are also seen in 20% of cases and are usually PAS-positive, diastase resistant, representing accumulation of A1AT. More lightly staining inclusions (8% of cases) that resemble ground glass cells of hepatitis B have been termed pale bodies and represent accumulations of fibrinogen. Copper-binding protein (Shikata orcein stain) and copper (rhodanine stain) have been detected in up to 28% of HCCs and appear to be related to the presence of bile within the tumor (138). Hemosiderin is rarely found in HCC, even in the context of hereditary hemochromatosis. Calcification is also quite rare in untreated cases.
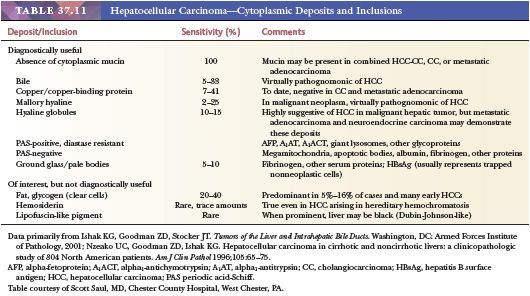
The following categories are recognized cytologic variants of HCC:
• Pleomorphic (giant cell). Tumor cells show marked variation in size, shape, and staining characteristics, and bizarre tumor giant cells are common in this rare variant (Fig. 37.13D). The tumor cells show marked loss of cell cohesion and a distinct trabecular pattern is difficult to identify in this high-grade variant. Extensive sectioning may be required to find evidence of typical HCC. Up to 30% of lower grade HCCs also contain multinucleated tumor giant cells without anaplastic features; these tumors should be graded according to the overall nuclear features (43).
• Clear cell. As the name implies, this variant is characterized by tumor cells with prominent clear cytoplasm resulting from accumulation of cytoplasmic glycogen and/or fat that is lost in the embedding process (Fig. 37.13E). Although HCCs predominantly composed of clear cells are seen in only about 16% of cases, foci of clear cell change are common in otherwise typical HCCs. Differentiating this type from metastatic renal, adrenal, or ovarian carcinoma may be problematic on purely histologic grounds, and the tumor may require extensive sampling to demonstrate foci of typical HCC. Immunohistochemical studies (see later and Table 37.12) demonstrating bile canaliculi (polyclonal CEA or p-CEA) or hepatocellular differentiation, such as HepPar-1 or glypican-3, may be required to establish the diagnosis of HCC. Elevated serum AFP level, presence of chronic liver disease, and the absence of an extrahepatic mass favor a hepatic primary tumor.
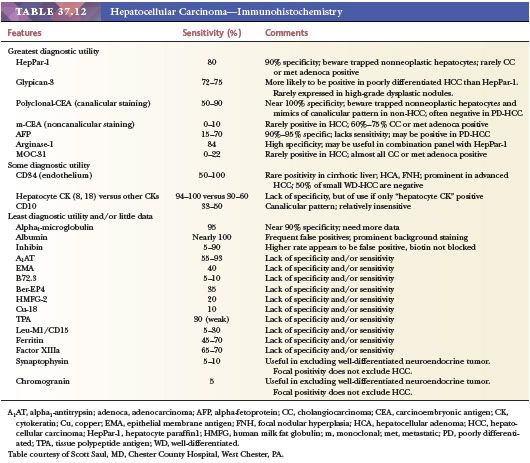
• Sarcomatoid (spindle cell, pseudosarcomatous). Up to 4% of HCCs exhibit a prominent sarcomatoid or spindle cell component characterized by spindle-shaped cells (Fig. 37.13F) that suggest a diagnosis of fibrosarcoma or undifferentiated pleomorphic sarcoma (malignant fibrous histiocytoma). Sometimes these tumors have been reported as carcinosarcoma or malignant mixed tumors, particularly when foci of more differentiated sarcomas (osteosarcoma, chondrosarcoma, leiomyosarcoma, rhabdomyosarcoma) are noted (43). In some cases, a transition between the spindle cell component and typical HCC is demonstrated, whereas in others, the components are separate; extensive sampling may be needed to demonstrate areas of typical HCC. Pleomorphic and osteoclast-like giant cells may be seen, resulting in overlap with the giant cell variant. Cytokeratin immunoreactivity, most commonly CAM 5.2, is detected in the spindle cells in about 60% of cases, supporting an epithelial origin, but the spindle cells also express mesenchymal markers such as vimentin, smooth muscle actin, and desmin. S-100 can be detected in a small number of cases. The serum AFP level is elevated in roughly half of reported patients, similar to that seen for typical HCC (139). Survival after hepatic resection appears to be shortened for this rare high-grade variant (139). Spindle cell change may be more common in tumors subjected to chemoembolization or preoperative chemotherapy (121).
IMMUNOHISTOCHEMISTRY AND SPECIAL STAINS. Although many cases of HCC are readily recognizable because of the characteristic trabecular architecture with little intervening stroma and demonstration of bile production by neoplastic cells, immunohistochemical studies and a panel of special stains may be invaluable in diagnostically challenging cases (Tables 37.12 and 37.13). Relatively few non-immunohistochemical stains are useful in evaluation of tumors suspected to be HCCs. Reticulin stain is often helpful in distinguishing well-differentiated HCC from nonneoplastic or benign nodules because the reticulin framework is typically reduced and incomplete in HCC; the stain also serves to highlight the trabecular structure and delineates thickened liver cell plates. Because HCCs do not produce mucin, demonstration of intracellular mucin by mucicarmine stain or Alcian blue is useful in distinguishing HCC from cholangiocarcinoma or metastatic adenocarcinoma.
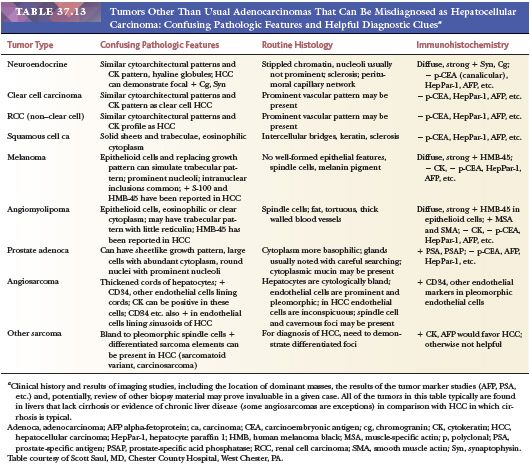
The immunohistochemical panel chosen for initial workup of HCC depends on whether the differential diagnosis is HCC versus a benign nodular lesion or distinction of HCC from other malignancies (Tables 37.12 to 37.14). For distinguishing HCC from other malignancies, the panel might include (a) antibodies against HepPar-1 (hepatocyte), glypican-3, and/or arginase-1 to show hepatocellular differentiation; (b) polyclonal or cross-reactive CEA antiserum or antibodies directed against CD10 to highlight inconspicuous bile canaliculi; (c) monoclonal CEA or MOC-31 to highlight metastatic adenocarcinoma; and (d) synaptophysin and chromogranin to rule out a low-grade metastatic neuroendocrine neoplasm (Table 37.12). To distinguish between low-grade HCC and benign nodules, glypican-3 is typically negative in high-grade dysplastic nodule and benign lesions and may be positive in early HCC. A combination of glutamine synthetase, glypican-3, and heat shock protein 70 has been recommended (101,121).
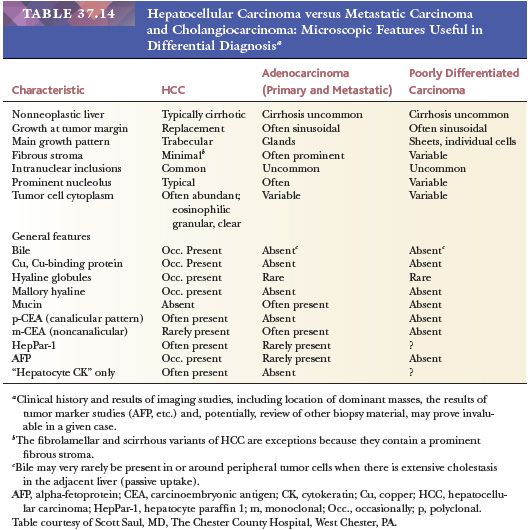
HepPar-1 (Hepatocyte paraffin 1) is a relatively hepatocyte-specific monoclonal antibody that reacts with a hepatocyte mitochondrial epitope that is resistant to formalin fixation and tissue processing and present in both adult and fetal liver. The granular and cytoplasmic expression may be patchy within tumors. HepPar-1 is useful in distinguishing HCC from cholangiocarcinoma and metastatic adenocarcinoma; it does not distinguish between HCC and benign hepatocellular proliferations. Sensitivity for HCC in recent series ranges from 73% (140) to 93% (141), with negative cases more likely to show a scirrhous architectural pattern or poor differentiation. Most nonhepatocytic neoplasms are negative for HepPar-1, although up to 6% of nonhepatic tumors in one series (142) and roughly 10% of cholangiocarcinomas are reported to be positive (143). Adenocarcinomas of the gastrointestinal tract and lung and neuroendocrine carcinomas may express HepPar-1 (142,144), and, as with many antibodies, HepPar-1 is probably best used as part of a panel. Scattered strongly positive cells in an otherwise negative tumor should be interpreted with caution, as they may represent entrapped hepatocytes.
Other useful markers of hepatocellular differentiation include glypican-3, a heparan sulfate proteoglycan normally expressed in fetal liver, and arginase-1, a manganese metalloenzyme, expressed in normal hepatocytes. Glypican-3 is generally negative in adjacent liver and in benign hepatic lesions, making it sometimes useful in diagnosis of well-differentiated HCC. However, patchy weak staining may be seen in hepatocytes in the setting of chronic hepatitis with pronounced necroinflammatory activity. Glypican-3 is especially useful for high-grade HCC, however, as it is more likely to be positive in poorly differentiated HCC (86% to 100%) compared to well-differentiated lesions (53% to 78%) (143). Like HepPar-1, glypican-3 is not entirely specific for HCC and has been reported to be positive in 14% of gastrointestinal and pancreatic neoplasms, particularly pancreatic acinar cell carcinoma (145). Immunohistochemistry for arginase-1 shows a similar profile to HepPar-1, with slightly improved sensitivity with arginase 1 for HCC (146,147).
Polyclonal anti-CEA antiserum or certain monoclonal CEA (m-CEA) antibodies that cross-react with canalicular biliary glycoprotein 1 highlight bile canaliculi (canalicular pattern) in 30% to 100% of HCCs in sections (Fig. 37.13G) and 47% to 83% in cell blocks or cytologic smears (148), depending on the degree of differentiation of the tumor. A similar pattern may be seen with antibodies directed against CD10; although CD10 expression may be somewhat less sensitive than p-CEA, interpretation is often easier because of less background cytoplasmic staining in the tumor cells. About 50% of poorly differentiated tumors lack immunoreactivity. A false-positive HCC interpretation of a canalicular pattern may result from entrapment of nonneoplastic hepatocytes within the tumor, misinterpretation of an incomplete membrane pattern as canalicular in location, or misinterpretation of periluminal immunoreactivity for p-CEA in adenocarcinomas as staining of dilated canaliculi. HCCs, unlike adenocarcinomas, rarely show cytoplasmic immunoreactivity with m-CEA antisera, and inclusion of such antibodies in a panel may be diagnostically useful.
Normal hepatocytes express CK8 and CK18, which theoretically should be useful in differentiating HCC from cholangiocarcinoma (which tends to express CK7 and CK19 and variably express CK20) and metastatic adenocarcinomas. However, cytokeratin expression profiles are variable in HCC as well as in other malignancies, limiting enthusiasm for this approach as an initial diagnostic strategy. A panel of CK7, CK20, and CK19 has been suggested as a practical approach (148); negative results for all three markers favors HCC, whereas CK19 and CK7 positivity favors cholangiocarcinoma; results for metastatic adenocarcinomas are variable depending on the primary site.
Other antibodies that may be useful include MOC-31, a cell surface glycoprotein that is expressed in cholangiocarcinomas and metastatic adenocarcinomas but less often in HCC (148). Cytoplasmic thyroid transcription factor-1 (TTF-1) has also been suggested as a relatively specific marker for HCC (149). AFP immunohistochemistry is highly specific but relatively insensitive, with positivity in only 17% to 68% of cases (148).
CD34 has been used to differentiate between hepatic adenoma and other benign hepatic nodules and HCC; HCC is more likely to show diffuse sinusoidal positivity indicative of capillarization of the sinusoids. Expression in benign hepatic nodules is more limited. However, results must be interpreted with caution, as there may be considerable overlap in degree of expression and pattern.
A variety of other antibodies of limited clinical utility have been applied to HCC. These include A1AT and alpha1-antichymotrypsin (A1ACT), which demonstrate a granular cytoplasmic nonspecific expression pattern; although most HCCs express A1AT, up to 70% of metastatic adenocarcinomas are positive as well (148). AE1/AE3, CAM 5.2, B72.3, inhibin, and factor XIIIa are also poorly discriminatory in distinguishing HCC from metastatic adenocarcinoma and cholangiocarcinoma (144).
In situ hybridization for albumin mRNA appears to be highly sensitive for hepatocellular differentiation but has not yet gained widespread use in the diagnostic setting. Up to 96% of HCCs are positive (148), as are HCAs and nonneoplastic liver. Immunohistochemistry for albumin is not useful because its abundance in the serum results in high background.
FINE-NEEDLE ASPIRATION. Aspirates of HCC are generally highly cellular due to the soft texture of the tumor and the lack of reticulin scaffolding, often resulting in a finely granular smear in well-differentiated tumors. Trabeculae or clusters of tumor cells are lined by a variable number of elongated endothelial cells (Fig. 37.14). The tumor cells are polygonal with central hyperchromatic nuclei and variably prominent nucleoli. The nuclear-to-cytoplasmic ratio is typically increased but varies with the degree of differentiation, and isolated stripped tumor cell nuclei are common. Intranuclear cytoplasmic invaginations and various cytoplasmic deposits such as bile, proteinaceous globules, and Mallory hyaline can be identified in cytologic preparations. Clear cell HCC may be confused with signet cell adenocarcinoma or pleomorphic liposarcoma. Correlation of cytologic findings with the microarchitecture demonstrated on smear preparations and in cell block material is critical to establishing the correct diagnosis in such cases showing variant cytologic features.
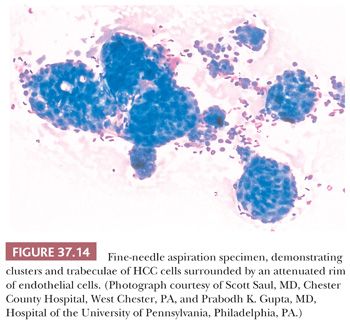
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


