13
Sterilization & Disinfection
CHAPTER CONTENTS
PRINCIPLES OF STERILIZATION & DISINFECTION
Sterilization is the killing or removal of all microorganisms, including bacterial spores, which are highly resistant. Sterilization is usually carried out by autoclaving, which consists of exposure to steam at 121°C under a pressure of 15 lb/in2 for 15 minutes. Surgical instruments that can be damaged by moist heat are usually sterilized by exposure to ethylene oxide gas, and most intravenous solutions are sterilized by filtration.
Disinfection is the killing of many, but not all, microorganisms. For adequate disinfection, pathogens must be killed, but some organisms and bacterial spores may survive. Disinfectants vary in their tissue-damaging properties from the corrosive phenol-containing compounds, which should be used only on inanimate objects, to less toxic materials such as ethanol and iodine, which can be used on skin surfaces. Chemicals used to kill microorganisms on the surface of skin and mucous membranes are called antiseptics.
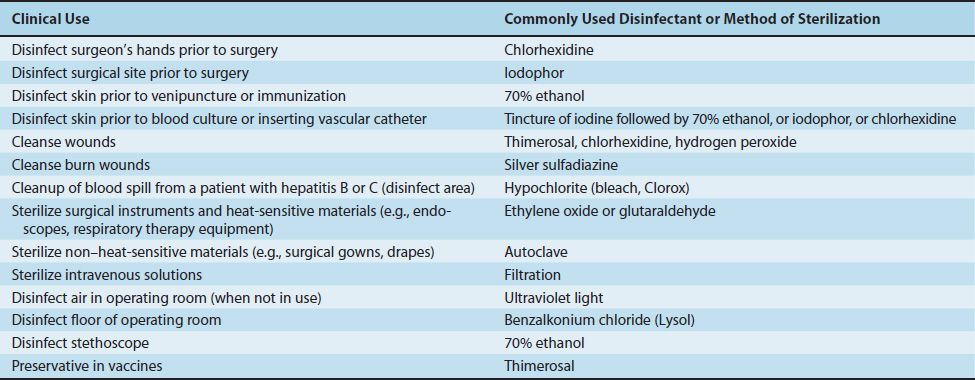
Table 13–1 describes the clinical uses of common disinfectants and modes of sterilization.
TABLE 13–1 Clinical Use of Disinfection and Sterilization
RATE OF KILLING OF MICROORGANISMS
Death of microorganisms occurs at a certain rate dependent primarily on two variables: the concentration of the killing agent and the length of time the agent is applied. The rate of killing is defined by the relationship
N α 1/CT
which shows that the number of survivors, N, is inversely proportionate to the concentration of the agent, C, and to the time of application of the agent, T. Collectively, CT is often referred to as the dose. Stated alternatively, the number of microorganisms killed is directly proportionate to CT. The relationship is usually stated in terms of survivors because they are easily measured by colony formation. Death is defined as the inability to reproduce. In certain circumstances, the physical remains of dead bacteria can still cause problems (see page 46).
CHEMICAL AGENTS
Chemicals vary greatly in their ability to kill microorganisms. A quantitative measure of this variation is expressed as the phenol coefficient, which is the ratio of the concentration of phenol to the concentration of the agent required to cause the same amount of killing under the standard conditions of the test.
Chemical agents act primarily by one of the three mechanisms: (1) disruption of the lipid-containing cell membrane, (2) modification of proteins, or (3) modification of DNA. Each of the following chemical agents has been classified into one of the three categories, but some of the chemicals act by more than one mechanism.
DISRUPTION OF CELL MEMBRANES
Alcohol
Ethanol is widely used to clean the skin before immunization or venipuncture. It acts mainly by disorganizing the lipid structure in membranes, but it denatures proteins as well. Ethanol requires the presence of water for maximal activity (i.e., it is far more effective at 70% than at 100%). Seventy percent ethanol is often used as an antiseptic to clean the skin prior to venipuncture. However, because it is not as effective as iodine-containing compounds, the latter should be used prior to obtaining a blood culture and installing intravenous catheters.
Detergents
Detergents are “surface-active” agents composed of a long-chain, lipid-soluble, hydrophobic portion and a polar hydrophilic group, which can be a cation, an anion, or a nonionic group. These surfactants interact with the lipid in the cell membrane through their hydrophobic chain and with the surrounding water through their polar group and thus disrupt the membrane. Quaternary ammonium compounds (e.g., benzalkonium chloride) are cationic detergents widely used for skin antisepsis. Benzalkonium chloride is the active ingredient in Lysol, a commonly used disinfectant for floors and other surfaces.
Phenols
Phenol was the first disinfectant used in the operating room (by Lister in the 1860s), but it is rarely used as a disinfectant today because it is too caustic. Chlorhexidine is a chlorinated phenol that is widely used as a hand disinfectant prior to surgery (“surgical scrub”) and in the cleansing of wounds. Hexachlorophene, which is a biphenol with six chlorine atoms, is used in germicidal soaps, but concern over possible neurotoxicity has limited its use. Phenols not only damage membranes, but also denature proteins.
MODIFICATION OF PROTEINS
Chlorine
Chlorine is used as a disinfectant to purify the water supply and to treat swimming pools. It is also the active component of hypochlorite (bleach, Clorox), which is used as a disinfectant in the home and in hospitals. Chlorine is a powerful oxidizing agent that kills by cross-linking essential sulfhydryl groups in enzymes to form the inactive disulfide.
Iodine
Iodine is the most effective skin antiseptic used in medical practice and should be used prior to obtaining a blood culture and installing intravenous catheters because contamination with skin flora such as Staphylococcus epidermidis can be a problem. Iodine, like chlorine, is an oxidant that inactivates sulfhydryl-containing enzymes. It also binds specifically to tyrosine residues in proteins.
Iodine is supplied in two forms:
(1) Tincture of iodine (2% solution of iodine and potassium iodide in ethanol) is used to prepare the skin prior to blood culture. Because tincture of iodine can be irritating to the skin, it should be removed with alcohol.
(2) Iodophors are complexes of iodine with detergents that are frequently used to prepare the skin prior to surgery because they are less irritating than tincture of iodine.
Heavy Metals
Mercury and silver have the greatest antibacterial activity of the heavy metals and are the most widely used in medicine. They act by binding to sulfhydryl groups, thereby blocking enzymatic activity. Thimerosal (Merthiolate) and merbromin (Mercurochrome), which contain mercury, are used as skin antiseptics. Silver nitrate drops are useful in preventing gonococcal ophthalmia neonatorum. Silver sulfadiazine is used to prevent infection of burn wounds.
Hydrogen Peroxide
Hydrogen peroxide is used as an antiseptic to clean wounds and to disinfect contact lenses. Its effectiveness is limited by the organism’s ability to produce catalase, an enzyme that degrades H2O2. (The bubbles produced when peroxide is used on wounds are formed by oxygen arising from the breakdown of H2O2 by tissue catalase.) Hydrogen peroxide is an oxidizing agent that attacks sulfhydryl groups, thereby inhibiting enzymatic activity.
Formaldehyde & Glutaraldehyde
Formaldehyde, which is available as a 37% solution in water (formalin), denatures proteins and nucleic acids. Both proteins and nucleic acids contain essential –NH2 and –OH groups, which are the main sites of alkylation by the hydroxymethyl group of formaldehyde. Glutaraldehyde, which has two reactive aldehyde groups, is 10 times more effective than formaldehyde and is less toxic. In hospitals, it is used to sterilize respiratory therapy equipment, endoscopes, and hemodialysis equipment.
Ethylene Oxide
Ethylene oxide gas is used extensively in hospitals for the sterilization of heat-sensitive materials such as surgical instruments and plastics. It kills by alkylating both proteins and nucleic acids (i.e., the hydroxyethyl group attacks the reactive hydrogen atoms on essential amino and hydroxyl groups).
Acids & Alkalis
Strong acids and alkalis kill by denaturing proteins. Although most bacteria are susceptible, it is important to note that Mycobacterium tuberculosis and other mycobacteria are relatively resistant to 2% NaOH, which is used in the clinical laboratory to liquefy sputum prior to culturing the organism. Weak acids, such as benzoic, propionic, and citric acids, are frequently used as food preservatives because they are bacteriostatic. The action of these acids is partially a function of the organic moiety (e.g., benzoate), as well as the low pH.
MODIFICATION OF NUCLEIC ACIDS
A variety of dyes not only stain microorganisms, but also inhibit their growth. One of these is crystal violet (gentian violet), which is used as a skin antiseptic. Its action is based on binding of the positively charged dye molecule to the negatively charged phosphate groups of the nucleic acids. Malachite green, a triphenylamine dyelike crystal violet, is a component of Löwenstein-Jensen’s medium, which is used to grow M. tuberculosis. The dye inhibits the growth of unwanted organisms in the sputum during the 6-week incubation period.
PHYSICAL AGENTS
The physical agents act either by imparting energy in the form of heat or radiation or by removing organisms through filtration.
HEAT
Heat energy can be applied in three ways: in the form of moist heat (either boiling or autoclaving) or dry heat or by pasteurization. In general, heat kills by denaturing proteins, but membrane damage and enzymatic cleavage of DNA may also be involved. Moist heat sterilizes at a lower temperature than dry heat, because water aids in the disruption of noncovalent bonds (e.g., hydrogen bonds), which hold protein chains together in their secondary and tertiary structures.
Moist heat sterilization, usually autoclaving, is the most frequently used method of sterilization. Because bacterial spores are resistant to boiling (100°C at sea level), they must be exposed to a higher temperature; this cannot be achieved unless the pressure is increased. For this purpose, an autoclave chamber is used in which steam, at a pressure of 15 lb/in2, reaches a temperature of 121°C and is held at that temperature for 15 to 20 minutes. This kills even the highly heat-resistant spores of Clostridium botulinum, the cause of botulism, with a margin of safety. To test the effectiveness of the autoclaving process, spore-forming organisms, such as members of the genus Clostridium, are used.
Sterilization by dry heat, on the other hand, requires temperatures in the range of 180°C for 2 hours. This process is used primarily for glassware and is used less frequently than autoclaving.
Pasteurization, which is used primarily for milk, consists of heating the milk to 62°C for 30 minutes followed by rapid cooling. (“Flash” pasteurization at 72°C for 15 seconds is often used.) This is sufficient to kill the vegetative cells of the milk-borne pathogens (e.g., Mycobacterium bovis, Salmonella, Streptococcus, Listeria, and Brucella), but not to sterilize the milk.
RADIATION
The two types of radiation used to kill microorganisms are ultraviolet (UV) light and X-rays. The greatest antimicrobial activity of UV light occurs at 250 to 260 nm, which is the wavelength region of maximum absorption by the purine and pyrimidine bases of DNA. The most significant lesion caused by UV irradiation is the formation of thymine dimers, but addition of hydroxyl groups to the bases also occurs. As a result, DNA replication is inhibited and the organism cannot grow. Cells have repair mechanisms against UV-induced damage that involve either cleavage of dimers in the presence of visible light (photoreactivation) or excision of damaged bases, which is not dependent on visible light (dark repair). Because UV radiation can damage the cornea and skin, the use of UV irradiation in medicine is limited. However, it is used in hospitals to kill airborne organisms, especially in operating rooms when they are not in use. Bacterial spores are quite resistant and require a dose up to 10 times greater than do the vegetative bacteria.
X-rays have higher energy and penetrating power than UV radiation and kill mainly by the production of free radicals (e.g., production of hydroxyl radicals by the hydrolysis of water). These highly reactive radicals can break covalent bonds in DNA, thereby killing the organism. Sulfhydryl-containing compounds, such as the amino acid cysteine, can protect DNA from free-radical attack. Another mechanism is a direct hit on a covalent bond in DNA, resulting in chain breakage, but this is probably less important than the mechanism involving free radicals.
X-rays kill vegetative cells readily, but spores are remarkably resistant, probably because of their lower water content. X-rays are used in medicine for sterilization of heat-sensitive items, such as sutures and surgical gloves, and plastic items, such as syringes.
FILTRATION
Filtration is the preferred method of sterilizing certain solutions (e.g., those with heat-sensitive components). In the past, solutions for intravenous use were autoclaved, but heat-resistant endotoxin in the cell walls of the dead gram-negative bacteria caused fever in recipients of the solutions. Therefore, solutions are now filtered to make them pyrogen-free prior to autoclaving.
The most commonly used filter is composed of nitrocellulose and has a pore size of 0.22 μm. This size will retain all bacteria and spores. Filters work by physically trapping particles larger than the pore size and by retaining somewhat smaller particles via electrostatic attraction of the particles to the filters.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


