46 This chapter describes the specialized services provided by hospital pharmacy departments in the provision of various aseptic dosage forms. These services may include: chemotherapy reconstitution services, centralized intravenous additive services (CIVAS), radiopharmacy services (see Ch. 45) and home-care services. In each case, the service involves the provision of aseptically-prepared medicines which are often tailored to the specific needs of individual patients. This chapter introduces the scope, practice and pharmaceutical challenges of aseptic compounding services. In the UK, the requirements for safe handling of cytotoxic drugs are set out and enforced by the Health and Safety Executive. Pharmacy staff preparing cytotoxic agents must be fully trained in the necessary aseptic and safe handling techniques and must be fully aware of the potential health risks and the precautions that are required. Current opinion suggests that resources should be invested in appropriate equipment, staff training and competency assessment. Published guidelines include the following areas of safe practice: Useful guidelines on cytotoxic handling include The Cytotoxics Handbook and further references are available in Appendix 4. On receipt of a prescription for a cytotoxic agent, a number of procedures must be undertaken. Figure 46.1 shows the areas of work in which a pharmacist may have involvement. Figure 46.1 Documentation required for cytotoxic services. (From Allwood et al. 2002, reproduced with permission.) Labels should include the following information:
Specialized services
 Pharmacy aseptic compounding services and the range of medicines prepared in them
Pharmacy aseptic compounding services and the range of medicines prepared in them
 Equipment and procedures used in centralized cytotoxic reconstitution services
Equipment and procedures used in centralized cytotoxic reconstitution services
 Occupational health risks of cytotoxic drugs and the effective management of these risks
Occupational health risks of cytotoxic drugs and the effective management of these risks
 Benefits of centralized, pharmacy-operated aseptic compounding services
Benefits of centralized, pharmacy-operated aseptic compounding services
 Scope and operation of a centralized intravenous additive service (CIVAS)
Scope and operation of a centralized intravenous additive service (CIVAS)
Introduction
Occupational exposure risks
Provision of a pharmacy-based chemotherapy preparation service
Training required for staff preparing cytotoxics
Validation of operator techniques
 The operator is asked to carry out broth transfer simulations where solutions are transferred from one vial or container to another
The operator is asked to carry out broth transfer simulations where solutions are transferred from one vial or container to another
 The broth-filled vials can then be incubated and examined for microbiological growth
The broth-filled vials can then be incubated and examined for microbiological growth
 Operators must achieve negative results (no growth after incubation) on each occasion before they are deemed capable of preparing cytotoxic agents
Operators must achieve negative results (no growth after incubation) on each occasion before they are deemed capable of preparing cytotoxic agents
 Typically, each operator and each process would be re-validated at least every 3 months and training procedures should be reviewed on a regular basis
Typically, each operator and each process would be re-validated at least every 3 months and training procedures should be reviewed on a regular basis
 Operators routinely incorporate environmental monitoring tests such as settle plates and finger-dab plates into the production schedule as part of the QA process
Operators routinely incorporate environmental monitoring tests such as settle plates and finger-dab plates into the production schedule as part of the QA process
 Expert guidance on the validation and monitoring of aseptic compounding has been published by the NHS
Expert guidance on the validation and monitoring of aseptic compounding has been published by the NHS
 Operator technique in the safe handling of cytotoxic drugs can be assessed by simulating aseptic transfer processes using a sterile solution containing a fluorescent dye as splashes or spillage can be visualized using a portable ultraviolet lamp
Operator technique in the safe handling of cytotoxic drugs can be assessed by simulating aseptic transfer processes using a sterile solution containing a fluorescent dye as splashes or spillage can be visualized using a portable ultraviolet lamp
 As with assessment of aseptic technique, safe handling should be evaluated using a combination of simulation and expert observation.
As with assessment of aseptic technique, safe handling should be evaluated using a combination of simulation and expert observation.
Documentation required for cytotoxics
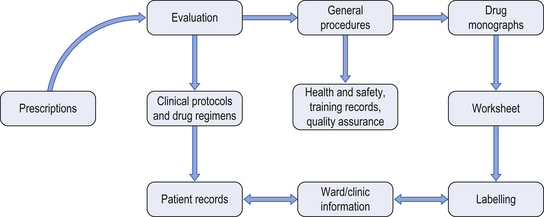
 Patient’s name, hospital number and ward or clinic name
Patient’s name, hospital number and ward or clinic name
 Drug name, total quantity and final volume of infusion
Drug name, total quantity and final volume of infusion
 Vehicle in which the drug is prepared (e.g. 0.9% sodium chloride)
Vehicle in which the drug is prepared (e.g. 0.9% sodium chloride)
 Batch number, expiry date and storage conditions required
Batch number, expiry date and storage conditions required
 Hospital pharmacy name and address
Hospital pharmacy name and address
 Route of administration and infusion rate
Route of administration and infusion rate
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Specialized services
Only gold members can continue reading. Log In or Register to continue













