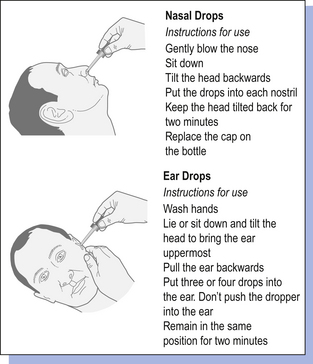
33 Solutions are homogeneous mixtures of two or more components. They contain one or more solutes dissolved in one or more solvents, usually solids dissolved in liquids. The solvent is often aqueous but can be oily, alcoholic or some other solvent. There are many types of pharmaceutical solutions. Solutions may be used as oral dosage forms, mouthwashes, gargles, nasal drops and ear drops and externally as lotions, liniments, paints, etc. Solutions may also be used in injections and ophthalmic preparations (see Chs 41, 42). The different forms of oral solutions are: Topical solutions for external use are considered in Chapter 36. Some topical solutions are designed for use in body cavities, such as the nose, mouth and ear. Gargles are used to relieve or treat sore throats and mouthwashes are used on the mucous membranes of the oral cavity, rather than the throat, to refresh and mechanically clean the mouth. Both are concentrated solutions, although gargles tend to contain higher concentrations of active ingredients than mouthwashes. Both are usually diluted with warm water before use. They may contain antiseptics, analgesics or weak astringents. The liquid is usually not intended for swallowing. Examples are Phenol Gargle BPC and Compound Sodium Chloride Mouthwash BP (see Example 33.7). Proprietary examples are chlorhexidine (Corsodyl®) mouthwash and povidone-iodine (Betadine®) mouthwash. An amber medicine bottle should be used for these extemporaneously prepared solutions. Most nasal preparations are solutions, administered as nose drops or sprays. They are usually formulated to be isotonic to nasal secretions (equivalent to 0.9% normal saline) and buffered to the normal pH range of nasal fluids (pH5.5–6.5) to prevent damage to ciliary transport in the nose. The most frequent use of nose drops is as a decongestant for the common cold or to administer local steroids for the treatment of allergic rhinitis. Examples are normal saline nose drops and ephedrine nose drops, 0.5% or 1%. Overuse of topical decongestants can lead to oedema of the nasal mucosa and they should only be used for short periods of time (about 4 days) to avoid rebound congestion, called rhinitis medicamentosa. The nasal route may also be useful for new biologically active peptides and polypeptides which need to avoid the first pass metabolism and destruction by the gastrointestinal fluids. The nasal mucosa rapidly absorbs applied medicaments to give a systemic effect. There are some products utilizing nasal delivery currently available on the market, e.g. desmopressin (e.g. Desmospray®, DDAVP®), used in the treatment of pituitary diabetes insipidus. Accurate dosage is achieved using metered spray devices. Ear drops are solutions of one or more active ingredient which exert a local effect in the ear, e.g. by softening earwax or treating infection or inflammation. They may also be referred to as otic or aural preparations. Propylene glycol, oils, glycerol (to increase viscosity) and water may be used as vehicles. Examples are aluminium acetate ear drops, almond oil ear drops and Sodium Bicarbonate Ear Drops BP (see Example 33.8). Patients should be advised not to share nasal sprays or nose and ear drops in order to minimize contamination and infection. Manufactured nasal sprays and nose and ear drops will usually contain instructions for administration. Patients should be given advice on how to administer extemporaneously prepared nose and ear drops, accompanied by written information if possible (Fig. 33.1). For nose drops, it may be easier if the patient is lying flat with the head tilted back as far as comfortable, preferably over the edge of a bed. The patient should remain in this position for a few minutes after the drops have been administered to allow the medication to spread in the nose. Enemas are oily or aqueous solutions that are administered rectally. They are usually anti-inflammatory, purgative, sedative or given to allow X-ray examination of the lower bowel. Examples are arachis oil enema and magnesium sulphate enema. Retention enemas are administered to give either a local action of the drug, e.g. prednisolone, or for systemic absorption, e.g. diazepam. They are used after defecation. The patient lies on one side during administration and remains there for 30 min to allow distribution of the medicament. Microenemas are single-dose, small-volume solutions. Examples are solutions of sodium phosphate, sodium citrate or docusate sodium. They are packaged in plastic containers with a nozzle for insertion into the rectum. Large-volume (0.5–1 L) enemas should be warmed to body temperature before administration. Strengths of pharmaceutical solutions can be expressed in a number of ways. The two most commonly used are in terms of amount of drug contained in 5 mL of vehicle or percentage strength (see Ch. 19). The saturation solubility of a chemical in a solvent is the maximum concentration of a solution, which may be prepared at a given temperature. For convenience, this is usually simply called solubility. Solubilities for medicinal agents in a given solvent are given in the British Pharmacopoeia (BP) and Martindale and other reference sources. Solubilities are usually stated as the number of parts of solvent (by volume) that will dissolve one part (by weight or volume) of the substance. In other situations, words are used to describe the solubility (see Examples 33.1 and 33.2). Using this information, it is often possible to calculate whether a solution can be prepared. Most solutions for pharmaceutical use are not saturated with solute.
Solutions
 Definitions of solutions and expressions of solubility
Definitions of solutions and expressions of solubility
 Advantages and disadvantages of using solutions
Advantages and disadvantages of using solutions
 Methods of controlling solubility
Methods of controlling solubility
 Use of preservatives and other ingredients in solutions
Use of preservatives and other ingredients in solutions
 Nasal, oral and aural solutions
Nasal, oral and aural solutions
Introduction
Solutions for oral dosage
 Syrups, which are aqueous solutions that contain sugar. An example is Epilim® syrup (sodium valproate)
Syrups, which are aqueous solutions that contain sugar. An example is Epilim® syrup (sodium valproate)
 Elixirs, which are clear, flavoured liquids containing a high proportion of sucrose or a suitable polyhydric alcohol and sometimes ethanol. Examples are phenobarbital elixir and chloral elixir (see Example 33.5)
Elixirs, which are clear, flavoured liquids containing a high proportion of sucrose or a suitable polyhydric alcohol and sometimes ethanol. Examples are phenobarbital elixir and chloral elixir (see Example 33.5)
 Linctuses, which are viscous liquids used in the treatment of cough. They usually contain a high proportion of sucrose, other sugars or a suitable polyhydric alcohol or alcohols. Examples are Simple Linctus BP and diamorphine linctus (see Example 33.4)
Linctuses, which are viscous liquids used in the treatment of cough. They usually contain a high proportion of sucrose, other sugars or a suitable polyhydric alcohol or alcohols. Examples are Simple Linctus BP and diamorphine linctus (see Example 33.4)
 Mixtures is a term often used to describe pharmaceutical oral solutions and suspensions. Examples are chloral hydrate mixture and ammonium and ipecacuanha mixture BP (see Example 33.3)
Mixtures is a term often used to describe pharmaceutical oral solutions and suspensions. Examples are chloral hydrate mixture and ammonium and ipecacuanha mixture BP (see Example 33.3)
 Oral drops are oral solutions or suspensions which are administered in small volumes, using a suitable measuring device. A proprietary example is Abidec® vitamin drops.
Oral drops are oral solutions or suspensions which are administered in small volumes, using a suitable measuring device. A proprietary example is Abidec® vitamin drops.
Solutions for other pharmaceutical uses
Mouthwashes and gargles
Containers for mouthwashes and gargles
Nasal solutions
Ear drops
Special labels and advice for nasal and aural preparations
Enemas
Expression of concentration
Formulation of solutions
Solubility
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Solutions
Only gold members can continue reading. Log In or Register to continue