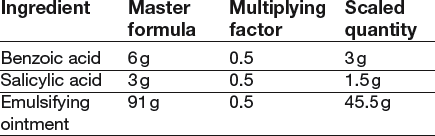
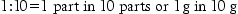19 Most pharmaceutical calculations are simple arithmetic. Calculating quantities in the dispensing process requires careful, methodical working which will minimize the risk of errors. Always try to relate the calculation to practice, visualize what you are doing and double-check everything. As in all dispensing procedures, an organized, methodical approach is essential: In some countries, the avoirdupois (or imperial) system (pounds and ounces) is still used in commerce and daily life. The imperial system of volume (pints and gallons) is still a common system for commerce and household measurement. Pharmacists need to know about these systems in order to avoid serious errors in interpretation of prescriptions. It is important to be able to change between the systems. Some conversion factors for the metric and avoirdupois systems are shown in Box 19.1 (see Examples 19.1–19.3). Ratio is the relative magnitude of two like quantities. Thus: Ratio strength is the expression of a concentration by means of a ratio, e.g. 1:10. Percentage strength is a ratio of parts per hundred, e.g. 10% (see Examples 19.4–19.6). Percentage weight in weight (w/w) is the number of grams of an ingredient in 100 grams (solid or liquid) (Example 19.7). Percentage weight in volume (w/v) is the number of grams of an ingredient in 100 mL of liquid (Example 19.8). Percentage volume in volume (v/v) indicates the number of millilitres (mL) of an ingredient in 100 mL of liquid (Example 19.9). Concentrations can also be expressed in moles or millimoles (see Ch. 41). When a mixture contains the molecular weight of a drug in grams in 1 litre of solution, the concentration is defined as a 1 molar solution (1 mol). It has a molarity of 1. Thus, for example, the molecular weight of potassium hydroxide (KOH) is the sum of the atomic weights of its elements, i.e. KOH = 39 + 16 + 1 = 56. Therefore a 1 molar solution (1 mol) of KOH contains 56 g of KOH in 1 litre of solution. A 1 millimole (mmol) solution of KOH contains one-thousandth of a mole in 1 litre = 56 mg (see Examples 19.14–19.16). In extemporaneous dispensing, a list of the ingredients is provided on the prescription or is obtained from a reference source. It may be that this ‘formula’ is for the quantity requested, but more often, the quantities provided by the master formula have to be scaled up or down, depending on the quantity of the product required. This can be achieved using proportion or by deriving a ‘multiplying factor’. The latter is the ratio of the required quantity divided by the formula quantity. The following examples illustrate this process (Examples 19.17 and 19.18). In most formulae where a combination of weights and volumes is required, the formula will indicate that the preparation is to be made up to the required weight or volume with the designated vehicle. However, occasionally, as can be seen in the next example, a combination of stated weights and volumes is used and it is not possible to indicate what the exact final weight or volume of the preparation will be. In these instances an excess quantity is normally calculated for and the required amount measured (Example 19.19)
Pharmaceutical calculations
Introduction
How to minimize errors
 Write out the calculation clearly – it is all too easy to end up reading from the wrong line
Write out the calculation clearly – it is all too easy to end up reading from the wrong line
 If you are transferring data from a reference source, double-check what you have written down is correct
If you are transferring data from a reference source, double-check what you have written down is correct
 Do not take short cuts – you are more likely to make a mistake
Do not take short cuts – you are more likely to make a mistake
 Try not to be totally dependent on your calculator – have an approximate idea of what the answer should be and then if you happen to hit the wrong button on the calculator you are more likely to be aware that an error has been made
Try not to be totally dependent on your calculator – have an approximate idea of what the answer should be and then if you happen to hit the wrong button on the calculator you are more likely to be aware that an error has been made
 Finally, always double-check your calculation. There is frequently more than one way of doing a calculation, so if you get the same answer by two different methods the chances are that your answer will be correct. Alternatively, try working it in reverse and see if you get the starting numbers
Finally, always double-check your calculation. There is frequently more than one way of doing a calculation, so if you get the same answer by two different methods the chances are that your answer will be correct. Alternatively, try working it in reverse and see if you get the starting numbers
Expressions of concentration
Expressions of strength
Percentage weight in weight (w/w)
Percentage weight in volume (w/v)
Percentage volume in volume (v/v)
Moles and molarity
Calculating quantities from a master formula
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Basicmedical Key
Fastest Basicmedical Insight Engine