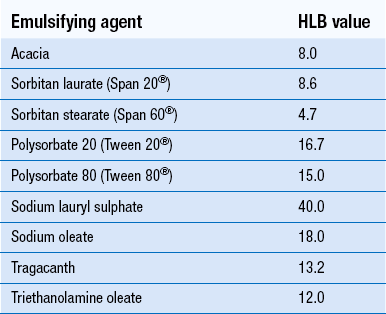
35 An emulsion consists of two immiscible liquids, one of which is uniformly dispersed throughout the other as fine droplets normally of diameter 0.1–100 μm. To prepare a stable emulsion, a third ingredient, an emulsifying agent, is required. Oral emulsions are stabilized oil-in-water dispersions that may contain one or more active ingredients. They are a useful way of presenting oils and fats in a palatable form. Emulsions for external use are known as lotions, applications or liniments if liquid, or creams if semi-solid in nature. Some parenteral products may also be formulated as emulsions. Most important of these is total parenteral nutrition (see Ch. 44). Pharmaceutically, the term ‘emulsion’, when no other qualification is used, is taken to mean an oil-in-water preparation for internal use. Emulsions have a wide range of uses, including: Examples of traditionally used emulsions for oral use are cod liver oil emulsion (see Example 35.1), Liquid Paraffin Oral Emulsion BP (see Example 35.2). An example of emulsion for external use is Oily Calamine Lotion BP (see Example 35.3). If the emulsion is for oral or intravenous administration it will always be oil-in-water. Intramuscular injections may be water-in-oil for depot therapy. When selecting emulsion type for preparations for external use, the therapeutic use, texture and patient acceptability will be taken into account. Oil-in-water emulsions are less greasy, easily washed off the skin and more cosmetically acceptable than water-in-oil emulsions. They have an occlusive effect, which hydrates the upper layers of the skin (called an emollient, see Ch. 36). Water-in-oil emulsions rub in more easily. Semi-synthetic polysaccharides. Low-viscosity grades of methylcellulose (see Example 35.2) and carboxymethylcellulose will form o/w emulsions. Sterol-containing substances. These agents act as water-in-oil emulsifying agents. Examples include beeswax, wool fat and wool alcohols (see Ch. 36). Anionic surfactants. These are organic salts which, in water, have a surface-active anion. They are incompatible with some organic and inorganic cations and with large organic cations such as cetrimide. They are widely used in external preparations as o/w emulsifying agents. They must be in their ionized form to be effective and emulsions made with anionic surfactants are generally stable at more alkaline pH. Some pharmaceutical examples of anionic surfactants include: The HLB (hydrophilic–lipophilic balance) system. An HLB number, usually between 1 and 20, is allocated to an emulsifying agent and represents the relative proportions of the lipophilic and hydrophilic parts of the molecule. The lower the number, the more oil soluble the emulsifying agent. Higher numbers (8–18) indicate a hydrophilic molecule which produces an o/w emulsion. Low numbers (3–6) indicate a lipophilic molecule which produces a w/o emulsion. Oils and waxy materials have a ‘required HLB number’ which helps in the selection of appropriate emulsifying agents when formulating emulsions. Liquid paraffin, for example, has a required HLB value of 4 to obtain a w/o emulsion and 12 for an o/w emulsion. Two or more surfactants can be combined to achieve a suitable HLB value and often give better results than one surfactant alone. HLB values of some commonly used emulsifying agents are given in Table 35.1.
Emulsions
 The uses of pharmaceutical emulsions
The uses of pharmaceutical emulsions
 The different types of emulsion and their identification
The different types of emulsion and their identification
 Selection of emulsifying agents and other ingredients
Selection of emulsifying agents and other ingredients
Introduction
Pharmaceutical applications of emulsions
 Oral, rectal and topical administration of oils and oil-soluble drugs
Oral, rectal and topical administration of oils and oil-soluble drugs
 Formulation of oil- and water-soluble drugs together
Formulation of oil- and water-soluble drugs together
 To enhance palatability of oils when given orally by disguising both taste and oiliness
To enhance palatability of oils when given orally by disguising both taste and oiliness
 Increasing absorption of oils and oil-soluble drugs through intestinal walls
Increasing absorption of oils and oil-soluble drugs through intestinal walls
 Intramuscular injections of some water-soluble vaccines: these provide slow release and therefore a greater antibody response and longer-lasting immunity
Intramuscular injections of some water-soluble vaccines: these provide slow release and therefore a greater antibody response and longer-lasting immunity
Emulsion types
Formulation of emulsions
Emulsifying agents
Naturally occurring emulsifying agents
Synthetic surfactants
 Alkali metal and ammonium soaps such as sodium stearate (o/w)
Alkali metal and ammonium soaps such as sodium stearate (o/w)
 Soaps of divalent and trivalent metals such as calcium oleate (w/o) (see Example 35.3)
Soaps of divalent and trivalent metals such as calcium oleate (w/o) (see Example 35.3)
 Amine soaps such as triethanolamine oleate (o/w)
Amine soaps such as triethanolamine oleate (o/w)
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Emulsions
Only gold members can continue reading. Log In or Register to continue