Seminoma
Steven S. Shen, MD, PhD
Mahul B. Amin, MD
Jae Y. Ro, MD, PhD
Key Facts
Terminology
Most common pure germ cell tumor composed of relatively uniform cells with abundant clear cytoplasm, well-defined cell borders, and nuclei with 1 or more prominent nucleoli
Clinical Issues
30-45% of testicular germ cell tumors
Macroscopic Features
Well circumscribed and homogeneous ± lobulation
Gray-white, tan, creamy, fleshy or firm, often bulging cut surface
Microscopic Pathology
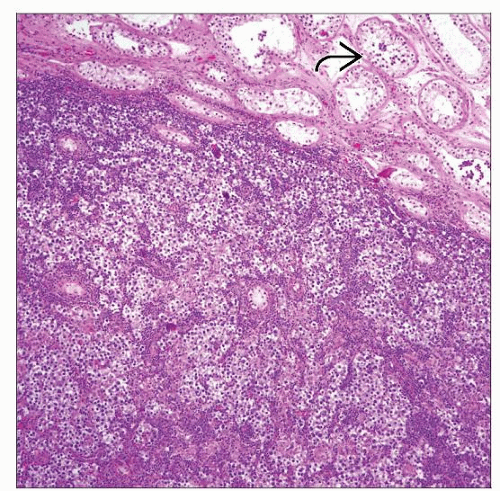
Fibrous septae divide sheets or nests of tumor cells into lobules
Tumor cells are evenly spread without nuclear overlap in well-fixed tissues
Prominent cytoplasmic membranes (distinct cell boundary)
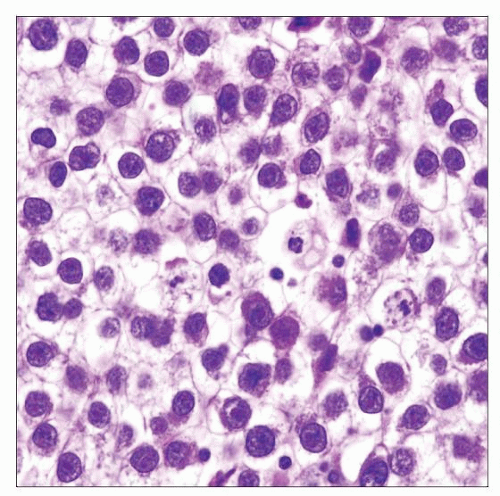
Large round-polygonal tumor cells with abundant clear cytoplasm
Lymphoplasmacytic infiltrate, occasionally extensive with germinal centers in fibrous septae
Granulomatous inflammation in approximately 30%; can be extensive, which can create diagnostic difficulty in recognizing tumor cells
Ancillary Tests
PAS positive with diastase sensitive
(+) for Oct3/4 (nuclear), Podoplanin(D2-40), & PLAP
(-) for cytokeratin (may be focal or weak), CD30(BerH2), & α-fetoprotein
TERMINOLOGY
Synonyms
Classic seminoma, typical seminoma
Germinoma in extragonadal sites
Dysgerminoma in females
Definitions
Most common pure germ cell tumor composed of relatively uniform cells with abundant clear cytoplasm, well-defined cell borders, and nuclei with 1 or more prominent nucleoli
CLINICAL ISSUES
Epidemiology
Incidence
30-45% of testicular germ cell tumors
Age
Most commonly in men 35-45 years old
Uncommon in men over 50 years and rare in children
Mean age 5-10 years older than nonseminomatous germ cell tumors
Presentation
Most commonly painless testicular mass (70%)
Other presentations
Scrotal pain (10%)
Symptoms of metastasis (10%)
Asymptomatic (4%)
Gynecomastia and exophthalmos (rare)
Mostly unilateral and rarely bilateral (about 2%); bilaterality more common than in nonseminomatous germ cell tumors
Spermatic cord involvement (rarer than in nonseminomatous germ cell tumors; < 5%)
Laboratory Tests
Serum markers may be elevated
Serum lactate dehydrogenase (LDH)
Human chorionic gonadotropin (hCG)
α-fetoprotein (AFP) should be normal for pure seminoma
AFP elevation in patient with pure seminoma is clinically treated as nonseminomatous germ cell tumor
Treatment
For patients with stage I seminoma, 3 options are available
Radical inguinal orchiectomy and surveillance with measurement of serum markers, chest x-ray, and CT
Radical inguinal orchiectomy with single dose carboplatin adjuvant therapy
Radical inguinal orchiectomy with radiation therapy
For patients with stage II seminoma
Radical inguinal orchiectomy followed by radiation therapy to retroperitoneal and ipsilateral pelvic lymph nodes, or combination chemotherapy
For patients with stage III seminoma
Radical inguinal orchiectomy followed by multidrug (bleomycin, etoposide, and cisplatin) chemotherapy
Prognosis
Excellent prognosis with 98% cure rate for stage I or II seminoma
Associated with pathologic stage, tumor size, rete testis invasion, and intertubular growth > 3 high-power fields
Lymphovascular invasion is important prognostic factor in univariate analysis but not independent prognostic factor
Concept of “anaplastic” seminoma (> 3 mitoses per high-power field) is not accepted as separate entity and not adverse prognostic factor
MACROSCOPIC FEATURES
General Features
Well circumscribed and homogeneous ± lobulation; 90% confined to testis
Gray-white, tan, creamy, fleshy or firm, often bulging cut surface; usually no hemorrhage or necrosis
Tumors with hemorrhage and necrosis often indicate nonseminomatous germ cell components
May have geographic infarct-type necrosis (usually large tumors)
Punctate hemorrhage (usually in areas of syncytiotrophoblasts)
Rare spermatic cord invasion (< 5%)
Size
Average 5.0 cm (range 2.0-24 cm)
MICROSCOPIC PATHOLOGY
Histologic Features
Main architectural growth patterns
Solid sheets or nests (most common)
Interstitial (in between seminiferous tubules; rare)
Tubular, alveolar, or pseudoglandular (rare)
Trabecular (rare)
Sclerotic (very rare)
Fibrous septae divide sheets or nests of tumor cells into lobules
Tumor cells are evenly spread without nuclear overlap in well-fixed tissues
Lymphoplasmacytic infiltrate, occasionally extensive, with germinal centers in fibrous septae
Granulomatous inflammation in approximately 30%; may be extensive, which can create diagnostic difficulty in recognizing tumor cells
Fibrosis and sclerosis may be prominent (burnt-out seminoma when no tumor cells present)
Hemorrhage and necrosis are rarely seen
Syncytiotrophoblastic giant cells may be seen in areas of hemorrhage
Intratubular germ cell neoplasia (ITGCN) in surrounding seminiferous tubules or pagetoid spread to rete testis
Cytologic Features
Large round-polygonal tumor cells with abundant clear cytoplasm
Prominent cytoplasmic membranes (distinct cell boundary)
Relatively uniform, large central nuclei with 1-2 prominent nucleoli
Mitotic figures range from rare to frequent
Some tumors can have larger cells, high N:C ratio, and more mitoses (> 3/high-power field); known as “anaplastic seminoma”
Rarely, tumor cells can have rhabdoid appearance with abundant eosinophilic cytoplasm and eccentrically located nuclei; often occurs in poorly fixed specimens
Predominant Pattern/Injury Type
Neoplastic
Predominant Cell/Compartment Type
Uncommitted large atypical malignant germ cells
ANCILLARY TESTS
Histochemistry
Periodic acid-Schiff without diastase
Reactivity: Positive
Staining pattern
Cytoplasmic
Immunohistochemistry
Positive for PLAP, Oct3/4, CD117, Podoplanin(D2-40), vimentin, SALL4
Negative for cytokeratin (may be focal or weak), α-fetoprotein, HCG, inhibin-α, CD30, glypican-3
DIFFERENTIAL DIAGNOSIS
Embryonal Carcinoma, Solid Pattern
Usually admixed with glandular, papillary, and solid growth patterns
Marked cellular pleomorphism, vesicular nuclei, nuclear crowding with overlapping and indistinct cell border, irregularly shaped nucleoli, frequent mitoses or apoptoses
Positive for cytokeratin and CD30(BerH2)
Yolk Sac Tumor, Solid Pattern
Variable growth patterns, most commonly microcystic and reticular
Schiller-Duval bodies, basement membrane deposition, and hyaline globules are characteristic, if present
Positive for cytokeratin, α-fetoprotein, and glypican-3
Malignant Lymphoma
Usually older age group, history of lymphoma, and frequent bilateral involvement
Predominantly interstitial pattern of tumor cells between seminiferous tubules
Cytokeratin and germ cell markers negative; CD45(LCA) and B- or T-cell markers positive (depending on type, B more common than T)
Frequent spermatic cord involvement (> 40%)
Spermatocytic Seminoma
Older age group (average: 56 years)
No association with ITGCN; intratubular growth may be seen
Presence of 3 distinct types of tumor cells
Lack of lymphocytic infiltration or granulomatous inflammation; no fibrous septa
PAS stain negative
Negative for germ cell tumor markers (PLAP, Oct3/4, Podoplanin[D2-40]); CD117 may be positive
Monophasic Choriocarcinoma
Extremely rare; primary or metastatic foci postchemotherapy
Mononucleated tumor cells of variable sizes
More frequent hemorrhage and necrosis
Positive for HCG and human placental lactogen (HPL)
Nonspecific Granulomatous Orchitis
Mixed population of inflammatory cells
Predominantly involves seminiferous tubules
Need to differentiate from burnt out seminoma
Sertoli Cell Tumor
Usually more prominent tubular or cystic growth
Particularly for those with solid or sheets of clear cells
Usually lack fibrous septae with lymphoplasmacytic and granulomatous inflammation
Usually positive for α-inhibin and cytokeratin, negative for PLAP, Podoplanin(D2-40), and Oct3/4
DIAGNOSTIC CHECKLIST
Pathologic Interpretation Pearls
Relatively uniform tumor cells with abundant clear cytoplasm, distinct cell boundaries, evenly spaced tumor cells without nuclear overlapping
Fibrous septae with lymphoplasmacytic infiltrates and granulomatous reaction
Diagnostically difficult patterns
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree