22
Sedative-Hypnotic Drugs*
CASE STUDY
At her annual physical examination, a 53-year-old middle school teacher complains that she has been having difficulty falling asleep, and after falling asleep, she awakens several times during the night. These episodes now occur almost nightly and are interfering with her ability to teach. She has tried various over-the-counter sleep remedies, but they were of little help and she experienced “hangover” effects on the day following their use. Her general health is good, she is not overweight, and she takes no prescription drugs. She drinks decaffeinated coffee but only one cup in the morning; however, she drinks as many as 6 cans per day of diet cola. She drinks a glass of wine with her evening meal but does not like stronger spirits. What other aspects of this patient’s history would you like to know? What therapeutic measures are appropriate for this patient? What drug, or drugs, (if any) would you prescribe?
Assignment of a drug to the sedative-hypnotic class indicates that it is able to cause sedation (with concomitant relief of anxiety) or to encourage sleep (hypnosis). Because there is considerable chemical variation within the group, this drug classification is based on clinical uses rather than on similarities in chemical structure. Anxiety states and sleep disorders are common problems, and sedative-hypnotics are widely prescribed drugs worldwide.
 BASIC PHARMACOLOGY OF SEDATIVE-HYPNOTICS
BASIC PHARMACOLOGY OF SEDATIVE-HYPNOTICS
An effective sedative (anxiolytic) agent should reduce anxiety and exert a calming effect. The degree of central nervous system (CNS) depression caused by a sedative should be the minimum consistent with therapeutic efficacy. A hypnotic drug should produce drowsiness and encourage the onset and maintenance of a state of sleep. Hypnotic effects involve more pronounced depression of the CNS than sedation, and this can be achieved with many drugs in this class simply by increasing the dose. Graded dose-dependent depression of CNS function is a characteristic of most sedative-hypnotics. However, individual drugs differ in the relationship between the dose and the degree of CNS depression. Two examples of such dose-response relationships are shown in Figure 22–1. The linear slope for drug A is typical of many of the older sedative-hypnotics, including the barbiturates and alcohols. With such drugs, an increase in dose higher than that needed for hypnosis may lead to a state of general anesthesia. At still higher doses, these sedative-hypnotics may depress respiratory and vasomotor centers in the medulla, leading to coma and death. Deviations from a linear dose-response relationship, as shown for drug B, require proportionately greater dosage increments to achieve CNS depression more profound than hypnosis. This appears to be the case for benzodiazepines and for certain newer hypnotics that have a similar mechanism of action.
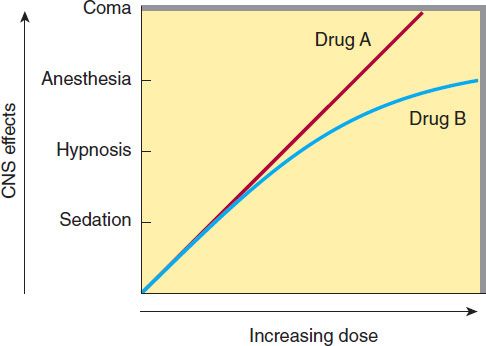
FIGURE 22–1 Dose-response curves for two hypothetical sedative-hypnotics.
CHEMICAL CLASSIFICATION
The benzodiazepines are widely used sedative-hypnotics. All of the structures shown in Figure 22–2 are 1,4-benzodiazepines, and most contain a carboxamide group in the 7-membered heterocyclic ring structure. A substituent in the 7 position, such as a halogen or a nitro group, is required for sedative-hypnotic activity. The structures of triazolam and alprazolam include the addition of a triazole ring at the 1,2-position.
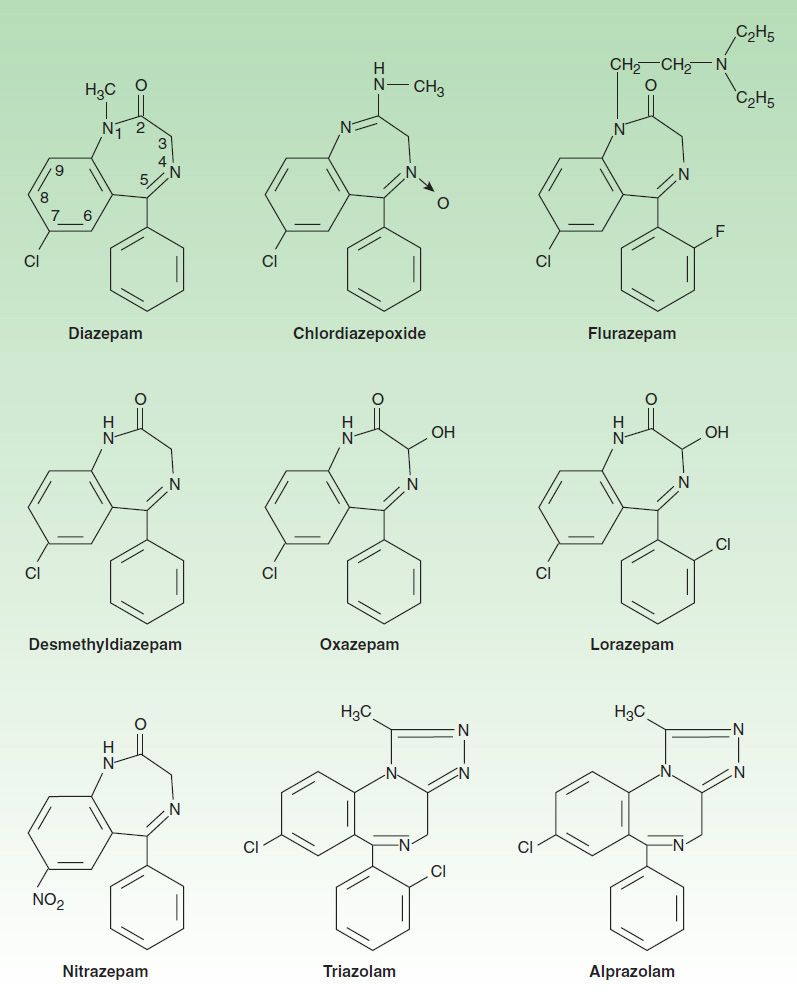
FIGURE 22–2 Chemical structures of benzodiazepines.
The chemical structures of some older and less commonly used sedative-hypnotics, including several barbiturates, are shown in Figure 22–3. Glutethimide and meprobamate are of distinctive chemical structure but are practically equivalent to barbiturates in their pharmacologic effects. They are rarely used. The sedative-hypnotic class also includes compounds of simpler chemical structure, including ethanol (see Chapter 23) and chloral hydrate.
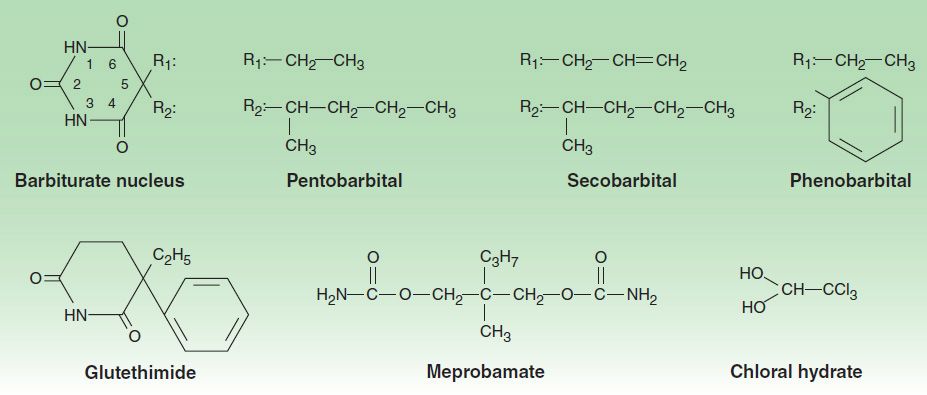
FIGURE 22–3 Chemical structures of some barbiturates and other sedative-hypnotics.
Several drugs with novel chemical structures have been introduced more recently for use in sleep disorders. Zolpidem, an imidazopyridine; zaleplon, a pyrazolopyrimidine; and eszopiclone, a cyclopyrrolone (Figure 22–4), although structurally unrelated to benzodiazepines, share a similar mechanism of action, as described below. Eszopiclone is the (S) enantiomer of zopiclone, a hypnotic drug that has been available outside the United States since 1989. Ramelteon and tasimelteon, melatonin receptor agonists, are newer hypnotic drugs (see Box: Ramelteon). Buspirone is a slow-onset anxiolytic agent whose actions are quite different from those of conventional sedative-hypnotics (see Box: Buspirone).
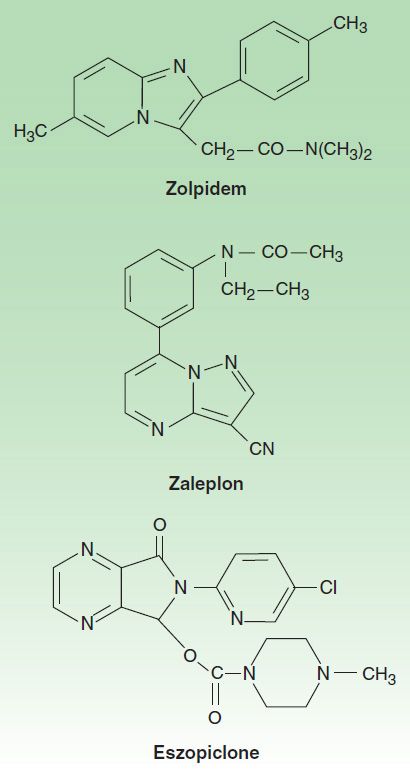
FIGURE 22–4 Chemical structures of newer hypnotics.
Other classes of drugs that exert sedative effects include antipsychotics (see Chapter 29), and many antidepressant drugs (see Chapter 30). The latter are currently used widely in management of chronic anxiety disorders. Certain antihistaminic agents including hydroxyzine and promethazine (see Chapter 16) are also sedating. These agents commonly also exert marked effects on the peripheral autonomic nervous system. Other antihistaminic drugs with hypnotic effects, eg, diphenhydramine and doxylamine, are available in over-the-counter sleep aids.
Pharmacokinetics
A. Absorption and Distribution
The rates of oral absorption of sedative-hypnotics differ depending on a number of factors, including lipophilicity. For example, the absorption of triazolam is extremely rapid, and that of diazepam and the active metabolite of clorazepate is more rapid than other commonly used benzodiazepines. Clorazepate, a prodrug, is converted to its active form, desmethyldiazepam (nordiazepam), by acid hydrolysis in the stomach. Most of the barbiturates and other older sedative-hypnotics, as well as the newer hypnotics (eszopiclone, zaleplon, zolpidem), are absorbed rapidly into the blood following oral administration.
Lipid solubility plays a major role in determining the rate at which a particular sedative-hypnotic enters the CNS. This property is responsible for the rapid onset of the effects of triazolam, thiopental (see Chapter 25), and the newer hypnotics.
All sedative-hypnotics cross the placental barrier during pregnancy. If sedative-hypnotics are given during the predelivery period, they may contribute to the depression of neonatal vital functions. Sedative-hypnotics are also detectable in breast milk and may exert depressant effects in the nursing infant.
B. Biotransformation
Metabolic transformation to more water-soluble metabolites is necessary for clearance of sedative-hypnotics from the body. The microsomal drug-metabolizing enzyme systems of the liver are most important in this regard, so elimination half-life of these drugs depends mainly on the rate of their metabolic transformation.
Melatonin receptors are thought to be involved in maintaining circadian rhythms underlying the sleep-wake cycle (see Chapter 16). Ramelteon, a novel hypnotic drug prescribed specifically for patients who have difficulty in falling asleep, is an agonist at MT1 and MT2 melatonin receptors located in the suprachiasmatic nuclei of the brain. Tasimelteon is similar and is approved for non-24 hour sleep-wake disorder. These drugs have no direct effects on GABAergic neurotransmission in the central nervous system. In polysomnography studies of patients with chronic insomnia, ramelteon reduced the latency of persistent sleep with no effects on sleep architecture and no rebound insomnia or significant withdrawal symptoms. The drug is rapidly absorbed after oral administration and undergoes extensive first-pass metabolism, forming an active metabolite with longer half-life (2–5 hours) than the parent drug. The CYP1A2 isoform of cytochrome P450 is mainly responsible for the metabolism of ramelteon, but the CYP2C9 isoform is also involved. Ramelteon should not be used in combination with inhibitors of CYP1A2 (eg, ciprofloxacin, fluvoxamine, tacrine, zileuton) or CYP2C9 (eg, fluconazole). Concurrent use with the antidepressant fluvoxamine increases the peak plasma concentration of ramelteon over 50-fold!
Ramelteon should be used with caution in patients with liver dysfunction. The CYP inducer rifampin markedly reduces the plasma levels of both ramelteon and its active metabolite. Adverse effects of ramelteon include dizziness, somnolence, fatigue, and endocrine changes.
1. Benzodiazepines—Hepatic metabolism accounts for the clearance of all benzodiazepines. The patterns and rates of metabolism depend on the individual drugs. Most benzodiazepines undergo microsomal oxidation (phase I reactions), including N-dealkylation and aliphatic hydroxylation catalyzed by cytochrome P450 isozymes, especially CYP3A4. The metabolites are subsequently conjugated (phase II reactions) to form glucuronides that are excreted in the urine. However, many phase I metabolites of benzodiazepines are pharmacologically active, some with long half-lives (Figure 22–5). For example, desmethyldiazepam, which has an elimination half-life of more than 40 hours, is an active metabolite of chlordiazepoxide, diazepam, prazepam, and clorazepate. Alprazolam and triazolam undergo α-hydroxylation, and the resulting metabolites appear to exert short-lived pharmacologic effects because they are rapidly conjugated to form inactive glucuronides. The short elimination half-life of triazolam (2–3 hours) favors its use as a hypnotic rather than as a sedative drug.
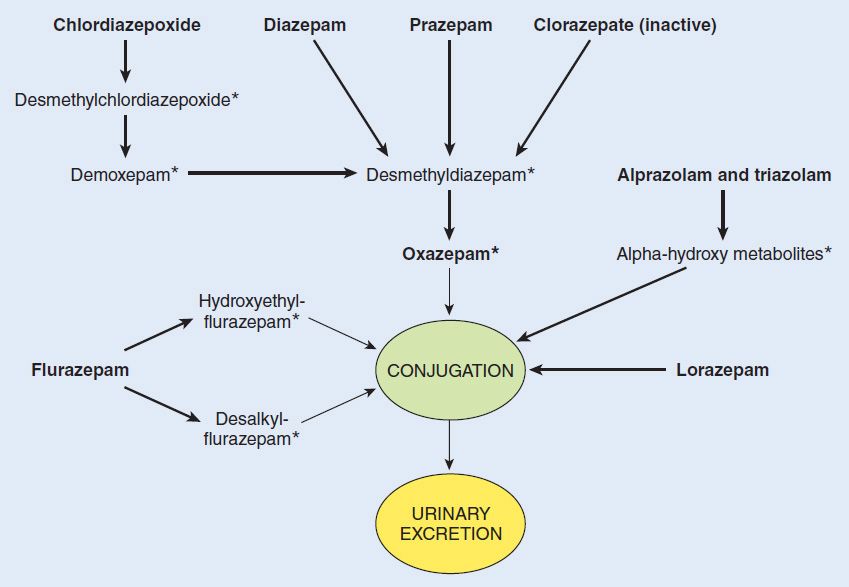
FIGURE 22–5 Biotransformation of benzodiazepines. Boldface, drugs available for clinical use in various countries; *, active metabolite.
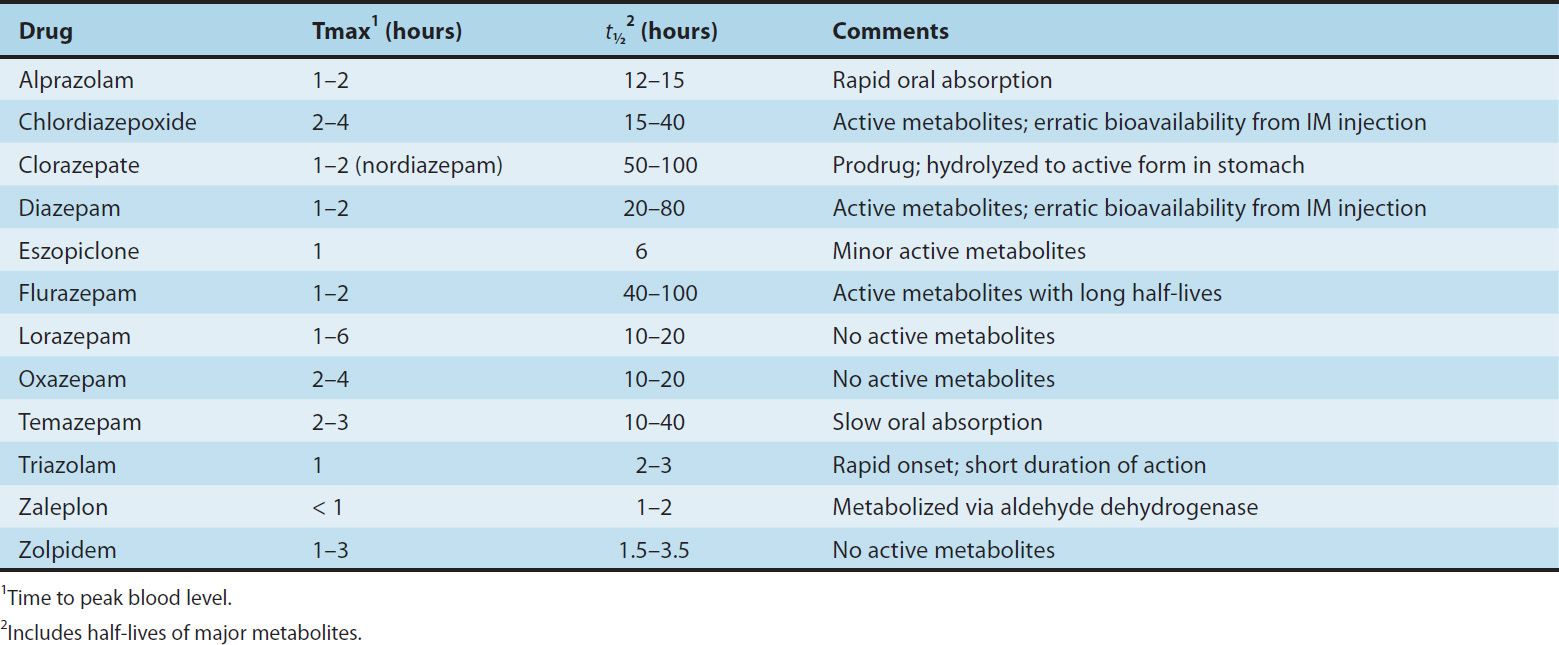
The formation of active metabolites has complicated studies on the pharmacokinetics of the benzodiazepines in humans because the elimination half-life of the parent drug may have little relation to the time course of pharmacologic effects. Benzodiazepines for which the parent drug or active metabolites have long half-lives are more likely to cause cumulative effects with multiple doses. Cumulative and residual effects such as excessive drowsiness appear to be less of a problem with such drugs as estazolam, oxazepam, and lorazepam, which have relatively short half-lives and are metabolized directly to inactive glucuronides. Some pharmacokinetic properties of selected benzodiazepines and newer hypnotics are listed in Table 22–1. The metabolism of several commonly used benzodiazepines including diazepam, midazolam, and triazolam is affected by inhibitors and inducers of hepatic P450 isozymes (see Chapter 4).
TABLE 22–1 Pharmacokinetic properties of some benzodiazepines and newer hypnotics in humans.
Buspirone
Buspirone has selective anxiolytic effects, and its pharmacologic characteristics are different from those of other drugs described in this chapter. Buspirone relieves anxiety without causing marked sedative, hypnotic, or euphoric effects. Unlike benzodiazepines, the drug has no anticonvulsant or muscle relaxant properties. Buspirone does not interact directly with GABAergic systems. It may exert its anxiolytic effects by acting as a partial agonist at brain 5-HT1A receptors, but it also has affinity for brain dopamine D2 receptors. Buspirone-treated patients show no rebound anxiety or withdrawal signs on abrupt discontinuance. The drug is not effective in blocking the acute withdrawal syndrome resulting from abrupt cessation of use of benzodiazepines or other sedative-hypnotics. Buspirone has minimal abuse liability. In marked contrast to the benzodiazepines, the anxiolytic effects of buspirone may take 3–4 weeks to become established, making the drug unsuitable for management of acute anxiety states. The drug is used in generalized anxiety states but is less effective in panic disorders.
Buspirone is rapidly absorbed orally but undergoes extensive first-pass metabolism via hydroxylation and dealkylation reactions to form several active metabolites. The major metabolite is 1-(2-pyrimidyl)-piperazine (1-PP), which has α2-adrenoceptor-blocking actions and which enters the central nervous system to reach higher levels than the parent drug. It is not known what role (if any) 1-PP plays in the central actions of buspirone. The elimination half-life of buspirone is 2–4 hours, and liver dysfunction may slow its clearance. Rifampin, an inducer of cytochrome P450, decreases the half-life of buspirone; inhibitors of CYP3A4 (eg, erythromycin, ketoconazole, grapefruit juice, nefazodone) can markedly increase its plasma levels.
Buspirone causes less psychomotor impairment than benzodiazepines and does not affect driving skills. The drug does not potentiate effects of conventional sedative-hypnotic drugs, ethanol, or tricyclic antidepressants; and elderly patients do not appear to be more sensitive to its actions. Nonspecific chest pain, tachycardia, palpitations, dizziness, nervousness, headache, tinnitus, gastrointestinal distress, and paresthesias and a dose-dependent pupillary constriction may occur. Blood pressure may be significantly elevated in patients receiving MAO inhibitors. Buspirone is an FDA category B drug in terms of its use in pregnancy.
2. Barbiturates—With the exception of phenobarbital, only insignificant quantities of the barbiturates are excreted unchanged. The major metabolic pathways involve oxidation by hepatic enzymes to form alcohols, acids, and ketones, which appear in the urine as glucuronide conjugates. The overall rate of hepatic metabolism in humans depends on the individual drug but (with the exception of the thiobarbiturates) is usually slow. The elimination half-lives of secobarbital and pentobarbital range from 18 to 48 hours in different individuals. The elimination half-life of phenobarbital in humans is 4–5 days. Multiple dosing with these agents can lead to cumulative effects.
3. Newer hypnotics—After oral administration of the standard formulation, zolpidem reaches peak plasma levels in 1–3 hours (Table 22-1). Sublingual and oral spray formulations of zolpidem are also available. Zolpidem is rapidly metabolized to inactive metabolites via oxidation and hydroxylation by hepatic CYP3A4. The elimination half-life of the drug is greater in women and is increased significantly in the elderly. A biphasic extended-release formulation extends plasma levels by approximately 2 hours. Zaleplon is metabolized to inactive metabolites mainly by hepatic aldehyde oxidase and partly by the cytochrome P450 iso-form CYP3A4. Dosage should be reduced in patients with hepatic impairment and in the elderly. Cimetidine, which inhibits both aldehyde dehydrogenase and CYP3A4, markedly increases the peak plasma level of zaleplon. Eszopiclone is metabolized by hepatic cytochromes P450 (especially CYP3A4) to form the inactive N-oxide derivative and weakly active desmethyleszopiclone. The elimination half-life of eszopiclone is prolonged in the elderly and in the presence of inhibitors of CYP3A4 (eg, ketoconazole). Inducers of CYP3A4 (eg, rifampin) increase the hepatic metabolism of eszopiclone.
C. Excretion
The water-soluble metabolites of sedative-hypnotics, mostly formed via the phase II conjugation of phase I metabolites, are excreted mainly via the kidney. In most cases, changes in renal function do not have a marked effect on the elimination of parent drugs. Phenobarbital is excreted unchanged in the urine to a certain extent (20–30% in humans), and its elimination rate can be increased significantly by alkalinization of the urine. This is partly due to increased ionization at alkaline pH, since phenobarbital is a weak acid with a pKa of 7.4.
D. Factors Affecting Biodisposition
The biodisposition of sedative-hypnotics can be influenced by several factors, particularly alterations in hepatic function resulting from disease or drug-induced increases or decreases in microsomal enzyme activities (see Chapter 4).
In very old patients and in patients with severe liver disease, the elimination half-lives of these drugs are often increased significantly. In such cases, multiple normal doses of these sedative-hypnotics can result in excessive CNS effects.
The activity of hepatic microsomal drug-metabolizing enzymes may be increased in patients exposed to certain older sedative-hypnotics on a long-term basis (enzyme induction; see Chapter 4). Barbiturates (especially phenobarbital) and meprobamate are most likely to cause this effect, which may result in an increase in their hepatic metabolism as well as that of other drugs. Increased biotransformation of other pharmacologic agents as a result of enzyme induction by barbiturates is a potential mechanism underlying drug interactions (see Chapter 66). In contrast, benzodiazepines and the newer hypnotics do not change hepatic drug-metabolizing enzyme activity with continuous use.
Pharmacodynamics of Benzodiazepines, Barbiturates, & Newer Hypnotics
A. Molecular Pharmacology of the GABAAReceptor
The benzodiazepines, the barbiturates, zolpidem, zaleplon, eszopiclone, and many other drugs bind to molecular components of the GABAA receptor in neuronal membranes in the CNS. This receptor, which functions as a chloride ion channel, is activated by the inhibitory neurotransmitter GABA (see Chapter 21).
The GABAA receptor has a pentameric structure assembled from five subunits (each with four membrane-spanning domains) selected from multiple polypeptide classes (α, β, γ, δ, ε, π, ρ, etc). Multiple subunits of several of these classes have been characterized, eg, six different α, four β, and three γ. A model of the GABAA receptor-chloride ion channel macromolecular complex is shown in Figure 22–6.
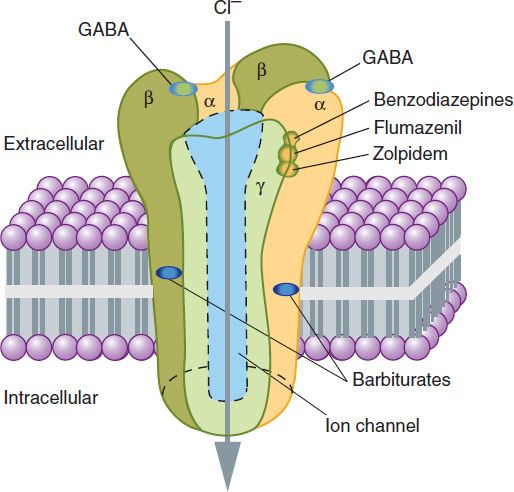
FIGURE 22–6 A model of the GABAA receptor-chloride ion channel macromolecular complex. A hetero-oligomeric glycoprotein, the complex consists of five or more membrane-spanning subunits. Multiple forms of α, β, and γ subunits are arranged in different pentameric combinations so that GABAA receptors exhibit molecular heterogeneity. GABA appears to interact at two sites between α and β subunits triggering chloride channel opening with resulting membrane hyperpolarization. Binding of benzodiazepines and the newer hypnotic drugs such as zolpidem occurs at a single site between α and γ subunits, facilitating the process of chloride ion channel opening. The benzodiazepine antagonist flumazenil also binds at this site and can reverse the hypnotic effects of zolpidem. Note that these binding sites are distinct from those of the barbiturates. (See also text and Box: The Versatility of the Chloride Channel GABA Receptor Complex.)
A major isoform of the GABAA receptor that is found in many regions of the brain consists of two α1 subunits, two β2 subunits, and one γ2 subunit. In this isoform, the two binding sites for GABA are located between adjacent α1 and β2 subunits, and the binding pocket for benzodiazepines (the BZ site of the GABAA receptor) is between an α1 and the γ2 subunit. However, GABAA receptors in different areas of the CNS consist of various combinations of the essential subunits, and the benzodiazepines bind to many of these, including receptor isoforms containing α2, α3, and α5 subunits. Barbiturates also bind to multiple isoforms of the GABAA
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


