Sclerosing Adenosis
Key Facts
Clinical Issues
Can present as a lesion on mammography, as a palpable mass, or as an incidental finding
Classified as proliferative disease without atypia
1.5-2x increased risk for development of invasive carcinoma in either breast
Most common benign lesion misdiagnosed as invasive carcinoma
Can be difficult to diagnose on core needle biopsies
Image Findings
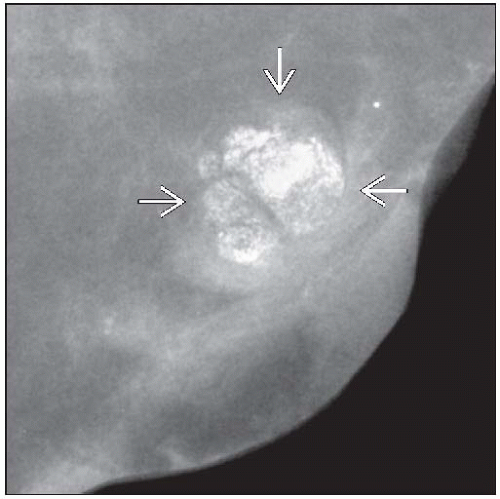
Microcalcifications (clustered amorphous) most common finding
Can also form rounded or lobulated masses
Microscopic Pathology
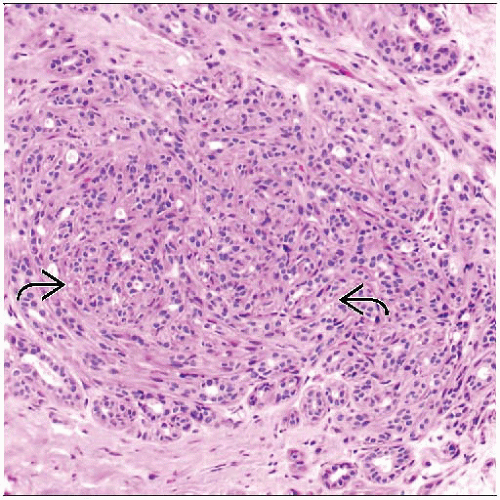
Circumscribed lobulocentric architecture
Lumens variably distorted and compressed by background collagen
Can closely mimic invasive carcinoma if involved by apocrine metaplasia, DCIS, or LCIS
Frequently associated with papillomas, complex sclerosing lesions, and fibroadenomas
Ancillary Tests
Immunohistochemistry to detect myoepithelial cells may be helpful to distinguish SA from invasive carcinoma
Expression of some markers is reduced
Top Differential Diagnoses
Normal breast
Invasive ductal carcinoma
Microglandular adenosis
TERMINOLOGY
Abbreviations
Sclerosing adenosis (SA)
Definitions
Lobulocentric proliferation of acini around a central duct with stromal sclerosis and compression of lumens
ETIOLOGY/PATHOGENESIS
Dysregulation of Estrogen Receptor
SA demonstrates increased expression of ER and Ki-67 over normal benign breast elements
SA and other proliferative lesions are more common in women receiving hormone replacement therapy
Association with obesity (increased endogenous estrogen levels)
Hormone imbalance and dysregulation of ER may play a role in development of SA
Risk for developing SA and other proliferative lesions may be increased in women who have > 25% fibroglandular breast tissue density
CLINICAL ISSUES
Epidemiology
Incidence
Frequent incidental finding in breast biopsies
Present in 12% of breast biopsies without cancer
Age
Most common in perimenopausal women
Presentation
Most common: Finding during screening mammography
Less commonly presents as a palpable mass
Termed nodular SA or adenosis tumor
May be associated with pain and tenderness
Can also be a frequent incidental finding in breast specimens removed for other indications
Prognosis
Benign proliferative lesion
Classified as proliferative disease without atypia
1.5-2x increased relative risk for development of invasive carcinoma or 5-7% actual lifetime risk
Increased risk is for both breasts
Core Needle Biopsy
Most common benign lesion mistaken for invasive carcinoma
Closely mimics carcinoma when involved by apocrine metaplasia, LCIS, or DCIS
More difficult to diagnose on core needle biopsy when borders and lobulocentric pattern may not be evaluable
Should always be considered in the differential diagnosis of invasive carcinomas
IHC for myoepithelial markers can be used to make the correct diagnosis
IMAGE FINDINGS
Mammographic Findings
Microcalcifications most common finding
Clustered amorphous calcifications typical
Pleomorphic and punctate calcifications may also be seen
Mass-forming lesions are generally circumscribed or lobulated
Less commonly, borders are ill defined or irregular
May be associated with calcifications
Rare cases present as architectural distortion
Ultrasonographic Findings
Circumscribed, hypoechoic, solid mass
MR Findings
Typical lesions are indistinguishable from breast parenchyma
May show enhancement in up to 30% of cases
MACROSCOPIC FEATURES
General Features
SA presenting as a mass by palpation or imaging may be grossly evident
Rubbery circumscribed nodule
Majority are small and not grossly apparent
MICROSCOPIC PATHOLOGY
Histologic Features
Arises within terminal duct lobular unit
Must be at least 2x larger than average lobule
However, lobules can vary greatly in size in same breast, making this requirement difficult to assess
Lobulocentric pattern is an important diagnostic feature
Refers to a cluster of tubules or acini
Often a central larger duct or ducts
Lobulocentric growth pattern is best appreciated at lower magnification
Acini appear to swirl around central duct
Acini are formed by luminal cells and myoepithelial cells
Acini conform in shape to one another and are tightly clustered
In less typical cases, acini on periphery can be located farther apart and appear to “wander” through surrounding breast tissue
Myoepithelial cells may be spindled in shape and can be prominent
2 cell layers may be best appreciated at periphery
May be difficult to see if center of lesion is sampled in a core needle biopsy
Sclerosis refers to stromal hyalinization that compresses and distorts acini
Luminal spaces may be completely obliterated
Acini may have appearance of solid cords in swirling pattern
Glandular compression and distortion may be most marked in center of lesions
Microcalcifications are present in > 50% of cases and may be prominent
Multiple confluent areas of SA are sometimes seen
When large, can form a mammographic density or palpable mass
Pseudoperineural invasion may be seen
Tubules are present within nerves
Not an indication of malignancy
Luminal cells lack atypia and may appear cuboidal or flattened
SA is frequently associated with other proliferative lesions
e.g., sclerosing papilloma, complex sclerosing lesion, fibroadenoma
SA can be involved by apocrine metaplasia
Term “apocrine adenosis” is used for these lesions
May be mistaken for carcinoma due to monomorphic appearance of cells with enlarged nuclei
SA can be involved by LCIS and DCIS
Such involvement may be mistaken for invasive carcinoma, particularly on needle biopsy
Features supporting SA include
Myoepithelial cells on H&E or by IHC
Lobulocentric architecture
Dense rather than desmoplastic stroma
Swirling back-to-back glands in concentric arrays
ANCILLARY TESTS
Immunohistochemistry
Myoepithelial markers
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree