40
RNA Nonenveloped Viruses
CHAPTER CONTENTS
PICORNAVIRUSES
Picornaviruses are small (20–30 nm) nonenveloped viruses composed of an icosahedral nucleocapsid and a single-stranded RNA genome. The genome RNA has positive polarity (i.e., on entering the cell, it functions as the viral mRNA). There is no polymerase within the virion. Picornaviruses replicate in the cytoplasm of cells. They are not inactivated by lipid solvents, such as ether, because they do not have an envelope.
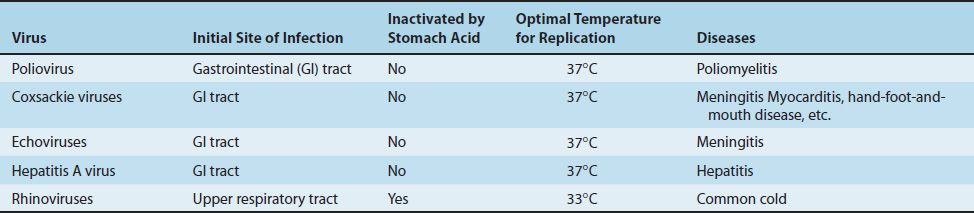
The picornavirus family includes two groups of medical importance: the enteroviruses and the rhinoviruses. Among the major enteroviruses are poliovirus, Coxsackie viruses, echoviruses, and hepatitis A virus (which is described in Chapter 41). Enteroviruses infect primarily the enteric tract, whereas rhinoviruses are found in the nose and throat (rhino = nose) (Table 40–1).
Enteroviruses replicate optimally at 37°C, whereas rhinoviruses grow better at 33°C, in accordance with the lower temperature of the nose. Enteroviruses are stable under acid conditions (pH 3–5), which enables them to survive exposure to gastric acid, whereas rhinoviruses are acid-labile. This explains why rhinovirus infections are restricted to the nose and throat.
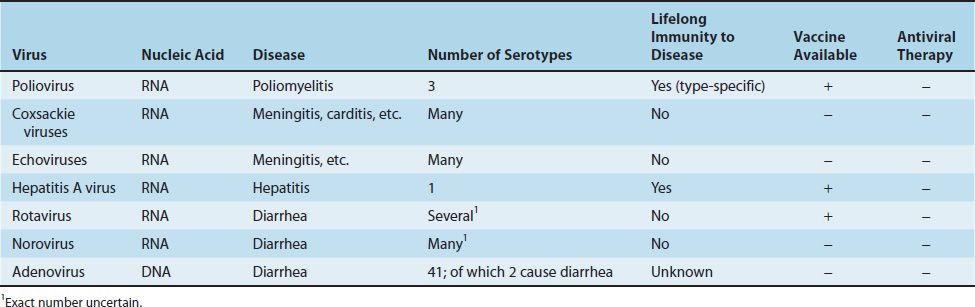
Important features of viruses that commonly infect the intestinal tract are summarized in Table 40–2. These include the picornaviruses but also rotavirus and norovirus, which are described later in this chapter, and adenovirus, which is described in Chapter 38.
ENTEROVIRUSES
1. Poliovirus
Disease
This virus causes poliomyelitis.
Important Properties
The host range is limited to primates (i.e., humans and non-human primates such as apes and monkeys). This limitation is due to the binding of the viral capsid protein to a receptor found only on primate cell membranes. However, note that purified viral RNA (without the capsid protein) can enter and replicate in many nonprimate cells—the RNA can bypass the cell membrane receptor (i.e., it is “infectious RNA”).
There are three serologic (antigenic) types based on different antigenic determinants on the outer capsid proteins. Because there is little cross-reaction, protection from disease requires the presence of antibody against each of the three types.
Summary of Replicative Cycle
The virion interacts with specific cell receptors on the cell membrane and then enters the cell. The capsid proteins are then removed. After uncoating, the genome RNA functions as mRNA and is translated into one very large polypeptide called noncapsid viral protein 00. This polypeptide is cleaved by a virus-encoded protease in multiple steps to form both the capsid proteins of the progeny virions and several noncapsid proteins, including the RNA polymerase that synthesizes the progeny RNA genomes. Replication of the genome occurs by synthesis of a complementary negative strand, which then serves as the template for the positive strands. Some of these positive strands function as mRNA to make more viral proteins, and the remainder become progeny virion genome RNA. Assembly of the progeny virions occurs by coating of the genome RNA with capsid proteins. Virions accumulate in the cell cytoplasm and are released upon death of the cell. They do not bud from the cell membrane.
Transmission & Epidemiology
Poliovirus is transmitted by the fecal–oral route. It replicates in the oropharynx and intestinal tract. Humans are the only natural hosts.
As a result of the success of the vaccine, poliomyelitis caused by naturally occurring “wild-type” virus has been eradicated from the United States and, indeed, from the entire Western hemisphere. The rare cases in the United States occur mainly in (1) people exposed to virulent revertants of the attenuated virus in the live vaccine and (2) unimmunized people exposed to wild-type poliovirus while traveling abroad. Before the vaccine was available, epidemics occurred in the summer and fall.
The World Health Organization set the eradication of paralytic polio by 2005 as a goal. Unfortunately, this goal was not achieved. In 1988, there were 388,000 cases of paralytic polio worldwide, whereas in 2005 there were fewer than 2000. Despite this remarkable decrease, paralytic polio continues to occur. As of 2012, there was still a total of approximately 200 cases each year in three countries: Afghanistan, Nigeria, and Pakistan. Thus far, smallpox is the only infectious disease that has been eradicated, a consequence of the worldwide use of the smallpox vaccine.
Pathogenesis & Immunity
After replicating in the oropharynx and small intestine, especially in lymphoid tissue, the virus spreads through the bloodstream to the central nervous system. It can also spread retrograde along nerve axons.
In the central nervous system, poliovirus preferentially replicates in the motor neurons located in the anterior horn of the spinal cord. Death of these cells results in paralysis of the muscles innervated by those neurons. Paralysis is not due to virus infection of muscle cells. The virus also affects the brainstem, leading to “bulbar” poliomyelitis (with respiratory paralysis), but rarely damages the cerebral cortex.
In infected individuals, the immune response consists of both intestinal IgA and humoral IgG to the specific sero-type. Infection provides lifelong type-specific immunity.
Clinical Findings
The range of responses to poliovirus infection includes (1) inapparent, asymptomatic infection; (2) abortive poliomyelitis; (3) nonparalytic poliomyelitis; and (4) paralytic poliomyelitis. Asymptomatic infection is quite common. Roughly 1% of infections are clinically apparent. The incubation period is usually 10 to 14 days.
The most common clinical form is abortive poliomyelitis, which is a mild, febrile illness characterized by headache, sore throat, nausea, and vomiting. Most patients recover spontaneously. Nonparalytic poliomyelitis manifests as aseptic meningitis with fever, headache, and a stiff neck. This also usually resolves spontaneously. In paralytic poliomyelitis, flaccid paralysis is the predominant finding, but brainstem involvement can lead to life-threatening respiratory paralysis. Painful muscle spasms also occur. The motor nerve damage is permanent, but some recovery of muscle function occurs as other nerve cells take over. In paralytic polio, both the meninges and the brain parenchyma (meningoencephalitis) are often involved. If the spinal cord is also involved, the term meningomyeloencephalitis is often used.
A postpolio syndrome that occurs many years after the acute illness has been described. Marked deterioration of the residual function of the affected muscles occurs many years after the acute phase. The cause of this deterioration is unknown.
No permanent carrier state occurs following infection by poliovirus, but virus excretion in the feces can occur for several months.
Laboratory Diagnosis
The diagnosis is made either by isolation of the virus or by a rise in antibody titer. Virus can be recovered from the throat, stool, or spinal fluid by inoculation of cell cultures. The virus causes a cytopathic effect (CPE) and can be identified by neutralization of the CPE with specific antisera.
Treatment
There is no antiviral therapy. Treatment is limited to symptomatic relief and respiratory support, if needed. Physiotherapy for the affected muscles is important.
Prevention
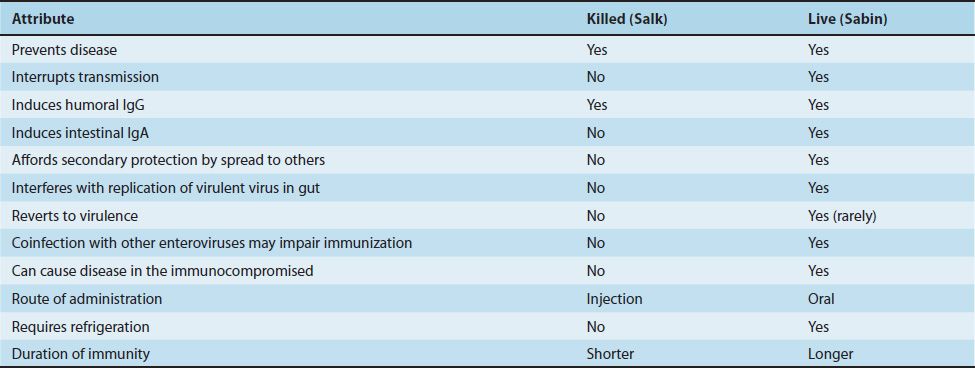
Poliomyelitis can be prevented by both the killed vaccine (Salk vaccine, inactivated vaccine, IPV) and the live, attenuated vaccine (Sabin vaccine, oral vaccine, OPV) (Table 40–3). Both vaccines induce humoral antibodies, which neutralize virus entering the blood and hence prevent central nervous system infection and disease. Both the killed and the live vaccines contain all three serotypes. At present, the inactivated vaccine is preferred for reasons that are described later.
The current version of the inactivated vaccine is called enhanced polio vaccine, or eIPV. It has a higher seroconversion rate and induces a higher titer of antibody than the previous IPV. eIPV also induces some mucosal immunity IgA, making it capable of interrupting transmission, but the amount of secretory IgA induced by eIPV is significantly less than the amount induced by OPV. OPV is therefore preferred for eradication efforts. The only version of polio vaccine currently produced in the United States is eIPV. In certain countries where polio remains endemic (e.g., India), a monovalent oral polio vaccine is used because the rate of seroconversion is higher with the monovalent vaccine than with the trivalent one.
In the past, the live vaccine was preferred in the United States for two main reasons: (1) It interrupts fecal–oral transmission by inducing secretory IgA in the gastrointestinal tract. (2) It is given orally and so is more readily accepted than the killed vaccine, which must be injected.
The live vaccine has four disadvantages: (1) Rarely, reversion of the attenuated virus to virulence will occur, and disease may ensue (especially for the type 3 virus). (2) It can cause disease in immunodeficient persons and therefore should not be given to them. (3) Infection of the gastrointestinal tract by other enteroviruses can limit replication of the vaccine virus and reduce protection. (4) It must be kept refrigerated to prevent heat inactivation of the live virus.
Outbreaks of paralytic polio caused by vaccine-derived poliovirus (VDPV) continue to occur, especially in areas where there are large numbers of unimmunized people. These VDPV strains have lost their attenuation by acquiring genes from wild-type enteroviruses by recombination. Outbreaks of VDPV-associated paralytic polio have been contained by campaigns to immunize people in the affected area with the oral (Sabin) vaccine that interrupts fecal–oral transmission.
The duration of immunity is thought to be longer with the live than with the killed vaccine, but a booster dose is recommended with both.
The currently approved vaccine schedule consists of four doses of inactivated vaccine administered at 2 months, 4 months, 6 to 18 months, and upon entry to school at 4 to 6 years. One booster (lifetime) is recommended for adults who travel to endemic areas. The use of the inactivated vaccine should prevent some of the approximately 10 cases per year of vaccine-associated paralytic polio that arise from reversion of the attenuated virus in the vaccine.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree