Chapter 15 Respiratory disease
Structure of the respiratory system
The trachea, bronchi and bronchioles
The first seven divisions are bronchi that have:
 walls consisting of cartilage and smooth muscle
walls consisting of cartilage and smooth muscle
 epithelial lining with cilia and goblet cells
epithelial lining with cilia and goblet cells
 submucosal mucus-secreting glands
submucosal mucus-secreting glands
 endocrine cells – Kulchitsky or APUD (amine precursor and uptake decarboxylation) containing 5-hydroxytryptamine.
endocrine cells – Kulchitsky or APUD (amine precursor and uptake decarboxylation) containing 5-hydroxytryptamine.
The next 16–18 divisions are bronchioles that have:
 no cartilage and a muscular layer that progressively becomes thinner
no cartilage and a muscular layer that progressively becomes thinner
 a single layer of ciliated cells but very few goblet cells
a single layer of ciliated cells but very few goblet cells
 granulated Clara cells that produce a surfactant-like substance.
granulated Clara cells that produce a surfactant-like substance.
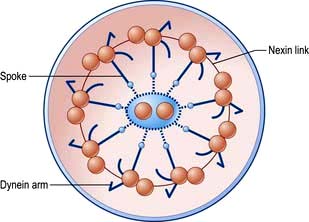
The ciliated epithelium is a key defence mechanism. Each cell bears approximately 200 cilia beating at 1000 beats per minute in organized waves of contraction. Each cilium consists of nine peripheral parts and two inner longitudinal fibrils in a cytoplasmic matrix (Fig. 15.1). Nexin links join the peripheral pairs. Dynein arms consisting of ATPase protein project towards the adjacent pairs. Bending of the cilia results from a sliding movement between adjacent fibrils powered by an ATP-dependent shearing force developed by the dynein arms (see also p. 22). Absence of dynein arms leads to immotile cilia. Mucus, which contains macrophages, cell debris, inhaled particles and bacteria, is moved by the cilia towards the larynx at about 1.5 cm/min (the ‘mucociliary escalator’, see below).
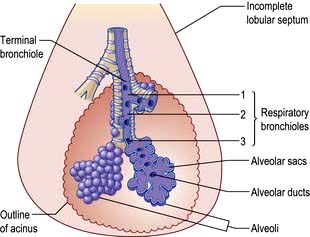
The bronchioles finally divide within the acinus into smaller respiratory bronchioles that have alveoli arising from the surface (Fig. 15.2). Each respiratory bronchiole supplies approximately 200 alveoli via alveolar ducts. The term ‘small airways’ refers to bronchioles of <2 mm; the average lung contains about 30 000 of these.
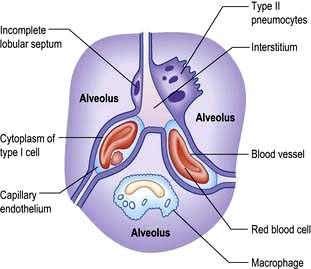
The alveoli
There are approximately 300 million alveoli in each lung. Their total surface area is 40–80 m2. The epithelial lining consists mainly of type I pneumocytes (Fig. 15.3). These cells have an extremely thin layer of cytoplasm, which only offers a thin barrier to gas exchange. Type I cells are connected to each other by tight junctions that limit the movements of fluid in and out of the alveoli. Alveoli are not completely airtight – many have holes in the alveolar wall allowing communication between alveoli of adjoining lobules (pores of Kohn).
The lungs
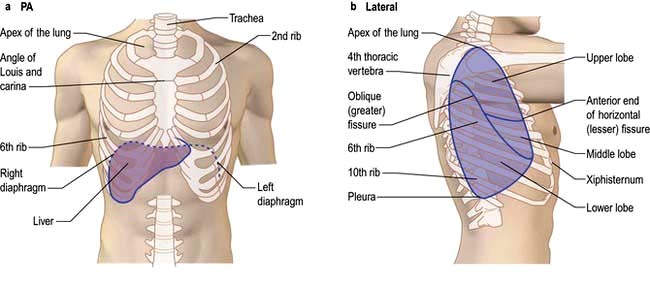
The lungs are separated into lobes by invaginations of the pleura, which are often incomplete. The right lung has three lobes, whereas the left lung has two. The positions of the oblique fissures and the right horizontal fissure are shown in Figure 15.4. The upper lobe lies mainly in front of the lower lobe and therefore physical signs on the right side in the front of the chest are due to lesions of the upper lobe or the middle lobe.
Physiology of the respiratory system
Breathing
Lung ventilation can be considered in two parts:
 The mechanical process of inspiration and expiration
The mechanical process of inspiration and expiration
 The control of respiration to a level appropriate for metabolic needs.
The control of respiration to a level appropriate for metabolic needs.
The control of respiration
 The pulmonary blood flow of 5 L/min carries 11 mmol/min (250 mL/min) of oxygen from the lungs to the tissues.
The pulmonary blood flow of 5 L/min carries 11 mmol/min (250 mL/min) of oxygen from the lungs to the tissues.
 Ventilation at about 6 L/min carries 9 mmol/min (200 mL/min) of carbon dioxide out of the body.
Ventilation at about 6 L/min carries 9 mmol/min (200 mL/min) of carbon dioxide out of the body.
 The normal pressure of oxygen in arterial blood (PaO2) is between 11 and 13 kPa.
The normal pressure of oxygen in arterial blood (PaO2) is between 11 and 13 kPa.
 The normal pressure of carbon dioxide in arterial blood (PaCO2) is 4.8–6.0 kPa.
The normal pressure of carbon dioxide in arterial blood (PaCO2) is 4.8–6.0 kPa.
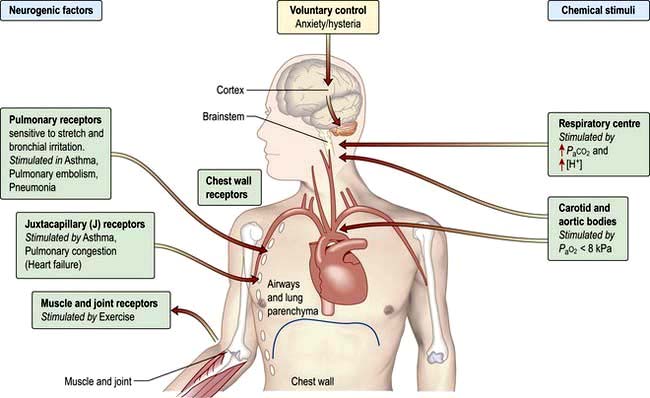
Ventilation is controlled by a combination of neurogenic and chemical factors (Fig. 15.5).
The airways of the lungs
Airflow
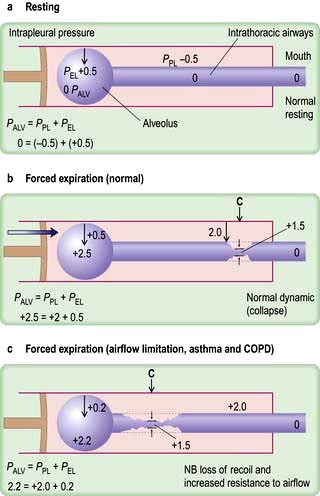
As air flows from the alveoli towards the mouth there is a gradual drop of pressure owing to flow resistance (Fig. 15.6a).
In forced expiration, as mentioned above, the driving pressure raises both the alveolar pressure and the intrapleural pressure. Between the alveolus and the mouth, there is a point (C in Fig. 15.6b) where the airway pressure equals the intrapleural pressure, and the airway collapses. However, this collapse is temporary, as the transient occlusion of the airway results in an increase in pressure behind it (i.e. upstream) and this raises the intra-airway pressure so that the airways open and flow is restored. The airways thus tend to vibrate at this point of ‘dynamic collapse’.
As lung volume decreases during expiration, the elastic recoil pressure of the lungs decreases and the ‘collapse point’ moves upstream (i.e. towards the smaller airways – see Fig. 15.6c). Where there is pathological loss of recoil pressure (as in chronic obstructive pulmonary disease, COPD), the ‘collapse point’ is located even further upstream and causes expiratory flow limitation. The measurement of the forced expiratory volume in 1 second (FEV1) is a useful clinical index of this phenomenon. To compensate, patients with COPD often ‘purse their lips’ in order to increase airway pressure so that their peripheral airways do not collapse. During inspiration, the intrapleural pressure is always less than the intraluminal pressure within the intrathoracic airways, so increasing effort does not limit airflow. Inspiratory flow is limited only by the power of the inspiratory muscles.
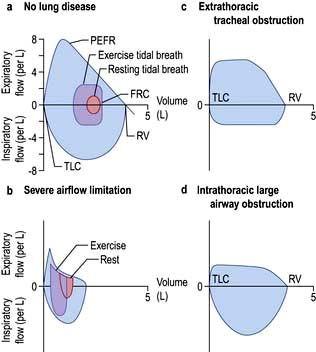
Flow-volume loops
The relationship between maximal flow rates and lung volume is demonstrated by the maximal flow-volume (MFV) loop (Fig. 15.7a).
In subjects with healthy lungs, maximal flow rates are rarely achieved even during vigorous exercise. However, in patients with severe COPD, limitation of expiratory flow occurs even during tidal breathing at rest (Fig. 15.7b). To increase ventilation these patients have to breathe at higher lung volumes and allow more time for expiration, both of which reduce the tendency for airway collapse. To compensate they increase flow rates during inspiration, where there is relatively less flow limitation.
The volume that can be forced in from the residual volume in 1 second (FIV1) will always be greater than that which can be forced out from TLC in 1 second (FEV1). Thus, the ratio of FEV1 to FIV1 is below 1. The only exception to this occurs when there is significant obstruction to the airways outside the thorax, such as tracheal tumour or retrosternal goitre. Expiratory airway narrowing is prevented by tracheal resistance and expiratory airflow becomes more effort-dependent. During forced inspiration this same resistance causes such negative intraluminal pressure that the trachea is compressed by the surrounding atmospheric pressure. Inspiratory flow thus becomes less effort-dependent, and the ratio of FEV1 to FIV1 exceeds 1. This phenomenon, and the characteristic flow-volume loop, is diagnostic of extrathoracic airways obstruction (Fig. 15.7c).
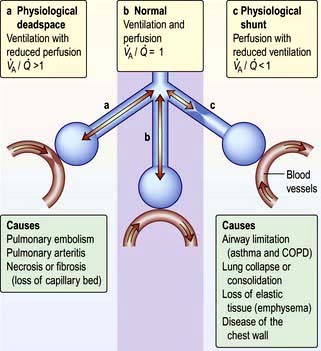
Ventilation and perfusion relationships
For optimum gas exchange there must be a match between ventilation of the alveoli (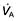 ) and their perfusion (
) and their perfusion (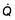 ). However, in reality there is variation in the (
). However, in reality there is variation in the (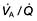 ) ratio in both normal and diseased lungs (Fig. 15.8). In the normal lung both ventilation and perfusion are greater at the bases than at the apices, but the gradient for perfusion is steeper, so the net effect is that ventilation exceeds perfusion towards the apices, while perfusion exceeds ventilation at the bases. Other causes of (
) ratio in both normal and diseased lungs (Fig. 15.8). In the normal lung both ventilation and perfusion are greater at the bases than at the apices, but the gradient for perfusion is steeper, so the net effect is that ventilation exceeds perfusion towards the apices, while perfusion exceeds ventilation at the bases. Other causes of (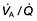 ) mismatch include direct shunting of deoxygenated blood through the lung without passing through alveoli (e.g. the bronchial circulation) and areas of lung that receive no blood (e.g. anatomical deadspace, bullae and areas of underperfusion during acceleration and deceleration, e.g. in aircraft and high performance cars).
) mismatch include direct shunting of deoxygenated blood through the lung without passing through alveoli (e.g. the bronchial circulation) and areas of lung that receive no blood (e.g. anatomical deadspace, bullae and areas of underperfusion during acceleration and deceleration, e.g. in aircraft and high performance cars).
Hypoxaemia occurs more readily than hypercapnia because of the different ways in which oxygen and carbon dioxide are carried in the blood. Carbon dioxide can be considered to be in simple solution in the plasma, the volume carried being proportional to the partial pressure. Oxygen is carried in chemical combination with haemoglobin in the red blood cells, with a non-linear relationship between the volume carried and the partial pressure (Fig. 15.5, p. 341). Alveolar hyperventilation reduces the alveolar PCO2 (PACO2) and diffusion leads to a proportional fall in the carbon dioxide content of the blood (PaCO2). However, as the haemoglobin is already saturated with oxygen, there is no significant increase in the blood oxygen content as a result of increasing the alveolar PO2 through hyperventilation. The hypoxaemia of even a small amount of physiological shunting cannot therefore be compensated for by hyperventilation.
Defence mechanisms of the respiratory tract
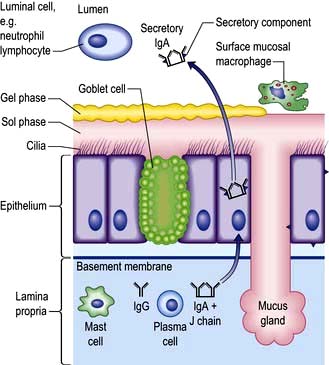
Pulmonary disease often results from a failure of the normal host defence mechanisms of the healthy lung (Fig. 15.9). These can be divided into physical, physiological, humoral and cellular mechanisms.
Humoral and cellular mechanisms
Nonspecific soluble factors
 α-Antitrypsin (α1-antiprotease, see p. 341) in lung secretions is derived from plasma. It inhibits chymotrypsin and trypsin and neutralizes proteases including neutrophil elastase.
α-Antitrypsin (α1-antiprotease, see p. 341) in lung secretions is derived from plasma. It inhibits chymotrypsin and trypsin and neutralizes proteases including neutrophil elastase.
 Antioxidant defences include enzymes such as superoxide dismutase and low-molecular-weight antioxidant molecules (ascorbate, urate) in the epithelial lining fluid. In addition, lung cells are protected by an extensive range of intracellular defences, especially members of the glutathione S-transferase (GST) superfamily.
Antioxidant defences include enzymes such as superoxide dismutase and low-molecular-weight antioxidant molecules (ascorbate, urate) in the epithelial lining fluid. In addition, lung cells are protected by an extensive range of intracellular defences, especially members of the glutathione S-transferase (GST) superfamily.
 Lysozyme is an enzyme found in granulocytes that has bactericidal properties.
Lysozyme is an enzyme found in granulocytes that has bactericidal properties.
 Lactoferrin is synthesized from epithelial cells and neutrophil granulocytes and has bactericidal properties.
Lactoferrin is synthesized from epithelial cells and neutrophil granulocytes and has bactericidal properties.
 Interferons are produced by most cells in response to viral infection and are potent modulators of lymphocyte function.
Interferons are produced by most cells in response to viral infection and are potent modulators of lymphocyte function.
 Complement in secretions is also derived from plasma. In association with antibodies, it plays a major role in cytotoxicity.
Complement in secretions is also derived from plasma. In association with antibodies, it plays a major role in cytotoxicity.
 Surfactant protein A (SPA) is one of four species of surfactant proteins which opsonizes bacteria/particles, enhancing phagocytosis by macrophages.
Surfactant protein A (SPA) is one of four species of surfactant proteins which opsonizes bacteria/particles, enhancing phagocytosis by macrophages.
 Defensins are bactericidal peptides present in the azurophil granules of neutrophils.
Defensins are bactericidal peptides present in the azurophil granules of neutrophils.
Innate and adaptive immunity
With infection, neutrophils migrate out of pulmonary capillaries into the air spaces and phagocytose and kill microbes with, for example, antimicrobial proteins (lactoferrin), degradative enzymes (elastase) and oxidant radicals. In addition, neutrophil extracellular traps (NET) ensnare and kill extracellular bacteria. Neutrophils also generate a variety of mediators, e.g. TNF-α, IL-1 and chemokines which activate dendritic cells and B cells and produce the T-cell-activating cytokine IL-12. The latter enhances neutrophil-mediated defence during pneumonia. Dendritic cells are antigen presenting cells and are key to the adaptive immune response (p. 58).
Symptoms
Runny, blocked nose and sneezing
Nasal symptoms (see also p. 691) are extremely common and both common colds and allergic rhinitis cause ‘runny nose’ (rhinorrhoea), nasal blockage and attacks of sneezing. In allergic rhinitis, symptoms may be intermittent, following contact with pollens or animal danders, or persistent, especially when house-dust mite is the allergen. Colds are frequent during the winter but if the symptoms persist for weeks the patient probably has perennial rhinitis rather than persistent viral infection.
Nasal secretions are usually thin and runny in allergic rhinitis but thicker and discoloured with viral infections. Nose bleeds and blood-stained nasal discharge are common and rarely indicate serious pathology. However, a blood-stained nasal discharge associated with nasal obstruction and pain may be the presenting feature of a nasal tumour (p. 1051). Nasal polyps typically present with nasal blockage and loss of smell.
Cough
Cough (see also p. 822) is the commonest symptom of lower respiratory tract disease. It is caused by mechanical or chemical stimulation of cough receptors in the epithelium of the pharynx, larynx, trachea, bronchi and diaphragm. Afferent receptors go to the cough centre in the medulla where efferent signals are generated to the expiratory musculature. Smokers often have a morning cough with a little sputum. A productive cough is the cardinal feature of chronic bronchitis, while dry coughing, particularly at night, can be a symptom of asthma. Cough also occurs in asthmatics after mild exertion or following forced expiration. Cough can also occur for psychological reasons without any definable pathology.
Sputum
Haemoptysis (blood-stained sputum) varies from small streaks of blood to massive bleeding.
 The commonest cause of mild haemoptysis is acute infection, particularly in exacerbations of chronic obstructive pulmonary disease (COPD) but it should not be attributed to this without investigation.
The commonest cause of mild haemoptysis is acute infection, particularly in exacerbations of chronic obstructive pulmonary disease (COPD) but it should not be attributed to this without investigation.
 Other common causes are pulmonary infarction, bronchial carcinoma and tuberculosis.
Other common causes are pulmonary infarction, bronchial carcinoma and tuberculosis.
 In lobar pneumonia, the sputum is usually rusty in appearance rather than frankly blood-stained.
In lobar pneumonia, the sputum is usually rusty in appearance rather than frankly blood-stained.
 Pink, frothy sputum is seen in pulmonary oedema.
Pink, frothy sputum is seen in pulmonary oedema.
 In bronchiectasis, the blood is often mixed with purulent sputum.
In bronchiectasis, the blood is often mixed with purulent sputum.
 Massive haemoptyses (>200 mL of blood in 24 hours) are usually due to bronchiectasis or tuberculosis.
Massive haemoptyses (>200 mL of blood in 24 hours) are usually due to bronchiectasis or tuberculosis.
 Uncommon causes of haemoptyses are idiopathic pulmonary haemosiderosis, Goodpasture’s syndrome, microscopic polyangiitis, trauma, blood disorders and benign tumours.
Uncommon causes of haemoptyses are idiopathic pulmonary haemosiderosis, Goodpasture’s syndrome, microscopic polyangiitis, trauma, blood disorders and benign tumours.
Breathlessness
Orthopnoea (see p. 675) is breathlessness on lying down. While it is classically linked to heart failure, it is partly due to the weight of the abdominal contents pushing the diaphragm up into the thorax. Such patients may also become breathless on bending over.
Hyperventilation is inappropriate overbreathing. This may occur at rest or on exertion and results in a lowering of the alveolar and arterial PCO2 (see p. 1178).
Paroxysmal nocturnal dyspnoea (see p. 798) is acute episodes of breathlessness at night, typically due to heart failure.
Examination of the respiratory system
The chest
Examination of the chest
Inspection
Cyanosis (see p. 676) is a dusky colour of the skin and mucous membranes, due to the presence of more than 50 g/L of desaturated haemoglobin. When due to central causes, cyanosis is visible on the tongue (especially the underside) and lips. Patients with central cyanosis will also be cyanosed peripherally. Peripheral cyanosis without central cyanosis is caused by a reduced peripheral circulation and is noted on the fingernails and skin of the extremities with associated coolness of the skin.
Finger clubbing is present when the normal angle between the base of the nail and the nail fold is lost. The base of the nail is fluctuant owing to increased vascularity, and there is an increased curvature of the nail in all directions, with expansion of the end of the digit. Some causes of clubbing are given in Table 15.1. Clubbing is not a feature of uncomplicated COPD.
Table 15.1 Some causes of finger clubbing
Palpation and percussion
Check the position of the trachea and apex beat. Examine the supraclavicular fossa for enlarged lymph nodes. The distance between the sternal notch and the cricoid cartilage (three to four finger breadths in full expiration) is reduced in patients with severe airflow limitation. Check chest expansion. A tape measure is used if precise or serial measurements are needed, e.g. in ankylosing spondylitis. Local discomfort over the sternochondral joints suggests costochondritis. In rib fractures, compression of the chest laterally and anteroposteriorly produces localized pain. On percussion, liver dullness is usually detected anteriorly at the level of the sixth rib. Liver and cardiac dullness disappear when lungs are over-inflated (Table 15.2).
Investigation of respiratory disease
Imaging
Chest X-ray
 Centring of the film. The distance between each clavicular head and the spinal processes should be equal
Centring of the film. The distance between each clavicular head and the spinal processes should be equal
 Penetration (check film is not too dark)
Penetration (check film is not too dark)
 View. Routine films are taken postero-anterior (PA), i.e. the film is placed in front of the patient with the X-ray source behind. Anteroposterior (AP) films are taken only in very ill patients who are unable to stand up or be taken to the radiology department; the cardiac outline appears bigger and the scapulae cannot be moved out of the way. Lateral chest X-rays were often performed in the past to localize pathology, but CT scans have replaced these.
View. Routine films are taken postero-anterior (PA), i.e. the film is placed in front of the patient with the X-ray source behind. Anteroposterior (AP) films are taken only in very ill patients who are unable to stand up or be taken to the radiology department; the cardiac outline appears bigger and the scapulae cannot be moved out of the way. Lateral chest X-rays were often performed in the past to localize pathology, but CT scans have replaced these.
X-ray abnormalities
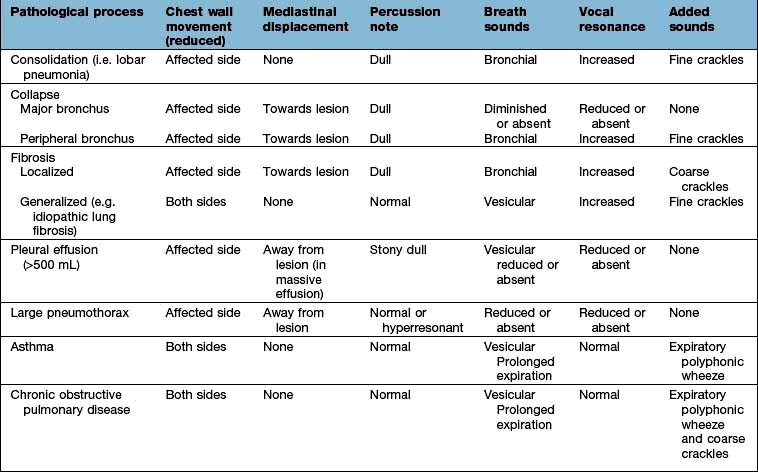
Collapse and consolidation
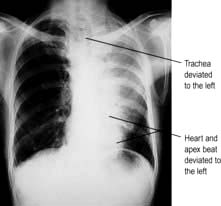
Simple pneumonia is easy to recognize (see Fig. 15.33) but look carefully for any evidence of collapse (Fig. 15.10, Table 15.3). Loss of volume or crowding of the ribs are the best indicators of lobar collapse. The lung lobes collapse in characteristic directions. The lower lobes collapse downwards and towards the mediastinum, the left upper lobe collapses forwards against the anterior chest wall, while the right upper lobe collapses upwards and inwards, forming the appearance of an arch over the remaining lung. The right middle lobe collapses anteriorly and inward, obscuring the right heart border. If a whole lung collapses, the mediastinum will shift towards the side of the collapse. Uncomplicated consolidation does not cause mediastinal shift or loss of lung volume, so any of these features should raise the suspicion of an endobronchial obstruction.
Table 15.3 Causes of collapse of the lung
Pleural effusion
Pleural effusions (see Fig. 15.45) need to be larger than 500 mL to cause much more than blunting of the costophrenic angle. On an erect film they produce a characteristic shadow with a curved upper edge rising into the axilla. If very large, the whole of one hemithorax may be opaque, with mediastinal shift away from the effusion.
Fibrosis
Localized fibrosis causes streaky shadowing, and the accompanying loss of lung volume causes mediastinal structures to move to the same side. More generalized fibrosis can lead to a honeycomb appearance (see p. 849), seen as diffuse shadows containing multiple circular translucencies a few millimetres in diameter.
Round shadows
Lung cancer is the commonest cause of large round shadows but many other causes are recognized (Table 15.4).
Table 15.4 Causes of round shadows (>3 cm) in the lung
Miliary mottling
This term, derived from the Latin for millet, describes numerous minute opacities, 1–3 mm in size. The commonest causes are tuberculosis, pneumoconiosis, sarcoidosis, idiopathic pulmonary fibrosis and pulmonary oedema (see Fig. 14.15), although pulmonary oedema is usually perihilar and accompanied by larger, fluffy shadows. Pulmonary microlithiasis is a rare but striking cause of miliary mottling.
Computed tomography
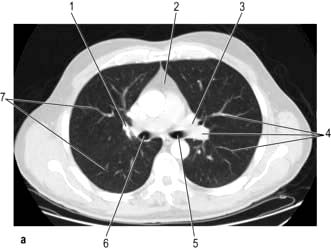
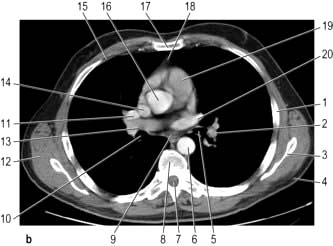
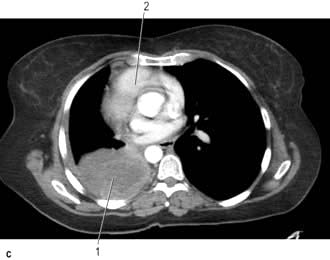
CT provides excellent images of the lungs and mediastinal structures (Fig. 15.11). Narrow slice, high-resolution CT scans show the lung parenchyma well, while thicker slice staging CT scans are used for diagnosis of malignant disease. Mediastinal structures are shown more clearly after injecting intravenous contrast medium.
 Evaluating diffuse disease of the lung parenchyma, including sarcoidosis, hypersensitivity pneumonitis, occupational lung disease, and any other form of interstitial pulmonary fibrosis.
Evaluating diffuse disease of the lung parenchyma, including sarcoidosis, hypersensitivity pneumonitis, occupational lung disease, and any other form of interstitial pulmonary fibrosis.
 Diagnosis of bronchiectasis. HRCT has a sensitivity and specificity >90%.
Diagnosis of bronchiectasis. HRCT has a sensitivity and specificity >90%.
 Distinguishing emphysema from diffuse parenchymal lung disease or pulmonary vascular disease as a cause of a low gas transfer factor with otherwise normal lung function.
Distinguishing emphysema from diffuse parenchymal lung disease or pulmonary vascular disease as a cause of a low gas transfer factor with otherwise normal lung function.
 Suspected opportunistic lung infection in immunocompromised patients
Suspected opportunistic lung infection in immunocompromised patients






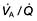 mismatch, the PaO2 and PaCO2 will still be normal at rest. Increasing the requirements for gas exchange by exercise will widen the
mismatch, the PaO2 and PaCO2 will still be normal at rest. Increasing the requirements for gas exchange by exercise will widen the 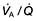 mismatch and the PaO2 will fall.
mismatch and the PaO2 will fall. 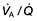 mismatch is by far the most common cause of arterial hypoxaemia.
mismatch is by far the most common cause of arterial hypoxaemia.