Women 20–40 years old
Back > face, extremities
History of biopsy or excision, with or without free margins
Appear within weeks–months after excision
Pigment usually within margins of scar
Pigment fades with time
Benign or atypical nevi most commonly recur
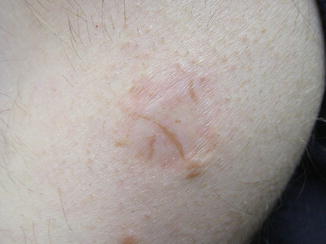
Fig. 11.1
Recurrent melanocytic nevus. Multiple irregularly shaped pigmented streaks are present within the margins of an atrophic scar. The patient had a history of shave excision of a junctional melanocytic nevus 17 years prior
Histopathologic Features
When entertaining the diagnosis of RMN, it is preferable, if not essential, to have for comparison slides of the patient’s initial biopsy for comparison. Any unusual features such as atypia initially present in the nevus would be expected to similarly manifest in a recurrence. Larger case series have shown up to 60 % of recurrent nevi are compound, followed by intradermal and then junctional nevi [29–31]. A distinguishing feature of recurrent nevi is the presence of a central dermal scar, interposed between a confluent junctional melanocytic component and deeper nests of residual melanocytes and melanophages—the so-called “trizonal”pattern [9]. Epithelioid melanocytes with bland and evenly staining nuclei are usually seen, though atypical histopathologic features such as confluence of melanocytic nests, cytologic atypia of melanocytes, suprabasal spread, and dermal mitotic figures are not uncommon in the junctional component, hence the historical pathologic correlate for the term “pseudomelanoma” [30, 38].
In one of the largest studies to date, 112 out of 357 recurrent nevi (26 %) demonstrated single-cell proliferation [29]. Extension and suprabasal spread of melanocytes down the adnexal structures was present in 6 % of cases. A confluent lentiginous growth pattern was observed in 2 % of the cases. 26 % of cases showed cytologic atypia, defined as hyperchromasia and nuclear enlargement. Usually, the irregular architectural and cytologic patterns seen in recurrent nevi are restricted to the epidermis and dermis immediately above the scar [39], and most RMN display a normal maturation pattern. However, focal lentiginous hyperplasia, moderate nuclear atypia, rare mitotic figures, and pagetoid spread (not beyond the granular layer) have been reported in decreasing order from 35 to 3 %, usually in isolation [30]. Furthermore, the appropriate diagnosis of RMN may be obscured by features consistent with intermediate/late stage regression of melanoma and dermal scar extending to the margin of the biopsy specimen [29]. Clinical-pathologic correlation is of particular importance in such cases as distinction from melanoma can be difficult without a pertinent history.
Reflectance confocal microscopy is an emerging in vivo adjunct for the characterization of pigmented lesions [40]. One case series of three patients with recurrent nevi showed some atypical but cytologically monomorphous junctional melanocytes consistent with histopathologic analysis, without any pagetoid or lateral spread of melanocytes or atypical nests [41] (Table 11.2, Fig. 11.2).
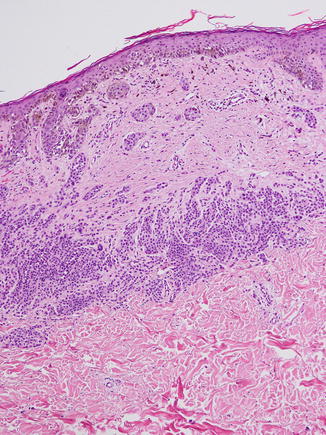
Table 11.2
Histopathologic features of recurrent nevi
Junctional confluence | |
Orderly central fibrosis | |
Dermal nests with normal maturation | |
Cytologic atypia usually contained in junctional component or superficial scar | |
Single-cell proliferation contained in the junctional component | |
Compound or intradermal nevi most common | |
Epithelioid melanocytes predominate | |
Focal hyperplasia, rare mitoses, or pagetoid spread may be seen in isolation | |

Fig. 11.2
Characteristic trizonal pattern of fibrosis in a recurrent nevus. Note the (1) confluent junctional component with epidermal pigmentation overlying an area of (2) central fibrosis and finally (3) nests of smaller nevus cells
Immunohistochemistry
Like their original nevi counterparts, RMN usually display a normal maturation gradient, with decreased expression of tyrosinase and gp100 deeper into the dermis and persistently low mitotic activity (<5 %) by Ki67 [15, 39]. Melanomas typically immunolabel strongly for all three stains, though exceptions have been described [42, 43]. Unfortunately, MART-1 (Melan-A) and S100 proteins have not been shown to be of use in distinguishing between these two entities [39]. Future utility of soluble adenylyl cyclase has been suggested in one small newer study, in which histopathologically benign recurrent nevi displayed an immunostaining pattern similar to the original lesion when compared to melanomas [44].
Special Types of Recurrent Nevi
Recurrent Atypical (Dysplastic) Nevus
Atypical nevi, first recognized in families with an increased risk of melanoma as nevi with unusual clinicopathologic features [45, 46] and autosomal dominant inheritance [47], present a challenge for many pathologists given their subjective and evolving controversial diagnostic criteria and association with melanoma [48]. They tend to show lentiginous and nesting proliferations, varying degrees of cytologic atypia with accompanying host inflammatory response, elongation and bridging of rete ridges, fibroplasia around rete pegs with proliferation of dermal capillaries, and horizontally arranged, usually spindle-shaped melanocytes [49]. In one study of mild-moderately atypical nevi followed for at least 2 years compared to benign nevi, fewer than 4 % of nevi in either group were found to recur clinically. Recurrence was associated with the use of shave biopsy technique for sampling but not the presence of positive margins or congenital features [50]. Other retrospective studies with up to 5 years follow-up have not shown melanoma to occur from excised atypical nevi, irrespective of margin involvement [51, 52].
Recurrent Spitz Nevi
Recurrent Spitz (spindled and epithelioid cell) nevi often demonstrate unusual features when compared to typical recurrent melanocytic nevi, not least of which is that their clinical presentation tends to be nodular rather than macular and can extend beyond the margins of the scar [53, 54]. Despite the presence of positive margins in many Spitz nevi, they infrequently recur. In a larger case series of 16 patients, the average time to recurrence was around 13 months, with predominately intradermal nests of spindled or epithelioid cells that extended into the deep reticular dermis and subcutis within an almost keloidal stroma [55]. These lesions histopathologically resembled desmoplastic Spitz nevus rather than the primary Spitz nevus. Other case series have shown less of a histopathological discordance between primary and recurrent Spitz nevi [56–58], and supported the observations of a desmoplastic stroma, a nodular growth pattern with low mitotic indices, maturation as assessed by immunostaining, and junctional nesting with intraepidermal spread above a dermal scar, such that many recurrent nevi can simulate melanoma. Recurrent Spitz nevi have reportedly resulted in metastasis and death [59], but in such extraordinary cases the diagnosis of Spitz nevus rather than melanoma must be questioned. The use of fluorescent in situ hybridization (FISH) or comparative genomic hybridization (CGH) can be used in conjunction with histopathologic and immunostains for further help in distinguishing between melanoma and recurrent Spitz nevi [57].
Recurrent Blue Nevi
There are even fewer reports of recurrent blue nevi than recurrent Spitz or atypical nevi, but they have been described as recurring from cutaneous (including 1 report of recurrence from a plaque-type blue nevus with malignant transformation and mortality [60]) and mucocutaneous surfaces as well as lymph nodes [61–63], the latter of which can further complicate the already difficult distinction between metastatic melanoma and benign nodal blue nevi [64]. Recurrences have been reported in all subsets and histotypes, and occur an average of 2.7 years after initial biopsy. Extension beyond the margins of the original scar has been described. In the largest case series reported to date, seven of nine recurrences resembled the original biopsy, occasionally with a more cellular deep component. The other two recurrences demonstrated a higher degree of cytologic atypia when compared to prior, although follow-up (mean 3.7 years) did not support malignant tumor progression [61].
Sclerosing Nevus with Features of Recurrent Nevi
Sclerosing nevi with pseudomelanomatous features, first described in 2008 [65] and similarly detailed in a larger case series as nevi with regression-like fibrosis in 2009 [66, 67], are relatively new entities to the recurrent nevi family characterized on the basis of their unique clinical and pathologic features including an absence of preceding trauma or biopsy. All of the 19 reported sclerosing nevi with pseudomelanomatous features had clinical features suggestive of regression in conjunction with architecturally disordered junctional and dermal melanocytic nests with pagetoid spread but few to no mitotic figures and no high-grade cytologic atypia or pleomorphism, flanking a central band of fibrous scar-like tissue. They were primarily from the truncal sites in young to middle-aged patients, as were the 90 reported nevi with regression-like fibrosis cases, and did not recur or metastasize at 2–9 year follow-up. Etiology is thought to be related to chronic, asymptomatic trauma over years, leading to atypical regenerative hyperplasia and fibrosis.
Epidermolysis Bullosa Nevi
Acquired nevi arising within scarred areas of former blistering in patients with hereditary epidermolysis bullosa have been shown to have clinical and histopathologic features similar to recurrent nevi, and these so-called “EB nevi” should be considered in the differential diagnosis of recurrent nevi when supported by history and clinical exam [68].
Differential Diagnosis
In addition to the special types mentioned above, the most important diagnoses to consider in the differential of a recurrent benign melanocytic nevus include melanoma arising from a dermal nevus, and a nevus with changes of regression.
The junctional confluence and atypia in melanomas tend to be more severe than in recurrent nevi, and should raise suspicion when seen in combination with prominent pagetoid spread and/or mitoses, features which individually can be seen in recurrent nevi but not altogether. While fibrosis can be seen in melanoma, it will not usually be in an orderly, trizonal pattern. A prominent lymphocytic infiltrate will also be seen [69]. Additional stains such as Ki-67, Melan-A, and HMB-45 (anti-gp100) should be employed if there is even the slightest doubt about whether or not a melanoma is present [29, 70].
RMN can have the same architecture as a regressed melanoma, and is more common in atypical nevi. Fibrosis is again present, but will be irregular and in association with prominent melanophages. The halo phenomenon can confound examination, but generally does not produce a fibrotic response. A melanoma with regression will have epidermal atrophy and neovascularity in addition to melanophages and irregular dermal fibrosis (Table 11.3, Figs. 11.3, 11.4, 11.5).
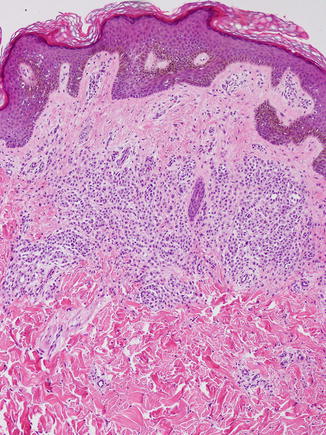
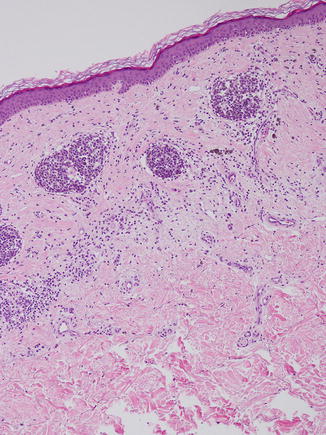
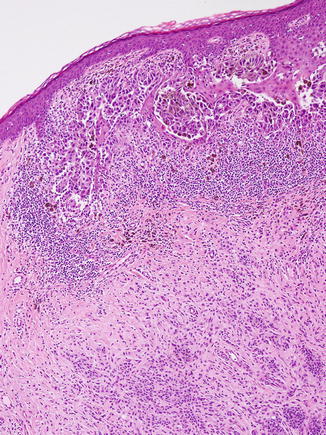
Table 11.3
Recurrent nevus vs melanoma
Recurrent nevus | Melanoma arising with a nevus | Nevus with regressiona | |
|---|---|---|---|
Pagetoid spread | Can be present in isolation | Often present with other concerning features | Absent |
Single-cell proliferation | Can be present in isolation | Often present with other concerning features | Absent |
Confluence | Present in junctional component | Present throughout | Absent |
Cytologic atypia | Present in junctional component | Present throughout | Absent |
Fibrosis | Central, trizonal | Haphazard | Usually non-fibrotic response |
Architectural disorder | Superficial | Throughout | Same as melanoma with regression |
Cell populations | Uniform | Two populations of large atypical melanoma cells and small nevus cells | Uniform |
Nesting | Confluent above scar, orderly below | Expansile | Orderly |
Patient demographics | Usually history of biopsy or trauma | Longstanding nevus that is evolving or suddenly symptomatic | Asymptomatic, younger patients |
Melanophages | Absent | Can be present or absent | Present |
Neovascularity | Absent | Present | Present |
Epidermal atrophy | Absent | Can be present or absent | Present |
Immunostaining | Normal | Abnormal | Normal |

Fig. 11.3
Recurrent atypical nevus. A trizonal pattern of fibrosis is observed, with cellular atypia and pleomorphism of both junctional and dermal nests

Fig. 11.4
Melanocytic nevus with features of regression. The fibrosis is disordered rather than trizonal, and inflammatory cells are present

Fig. 11.5
Melanoma arising within a nevus. A dense lymphocytic infiltrate and erratic fibrosis are present alongside poorly cohesive melanocytic cells that display bizarre architectural and cellular disorder
Treatment
Complete re-excision of all recurrent nevi should be considered, both for ease of future surveillance as well as to limit the number of additional invasive procedures to the patient. If a nevus with regression is suspected, clear surgical margins are recommended as what has already regressed is unknown and a component of regressed malignant melanoma cannot be excluded. When excising congenital nevi, it has been recommended to biopsy for histopathologic subtype beforehand, as over one-third of lesions in one study showed a depth of invasion into the deep dermis and subcutis that predicted a higher likelihood of recurrence [71].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


