Common acquired nevi
Dysplastic nevi
Melanoma
Well nested at peripheral junctional component (circumscribed)
Mild–moderate: circumscribed
Single-cell, non-nested melanocytes predominate
Moderate–severe: poorly circumscribed
Maturation (senescence) transition from pigmented nested melanocytes in superficial dermis to singly dispersed small melanocytes at base
Maturation preserved
Minimal maturation; presence of nests, pigment, or mitotic figures at base of lesion
Symmetric
Mild–moderate: symmetric
Asymmetric
Moderate–severe: asymmetric
Nests equidistant with round-oval shape and similar size
Mild–moderate: equidistant, uniform nests
Elongated nests with irregular shapes in random, haphazard distribution
Moderate–severe: extensive bridging, variability in nest size and distance
Nests usually at rete tips
Mild–moderate: nests usually at rete tips
Nests at arch of dermal papillae, and sides of rete
Moderate–severe: nests at arch of dermal papillae, and sides of rete
Nests in dermis cohesive and smaller than junctional nests (if nevus is compound)
Discohesion and confluence to varied degrees depending on severity
Confluence of melanocytes at DEJ and down adnexae, and discohesion of nests
Absent pigment deep in neoplasm
Absent pigment deep in neoplasm
Presence of pigmented and large nests deep in neoplasm
Minimal mitotic figures
Variable mitotic figures; minimal involvement of depth
Notable mitotic figures including the deepest aspect
Minimal cycling cells (quiescence)
Variable cycling cells
Numerous cells active in cell cycle
Minimal suprabasal (pagetoid) spread (except special sites)
Focal suprabasal (pagetoid) spread in center of lesion
Extensive suprabasal (pagetoid) spread
Minimal inflammatory infiltration (except in halo nevi)
Variable inflammatory cells
Inflammatory infiltrate, sometimes with numerous plasma cells; however, lesions can have minimal inflammation
gp100 (HMB-45) expression top-heavy, with loss of signal at increased depth
gp100 (HMB-45) expression top-heavy, with loss of signal at increased depth
gp100 (HMB-45) expression is patchy
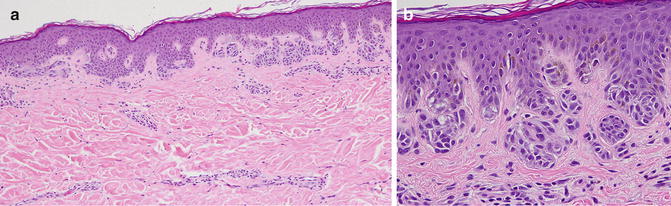
Fig. 9.1
Compound mild dysplastic nevus histology. (a) ×10 magnification—Proliferation of melanocytes along the dermal–epidermal junction and as dermal nests, with minimal cytologic atypia. Mild architectural disorder with shouldering focal bridging of nests, elongated rete ridges, lamellar and concentric fibrosis around rete and a mild dermal inflammatory infiltrate. (b) at ×40 magnification—mild pleomorphism with scattered hyperchromatic cells
Architectural disorder: There is a crowded, lentiginous proliferation of spindled or epithelioid melanocytes in a horizontal arrangement within the epidermis. These cells have finely granular melanin in the cytoplasm, and are arranged either in nests or as single cells, sometimes reaching confluence. The nests can vary in size and are dispersed haphazardly (unlike the ordered architecture of common acquired nevi) at various distances along the sides and tips of elongated rete ridges. There are variable degrees of cohesion within the nests, bridging between nests, and cytoplasmic shrinkage artifacts of the melanocytes. The junctional nests extend beyond the dermal component by at least three rete ridges (“shouldering”) and the lesion may or may not be well-circumscribed (i.e. nested at both lateral edges).
Cytologic atypia: Occasional melanocytes exhibit abundant cytoplasm, nuclei larger than those of adjacent keratinocytes, hyperchromasia, and prominent nucleoli; however, the consistently high-grade, extensive atypia characteristic of melanoma is not observed. DN may have a limited degree of histopathologic overlap with superficial spreading melanoma or lentigo maligna melanoma (Fig. 9.2) but the latter exhibits increased consistency and severity of atypia extensively throughout the lesion, with peripheral lentiginous proliferation (poor circumscription), and a greater degree of cytologic atypia in the junctional component. A melanocytic neoplasm exhibiting extensive features of architectural disorder and cytological atypia should indeed raise concern for melanoma arising within a DN [8]. The degrees of atypia exhibited by DN can range from a sparse presence of one or more features, to extensive manifestation of multiple atypical features, resulting in a spectrum of histopathologic phenotypes.
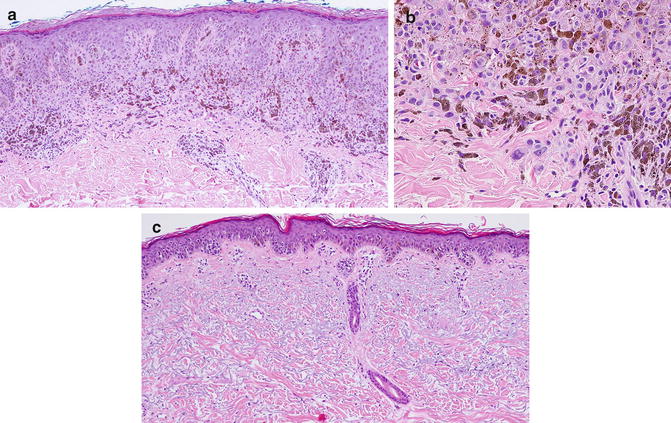
Fig. 9.2
(a-b) Superficial spreading melanoma histology. (a) ×10 magnification—Sheets of large pigmented melanocytes with very severe cytologic atypia including large pleomorphic bizarre nuclei and prominent nucleoli; severe architectural disorder with extensive pagetoid spread, and heavily pigmented melanocytes at the base. Dermal infiltrating lymphocytes and some plasma cells are noted. (b) ×40 magnification—sheets of atypical pigmented melanocytes; note bizarre melanocytes at base of specimen. (c) Lentigo maligna melanoma histology. ×10 magnification—Lentiginous proliferation of atypical melanocytes both as single cells and in poorly cohesive nests along the dermoepidermal junction and in suprabasilar regions, on a background of dermal solar elastosis. Note extension down adnexal structures (eccrine duct)
Stromal response: The superficial dermis around DN exhibits concentric fibrosis (condensation of dense, hypocellular collagen around elongated rete ridges) and lamellar fibroplasia (delicate layered or laminated collagen in a linear array). There are increased fibroblasts in papillary dermis, fibrosis in the upper reticular dermis with widely spaced nests in the dermal component if present, and a patchy lymphocytic infiltrate, and telangiectasia.
Comparison of Dysplastic Nevi with Common Acquired Nevi
Dysplastic nevi (DN) display various features that distinguish them from common acquired nevi (Table 9.1). Characteristic aspects of DN include the presence of varying levels of disordered architecture and atypical cytology, as previously discussed; a higher proliferation index; distinctive gene expression patterns including the presence of p16-INK4A gene mutation (or deletion in some cases), altered p53 expression; evidence of increased microsatellite instability; and increased presence of reactive oxygen species [7]. Expression of HMSA-2, a protein involved in melanogenesis, is present in both DN and melanoma but not in common acquired nevi [9]. There is also a lack of expression of collagen IV around the nests of common acquired nevi, but a continuous pattern of staining surrounding the junctional nests in a concentric fashion in most DN, with the remainder having a discontinuous pattern [10]. DN and common acquired nevi also share certain similarities including the presence of clonality of melanocytes; expression of apoptotic regulators and senescence marker; similar BRAF mutation rates; loss of PTEN expression; and similar rates for recurrence after biopsy [7].
Correlation of Architectural and Cytologic Dysplasia
One point of contention has been whether the diagnosis of DN must be based on cytologic or architectural features alone, or should incorporate both features. A study attempted to develop objective, reproducible criteria for grading DN and correlate architectural disorder with cytologic atypia [11]. The resulting Duke system for grading DN used a binary scoring system in which each factor was given a value of 0 or 1. The features given a value of 1 included;
Architectural disorder: Junctional component not nested at both edges (poor circumscription), poor overall symmetry, less than 50 % of nests cohesive (poor cohesion), suprabasal spread prominent (in more than 2 high power field (hpf)) or present at the edge, confluence of more than 50 % of the proliferation as bridges or single cells, single-cell proliferation not focal or absent. Mild disorder (score of 0–1), moderate disorder (score of 2–3), and severe disorder (score of 4–6) were delineated.
Cytologic atypia (determined in more than 50 % of cells within 2hpf of the most atypical areas): Nuclei not round/oval and euchromatic, nuclei size greater than basal keratinocyte nuclei, nucleoli prominent, and cell diameter greater than twice the size of the basal keratinocyte nuclei. Mild atypia (score 0–1), moderate atypia (score 2), and severe atypia (score 3–4) were delineated.
This study suggested that both architectural disorder and cytologic atypia were important in combination to increase the sensitivity of evaluation and diagnosis of DN (Fig. 9.3). In this study, confluence of junctional component and poor circumscription were the most frequent features of architectural disorder noted, followed by single-cell proliferation and asymmetry [11]. The data indicated that on average architectural disorder and cytological atypia tended to correlate. The authors proposed that both criteria should be considered for a complete histopathologic evaluation of DN because grading architecture may permit better clinical-pathologic correlation [7, 11, 12].
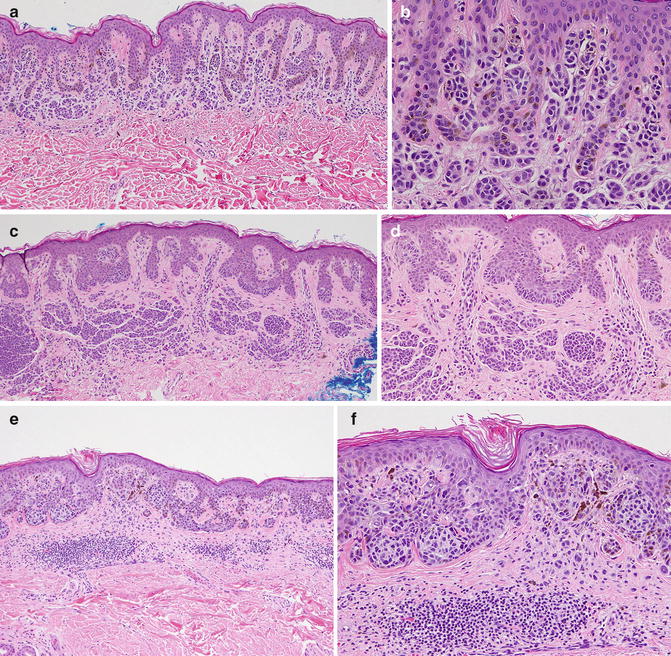
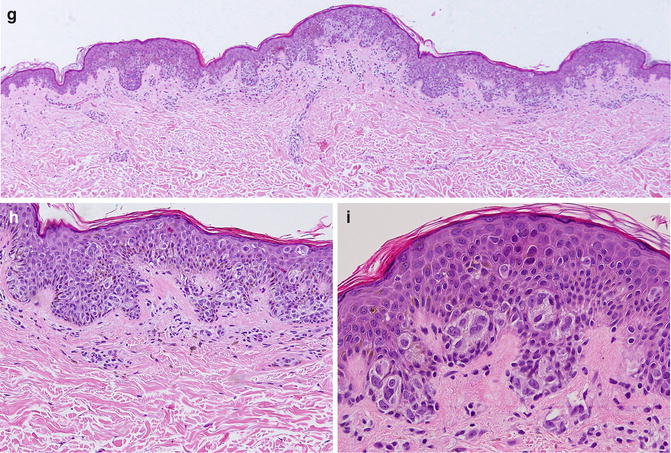


Fig. 9.3
(a-f) Comparision of different grade of dysplasia in DN (a) Compound mild DN. x10 magnification-Compound mild dysplastic nevi. ×20—Architectural disorder with elongation of rete, bridging of similar-sized cohesive nests, concentric fibrosis around rete ridges and scattered single melanocytes along dermal–epidermal junction. Presence of inflammatory infiltrate is minimal. (b) ×40 magnification of (a) —Mild pleomorphism with scattered hyperchromatic nuclei of melanocytes. (c) Compound moderate DN. ×10 magnification—Architectural disorder with more extensive cohesive nest of melanocytes and single cells along the DEJ extending up the rete ridges, bridging, concentric fibrosis, and an inflammatory infiltrate. (d) ×20 magnification of (c) More atypical melanocytes with hyperchromatic nuclei, as single cells and nests, extending up the rete ridges. (e) Compound severe DN. ×10 magnification—Architectural disorder with extensive bridging; proliferation of melanocytes in cohesive nests and as single cells along the DEJ and a few suprabasilar melanocytes. Notable dermal inflammatory infiltrate. (f) ×20 magnification of (e) Multiple melanocytes with pleomorphic hyperchromatic large nuclei and prominent nucleoli, more extensively through the lesion. (g–i) superficial spreading melanoma. (g) ×10 magnification —Sheets of large pigmented melanocytes with very severe architectural disorder with extensive pagetoid spread, and heavily pigmented melanocytes at the base. (h) ×20 magnification of (g) Dermal infiltrating lymphocytes and some plasma cells are noted. (i) ×40 magnification of (g) —Cells with severe cytologic atypia inclusing large pleomorphic bizzare-shaped nuclei with prominent nucleoli, present throught lesion including the base
Ancillary Studies
Immunohistochemistry in Melanocytic Lesions
The histopathologic diagnosis of a significant proportion of melanocytic lesions are clear-cut on hematoxylin-eosin staining and may be confidently classified as nevi (whether dysplastic or non-dysplastic) versus melanoma (Fig. 9.3). Only a minority of lesions, such as nevi with a high degree of dysplasia and spitzoid melanocytic proliferation, necessitate use of ancillary techniques to aid in the diagnosis. Among those techniques, immunohistochemistry is the method most widely employed. Melanocyte differentiation antigens such as Mart-1/Melan-A, tyrosinase, and gp100 (HMB-45) help highlight melanocytes to better ascertain architectural behavior, such as pagetoid spread, lentiginous growth, and lack of maturation. They may be used in combination with proliferation markers (e.g., Ki-67) to determine biologic behavior. There is no single marker, or combination, that establishes an unequivocal diagnosis of melanoma or nevus. Analysis of the pattern of expression and localization can be correlative with morphologic features to facilitate getting to a diagnosis.
HMB-45
HMB-45 antibody, which recognizes the gp100 protein, originally produced by Gown and Vogel in 1980s, defines an oncofetal premelanosomal antigen, positive in fetal melanocytes and melanoma but typically negative in adult resting melanocytes [13]. This marker is valuable in determining the biologic behavior of maturation. In DN, there is a progressive morphologic change in the melanocytes with increased depth. This maturation (senescence) is captured by the antibody HMB-45, which highlights melanocytes in a top-heavy pattern, with loss of staining with increasing depth into the dermis. Overall sensitivity is approximately 85 % but this significantly decreases in spindle-cell or desmoplastic melanomas [14]. In contrast, primary cutaneous melanoma usually expresses gp100 in a patchy pattern throughout the dermal component. HMB-45 can also be used to highlight the intraepidermal component and can label confluence/lentiginous proliferation, and suprabasilar spread of melanocytes, which are features characteristic of melanoma.
Mart-1/Melan-A
Mart-1, also known as Melan-A, is a small cytoplasmic protein, not localized to premelanosomes, initially identified as a target for cytotoxic T-cells [15] and expressed in adult resting melanocytes as well as melanoma. Staining for this melanocyte differentiation antigen has an overall sensitivity of ~85 %, greatest in large-cell, undifferentiated malignancies [16, 17]. Anti-Mart-1 antibodies are positive not just in melanocytes but in adrenocortical adenomas/carcinomas as well as sex-cord stromal tumors of ovary, which may be a pitfall in cases of metastasis of these malignancies to the skin [18]. Anti-Mart-1 antibodies can label confluence/lentiginous proliferation, and suprabasilar spread of melanocytes, highlighting their extent, which can help distinguish DN from melanoma. Also, as a potential pitfall, there is labeling of melanophages by anti-Mart-1 antibodies, which may represent melanocytic antigens that have been phagocytized by macrophages.
MIB-1/Ki-67
MIB-1, also known as Ki-67, is a proliferation marker of cycling cells. The practice of co-labeling the nuclear Ki-67 stain with a cytoplasmic melanocytic marker such as Mart-1/Melan-A, greatly improves the identification of proliferating melanocytes. In DN, Ki-67-positive cells are few (usually <5 %) and are typically located close to the dermoepidermal junction and adnexal epithelium, or in the superficial dermis, but are absent in the deeper portion of the lesion. In contrast, melanomas have a random pattern of immunoreactivity (average ~16 % in “hot spots”), with proliferating cells present at all levels of the lesion, especially at depth, indicating a lack of maturation/senescence [19].
The p16-INK4A Protein
The p16-INK4A product of CDKN2A is a cyclin-dependent kinase inhibitor, which has critical functions at the G1-S checkpoint of the cell cycle, This enzyme blocks the cell cycle at the G1-S checkpoint by inhibiting CDK (cyclin-dependent kinases), including CDK4, and cyclins such as cyclin D1. This suppresses the proliferation of cells with damaged DNA or with activated oncogenes and is also activated when cells are old or crowded. The p16-INK4A protein is frequently inactivated in human tumors, including melanoma, and inherited mutations are associated with increased melanoma susceptibility [20, 21]. Common acquired nevi show minimal loss of p16-INK4A, while allelic loss of this locus is common in DN and in primary and metastatic melanomas [22]. The expression pattern can be nuclear or cytoplasmic.
Microphthalmia Transcription Factor (MITF)
Clark and colleagues proposed that failure of melanocytes to differentiate is necessary for dysplasia [23]. MITF is a nuclear transcription factor that regulates development, differentiation, and survival of melanocytes [24]. MITF plays a key role in the pathway leading to melanin production. Specifically, signaling initiated by alpha-MSH binding to the MCR1 transmembrane receptor results in MITF activation and subsequent transcription of genes necessary for melanin synthesis, including the key enzyme, tyrosinase [25], as well as other melanocyte-specific genes such as MART1 and SILV (silver homolog). MITF expression can also result in cell-cycle arrest by the induction of p16-INK4A [26, 27]. MITF is amplified or mutated in ~10 % of primary cutaneous melanoma and ~20 % of metastatic melanoma [28, 29]. MITF amplification occurs in tumors with poor prognosis, being associated with resistance to therapy [28]. There is paradoxically a decrease in genes regulated by MITF, including SILV, TRPM1 (melastatin), and MART1 in certain melanoma subsets and this is thought to accompany the progression from nevus to melanoma, as well as to be a poor prognostic indicator [30, 31].
-Hydroxymethylcytosine
5-Hydroxymethylcytosine is a recently described marker that correlates with the level of dysplasia [32]. This proves very useful in challenging lesions, including distinguishing DN from melanoma. Tumor cells in various human cancers exhibit global hypomethylation as well as selective hypermethylation at promoter regions of tumor suppressors, resulting in gene silencing and malignant transformation [33]. Progressive loss of 5-hydroxymethylcytosine was noted in one study to be associated with increasing levels of dysplasia, with the common acquired nevi expressing the marker to the strongest extent and near-complete loss in melanoma. Specifically, 5-hydroxymethylcytosine staining was highest in normal resident basal layer melanocytes, with 100 % staining darkly. Common acquired (non-dysplastic) nevi and low-grade DN (defined as those with mild or focally moderate cytologic atypia) showed 60 % of melanocytes staining darkly in this study [32]. High-grade DN (defined as those with diffusely moderate or severe atypia) ranged from 90 % lightly stained to 10 % negatively stained melanocytes. 5-Hydroxymethylcytosine exhibited near total loss in melanoma, being associated particularly with poor prognosis in superficial spreading melanoma and nodular melanoma [34]. In addition, increased nuclear size, a feature of dysplasia, had an inverse correlation with the expression of 5-hydroxymethylcytosine while being directly proportional to the degree of dysplasia [32]. Interestingly, DN showed darker staining in deep aspects of the neoplasm than the superficial aspect, likely highlighting maturation. In melanoma arising within a nevus, where delineating the extent of the melanoma for Breslow depth determination may prove challenging, there was a strong staining of 5-hydroxymethylcytosine levels in the nevus component and loss in the melanoma component [32].
SOX Proteins
SOX (Sry-HMG-box) proteins are a family of transcription factors involved in regulating a variety of biologic events including lineage restriction and terminal differentiation, through a precise pattern of expression that is cell-type specific [35]. SOX10 plays a key role in the transcriptional control of MITF, which is the master regulatory gene for melanogenesis [36]. However, SOX9, SOX18, and SOX5 have also been implicated in regulating aspects of the melanocyte life cycle. SOX9 (a nuclear stain), and SOX10 (a perinuclear and cytoplasmic stain) have been reported to be expressed in various stages of melanoma progression and in established melanoma cell lines [37–39]. Studies showed that SOX10-positive melanocytes were present in 31 % of nevi, 43 % of primary melanoma, and 50 % of metastatic melanoma [38, 39]. However, SOX9 expression was observed in a majority (~75 %) of the melanocytic neoplasms, with moderate decrease as the severity of melanoma progressed [40]. SOX9 expression has been shown to reduce proliferation of multiple melanoma cell lines [37]. The SOX protein expression in DN have not yet been fully elucidated and the combination of SOX 9 and SOX 10 expression patterns by immunohistochemistry may prove useful in better delineating the spectrum or grades of atypia that characterize DN and melanoma.
Angiogenesis Markers
Angiogenesis and microvascular density (MVD) are important characteristics in tumorigenesis, with roles in the multifactorial transition from benign to malignant states [41]. These features have been shown to affect the prognosis of malignant tumors and skin neoplasms including melanoma [42]. Benign nevi (dysplastic and non-dysplastic) have similar MVD and mean major diameters of blood vessels. However, these parameters, in addition to total vascular area, are significantly increased in melanoma [43]. Therefore melanomas, unlike DN, have a greater number of vessels over a larger area, providing evidence for correlation of malignancy with increased vascularity.
Survivin
Survivin is an antiapoptotic protein that has been detected in DN by immunohistochemistry. In one study, the majority of DN with a nuclear and cytoplasmic staining pattern for this marker had severe dysplasia [44]. A lack of nuclear staining was specifically described in benign melanocytic nevi, while 67 % of melanoma showed a positive nuclear stain, with an average index of 7 % [19].
Phosphohistone H3 (PHH3)
Phosphohistone H3 (PHH3) is a proliferative marker that highlights cells specifically in the M-phase of the cell cycle. A study by Nasr et al. showed a lack of PHH3 expression in the dermis of compound and dysplastic nevi, however an average of positivity of 25 cells/10 hpf was noted in melanomas [19]. Use of PHH3 expression can be of value in identifying the “hot spot” of greatest mitotic index within a tumor.
Confocal Microscopy in Melanocytic Neoplasms
Confocal microscopy uses point illumination via a pinhole to eliminate out-of-focus signals. The pinhole is conjugate to the focal point of the lens, allowing for optimal resolution [45]. Melanin offers the strongest contrast due to a high refractive index; therefore the cytoplasm of melanocytes is intensely white (Fig. 9.4). However, keratin has a lower refractive index and therefore less contrast, so keratinocyte cytoplasms appear darker. Nuclei appear dark and dermal collagen fibers appear very bright [46]. In vivo reflectance confocal microscopy (RCM) is a noninvasive tool that generates stacks of optical horizontal (z-axis) sections within the depth of intact living tissue. This proves to be a useful tool for studying the skin surface, and was first used in human skin in 1995 [47]. RCM enables visualization of the skin layers to a cellular level resolution (0.5–1.0 μm in the lateral dimension and 4–5 μm axially). The imaging depth is limited to 200–300 μm corresponding to depth at the dermoepidermal junction and papillary dermis, using the current commercially available RCM model [48]. An advantage of RCM is that the technology allows a section of skin to be assessed without processing (which may introduce artifact), and re-examination in order to evaluate dynamic changes over time. Characteristic architectural and cytologic features have been described, with histopathologic and dermoscopic correlates (Table 9.2).
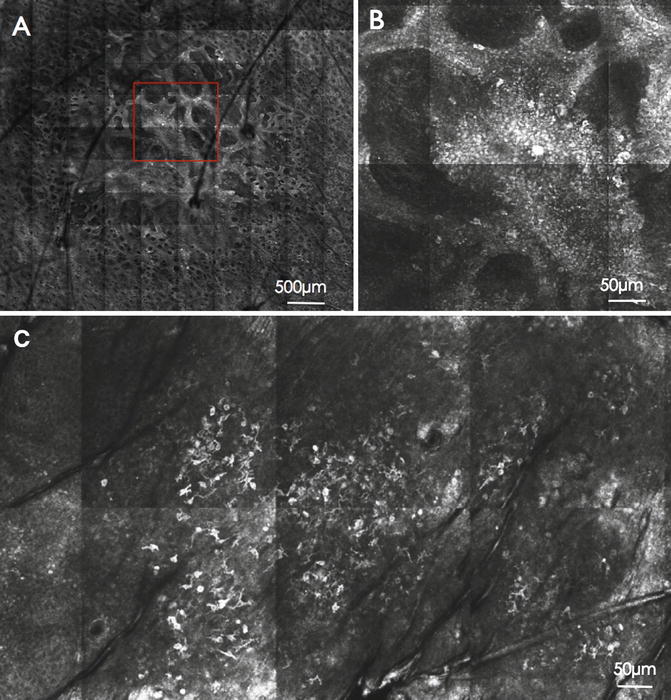

Fig. 9.4
(a) Confocal image of a dysplastic nevus: overview highlighting the meshwork pattern (500 μm). (b) Magnified view of the red box insert of (a): cytologic atypia (50 μm). (c) Confocal image of a melanoma: Florid and widespread pagetoid cells (50 μm). Courtesy Caterina Longo M.D., Ph.D. Caterina Longo, M.D., Ph.D. Skin Cancer Unit, Arcispedale Santa Maria Nuova-IRCCS, viale Risorgimento 80, 42100 Reggio Emilia, Italy
Table 9.2
Confocal terminology with dermoscopic and histopathologic correlates
Histopathologic features | Dermoscopic features | Confocal microscopic features |
|---|---|---|
Elongated rete with increased melanocytes | Typical pigment network | Edged papillae and ringed pattern at DEJ |
Rete ridges disarranged with atypical melanocytes. Usually in melanoma | Atypical pigment network | Non-edged papilla; irregular size and shape of dermal papillae without a clear border |
Uniform nests at DEJ/papillary dermis | Regular pigment globules | Compact aggregates with sharp margins made of monomorphous polygonal cells |
Combination of typical nests and compact aggregates of pleomorphic melanocytes | Irregular pigment globules | Irregular clusters with regular cytology (in benign) and atypical pleomorphic cells (in melanoma) |
(A) Pagetoid spread | Pigmented dots | (A) Bright cells in superficial layers |
(B) Dermal plump bright cells | ||
(B) Melanophages in dermis | ||
Peripheral elongated and parallel epidermal rete with nests | (A) Radial streaming | Parallel elongated lines of elongated cells projected toward the periphery |
Uniform nests at peripheral | (B) Peripheral globules | Dense peripheral clusters |
Well-defined nests at tips of enlarged and parallel rete | (C) Pseudopods | Globular-like bulging structures |
Pigmented melanocytes in a uniform epidermal architecture | Light brown pigmentation | Regular honeycombed pattern |
Keratinocyte pigmentation and transepidermal melanin loss with pagetoid spread of cells | Diffuse dark pigmentation and pigment blotches | Bright cobblestone pattern; suprabasal spread |
Ortho/parakeratosis with pagetoid cells; marked basal melanocyte atypia; disarranged pattern of DEJ, malignant cells in nests, solitary in dermis with melanophages and inflammatory cells | Blue-white veil | Irregular pattern, round bright cells in superficial layers; non-edged papillae; cytologic atypia in basal layer; dishomogenous nest; nucleated and plump bright cells in papillae |
Melanophages and inflammatory cells in dermis | Blue areas | Plump bright cells in dermal papillae |
Thin epidermis, fibroplasia with inflammatory infiltrate with melanophages | Regression | Coarse network of ill-defined grainy fibers in dermis with intermingled bright spots and plump bright cells |
Confocal Microscopy in Melanoma
In vivo RCM plays an important role in the characterization of the superficial aspect of melanoma, where a large number of characteristic findings are visible. However, Breslow depth determination, which has prognostic significance, is currently not possible. In the radial growth phase of melanoma, the epidermal pattern can be a disarranged honeycombed or cobblestoned pattern (i.e. an irregular epidermal pattern due to irregularly shaped keratinocytes) [49]. There can be suprabasilar/pagetoid migration of malignant melanocytes, evident as bright cells in superficial layers (Fig. 9.4). Atypical melanocytes are also noted along DEJ and in superficial layers, forming a nested and confluent proliferation (Fig. 9.3). In addition, atypical nucleated cells tend to infiltrate the dermal papillae and correspond histopathologically to nested melanocytic proliferation in upper dermis that invade and cause disarray of rete ridges. In almost 50 % of melanoma with microinvasion, regression is present, with an inflammatory infiltrate, and this is visualized by small bright inflammatory cells and plump bright cells (melanophages) with coarse bright collagen fibers [50]. In hypopigmented and amelanotic melanoma, confocal microscopy may still show features of melanoma due to melanin refractivity [49].
Confocal Microscopy in Grading of Dysplastic Nevi
Biopsy of melanocytic lesions for histopathologic assessment is the gold standard but it interferes with the natural evolution of the lesion in vivo. Therefore histopathology-based assessments of the dynamics of the any given DN, either as a benign entity or as a precursor to melanoma, are limited. The advantage of in vivo observation in real time of tumor at the bedside is opening the clinical application of RCM for evaluation of melanocytic lesions and monitoring evolution, which is a necessary component to better understanding DN. Although limited by a small sample size, Pellacani et al. first demonstrated that histopathologic criteria i.e. architectural and cytologic features outlined by the Duke grading system had significant RCM correlates [51]. DN viewed by confocal microscopy were characterized in this study predominantly by a ringed pattern in association with a meshwork pattern, in addition to atypical junctional cells in the center of the lesion and irregular junctional nests with short interconnections (Fig. 9.3). In general, DN had cytologic atypia and atypical junctional nests, i.e. an irregular pattern with short interconnections and/or with nonhomogenous cellularity. However, pagetoid spread, widespread cytologic atypia at the junction, and non-edged papillae were suggestive of melanoma [51].
There were characteristic findings for common acquired nevi, melanoma, as well as the different grades of DN (Table 9.3). Suprabasal melanocytes most directly correlated with the level of dysplasia, i.e., 0 % in common acquired nevus and nevus with mild dysplasia 7 % in nevus with moderate dysplasia, 40 % and 100 % in nevus with severe dysplasia and melanoma, respectively [51]. Marked architectural disorder was observed in all but one melanoma (which showed severe cytologic atypia), in all severe DN, in 3 of 15 moderate DN, and in one common acquired nevus. Marked cytologic atypia was observed in some DN and melanoma but not in common acquired nevi or mildly dysplastic nevi [51].
Table 9.3




Differences among common acquired nevi, dysplastic nevi, and melanoma by in vivo RCM
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


