45 Elements that emit radiation are known as radionuclides and have a number of applications in medicine. Radiopharmacy in hospital practice is concerned with the manufacture or preparation of radioactive medicines known as radiopharmaceuticals. These have two main applications in medicine: Diagnostic radiopharmaceuticals may be classified into two types: It is important to note that for the safe production of radiopharmaceuticals, the radiopharmacy must be designed to comply with, and procedures must follow, good manufacturing practice and good radiation protection practice. Radiopharmacists working in this field are part of a multidisciplinary team which includes physicians, physicists, radiochemists and technicians from the field of pharmacy as well as nuclear medicine. As part of this team, they not only ensure that the radiopharmaceuticals will give high-quality clinical information, but also that they are safe for both patient and user alike. Radionuclides which decay by beta−-decay tend to have nuclei that are neutron rich. They attempt to reach a more stable state by the transformation of a neutron into a proton with the emission of a beta− -particle (e.g. 32P: 3215P → 3216S + beta−). Despite beta−-particles having a range in air of up to several metres, their range in tissues is only a few millimetres. Because of this and their highly ionizing nature, beta−-emitters tend to be used in therapeutic radiopharmaceuticals (Table 45.1). The most widely used example of this is 131I- sodium iodide, which is used in the treatment of hyperactive thyroid disease and in certain thyroid tumours. Here the physiological property of thyroid tissue is exploited to target the radionuclide to the site of action. Since thyroid tissue avidly takes up iodine in the normal synthesis of the hormone levothyroxine, radioactive iodine is also taken up and held in the thyroid tissue. Hence, the radiation damage is targeted to the thyroid tissue specifically and the normal excretion of any excess iodine results in no significant damage to other organs and tissues. When used in conjunction with a specialized gamma-camera with detectors placed 180° apart, it is possible to create images in all three dimensions with the position of the radiopharmaceutical being very precisely known. This type of imaging technique is known as positron emission tomography (PET). There are a number of positron emitting radionuclides which are becoming important tools in diagnostic imaging. Currently 18F-labelled glucose, known as 18F-fluoro deoxy-glucose (18F-FDG), is the most commonly used PET radiopharmaceutical in hospital practice and as a result the production processes for it will be described in simplified form and used as an example (see below). However, it should be noted there are four main positron emitters used to prepare radiopharmaceuticals (see Table 45.1). PET imaging with 18F-FDG, in combination with X-ray computerized tomography (CT) is rapidly becoming an important imaging technique in the diagnosis of cancer. Radionuclides which decay by electron capture are useful in diagnostic imaging since they emit gamma-rays; examples are given in Table 45.1. A simplified decay scheme for 99mTc-technetium is shown in Figure 45.1 where 99mTc’s parent radionuclide, molybdenum (99Mo), decays by beta−-emission to the ground state 99Tc either directly or indirectly. Radionuclides which decay by this process are used in diagnostic imaging since they emit gamma-rays (see Table 45.1). It should be noted that 99mTc is the most widely used radionuclide in hospital radiopharmacy today, making up the radionuclide component of around 90% of the radiopharmaceuticals produced. For these reasons the production processes for 99mTc-radiopharmaceuticals will be especially emphasized (see below). Table 45.2 Examples of 99mTc-radiopharmaceuticals
Radiopharmacy
 Types of radionuclides and the principles of their medical use
Types of radionuclides and the principles of their medical use
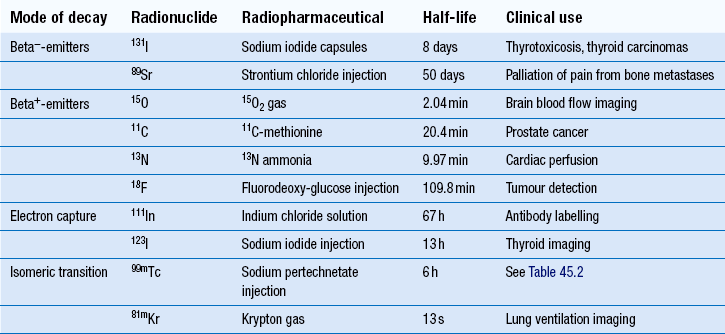
 Examples of alpha-emitters, beta−– and beta+-emitters, electron capture and isomeric transitions
Examples of alpha-emitters, beta−– and beta+-emitters, electron capture and isomeric transitions
 Radionuclide production of beta+-emitters
Radionuclide production of beta+-emitters
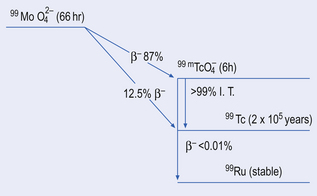
 Principles of using a molybdenum-technetium generator
Principles of using a molybdenum-technetium generator
 Preparation of 99mTc radiopharmaceuticals
Preparation of 99mTc radiopharmaceuticals
Introduction
 As an aid to the diagnosis of disease (diagnostic radiopharmaceuticals)
As an aid to the diagnosis of disease (diagnostic radiopharmaceuticals)
 In the treatment of disease (therapeutic radiopharmaceuticals).
In the treatment of disease (therapeutic radiopharmaceuticals).
 Radiopharmaceuticals used in tracer techniques for measuring physiological parameters (e.g. 51Cr-EDTA for measuring glomerular filtration rate)
Radiopharmaceuticals used in tracer techniques for measuring physiological parameters (e.g. 51Cr-EDTA for measuring glomerular filtration rate)
 Radiopharmaceuticals for diagnostic imaging (e.g. 99mTc-methylene diphosphonate (MDP) used in bone scanning).
Radiopharmaceuticals for diagnostic imaging (e.g. 99mTc-methylene diphosphonate (MDP) used in bone scanning).
Radionuclides used in nuclear medicine
Beta-emitters
Beta−-emitters
Beta+-emitters (positrons)
Electron capture
Isomeric transition
Principles of 99mTc-radiopharmaceutical production
 It has a 6-h half-life (T1/2); long enough to allow imaging to take place in the working day, while also being short enough that patients are not radioactive for long periods (in 24 h, or 4 half-lives, the radioactivity will have decayed by 94%)
It has a 6-h half-life (T1/2); long enough to allow imaging to take place in the working day, while also being short enough that patients are not radioactive for long periods (in 24 h, or 4 half-lives, the radioactivity will have decayed by 94%)
 99mTc emits gamma-rays of 140 keV energy: ideal for use with the modern gamma-camera
99mTc emits gamma-rays of 140 keV energy: ideal for use with the modern gamma-camera
 There are no particulate emissions that, if present, would add to the patient’s radiation dose
There are no particulate emissions that, if present, would add to the patient’s radiation dose
 By purchasing a device known as a 99Mo/99mTc -generator, 99mTc can be made readily available to the hospital site in a sterile and pyrogen-free form
By purchasing a device known as a 99Mo/99mTc -generator, 99mTc can be made readily available to the hospital site in a sterile and pyrogen-free form
 99mTc has versatile coordination chemistry and will allow a large number of ligands to complex with it. By using different ligands in the radiopharmaceutical’s formulation, a wide range of radiopharmaceuticals can be prepared in the radiopharmacy, providing for the many different investigations carried out in nuclear medicine departments (Table 45.2).
99mTc has versatile coordination chemistry and will allow a large number of ligands to complex with it. By using different ligands in the radiopharmaceutical’s formulation, a wide range of radiopharmaceuticals can be prepared in the radiopharmacy, providing for the many different investigations carried out in nuclear medicine departments (Table 45.2).
Radiopharmaceutical
Organ or tissue of distribution
Main clinical application
99mTc-sodium pertechnetate
Thyroid
Imaging the thyroid gland and ectopic tissue
Salivary gland
Dynamic images of accumulation and drainage to show gland function
Gastric mucosa
Presence of Meckel’s diverticulum containing gastric mucosa
99mTc-methylene diphosphonate (MDP)
Skeleton
Bone metastases from carcinoma of lung, breast and prostate
99mTc-macro-aggregates of albumin (MAA)
Lung blood flow
Lung perfusion studies most commonly for the diagnosis of pulmonary embolism
99mTc-exametazime (HMPAO)
Brain blood flow
Regional cerebral imaging in stroke and tumours
Diagnosis of Alzheimer’s dementia
99mTc-exametazime (HMPAO) labelled leucocytes
Infection or inflammation
Identification of abscesses associated with pyrexia of unknown origin. Extent of inflammatory bowel disease
99mTc-tetrofosmin
Heart
Cardiac perfusion imaging
99mTc-sestamibi (MIBI)
Heart
Cardiac perfusion imaging
99mTc-tin colloid
Liver
Location of hepatic tumours, abscesses and cysts. Detection of cirrhosis
99mTc-mercapto triglycine (MAG 3)
Kidney
Dynamic studies to study kidney function
99mTc-dimercapto-succinic acid (DMSA)
Kidney
Static imaging showing the kidney structure ![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Radiopharmacy
Only gold members can continue reading. Log In or Register to continue