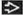Radial Sclerosing Lesion/Radial Scar
Key Facts
Terminology
Radial sclerosing lesion (RSL)
Radially organized combination of epithelial proliferation, central stromal fibrosis, and sclerosis resulting in mass-like lesion
Clinically, mammographically, and pathologically RSL may mimic invasive carcinoma
Clinical Issues
Most RSLs are microscopic findings
Larger RSL may present as mammographic or palpable mass
Risk factor for subsequent development of breast carcinoma
Surgical excision recommended for RSL diagnosed by core needle biopsy
Both in situ and invasive carcinomas have been reported in association with RSL
Image Findings
Irregular appearance on mammography
Microscopic Pathology
Central nidus, varying degrees of fibrosis and fibroelastosis in stellate or radial configuration
Associated proliferative epithelial component
Varying degrees of proliferative epithelial changes
Smaller ducts can become entrapped in dense fibrous stroma within central fibrotic region
Top Differential Diagnoses
Tubular carcinoma
TERMINOLOGY
Abbreviations
Radial sclerosing lesion (RSL)
Synonyms
Radial scar
Complex sclerosing lesion (CSL)
Definitions
RSL shows variable combinations of the following elements
Epithelial proliferation, which may be florid
Stromal fibrosis and dense sclerosis
As a result, RSL shows irregular contours
Clinically, mammographically, and pathologically, this lesion closely resembles invasive carcinoma
“Radial scar” is term used for same lesion; “scar” does not imply prior surgery or trauma
CSL is less specific term
Sometimes defined as a RSL > 1 cm in size
CSL may also include lesions consisting of confluent sclerosing adenosis, sclerosing papillomas, &/or multiple RSLs
CLINICAL ISSUES
Presentation
Most RSLs are microscopic findings
Larger RSLs may present as mammographic density or even palpable mass
Both in situ and invasive carcinomas have been reported in association with RSL
Treatment
Surgical approaches
Most authors recommend surgical excision for RSL diagnosed by needle core biopsy
Some RSLs are reported to coexist with malignancy
RSL on core needle biopsy may be difficult to distinguish from invasive carcinomas
Prognosis
RSL is histologic risk factor for subsequent development of breast carcinoma
Presence of epithelial atypia, increased size, and multiple lesions are likely associated with increased risk for development of malignancy
However, additional risk after adjusting for proliferative disease and atypical hyperplasia is small
IMAGE FINDINGS
Mammographic Findings
RSL presents as irregular mass
Length of radiating arms is long in relation to size of mass, as compared to carcinomas
Center of mass may be lucent
Microcalcifications are present in some lesions
Typically seen in association with accompanying sclerosing adenosis or ADH
Most mammographically detected lesions are < 2 cm
Average size: < 1 cm
Associated carcinomas more common with RSL > 2 cm
Ultrasonographic Findings
RSL most commonly seen as hypoechoic area/mass
Parenchymal distortion without hypoechoic mass may be seen
MR Findings
Irregular enhancing lesion by MR
RSLs tend to show less enhancement by MR, as compared with invasive carcinoma
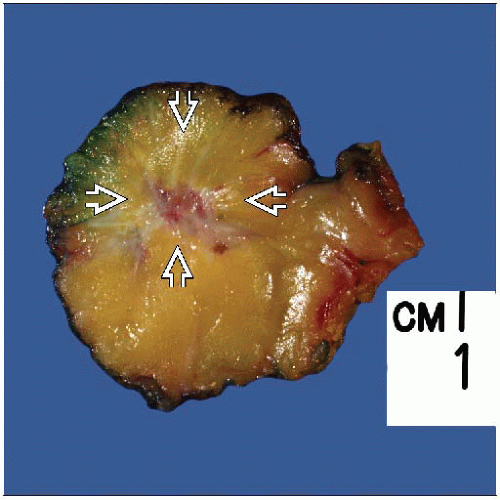
MACROSCOPIC FEATURES
General Features
Irregular firm mass
Yellow-white color, indurated with central retraction
Lesions are usually firm but not as hard as invasive carcinomas
However, lesions may grossly be indistinguishable from invasive carcinoma
Size
Most lesions are < 2 cm
MICROSCOPIC PATHOLOGY
Histologic Features
Central nidus, sclerotic zone composed of varying degrees of fibrosis and fibroelastosis in stellate or radial configuration
Dense granular eosinophilic stroma, sometimes showing weakly basophilic quality
Elastic component of stroma may be highlighted by van Gieson stain, which shows dense curvilinear elastic fibrils
Stroma usually hypocellular
Associated proliferative epithelial component
Ducts and lobular elements radiate from central fibroelastotic zone
Demonstrates varying degrees of benign alteration and proliferative changes
Florid ductal epithelial hyperplasia, sclerosing adenosis, and epithelial cyst formation
Radiating epithelial components appear to expand or enlarge moving away from central fibroelastotic region
ADH, ALH, in situ and invasive carcinoma can occur in association with RSL
Atypical hyperplasias and carcinomas more common in larger lesions
Atypical hyperplasias and associated carcinomas more common in women > 50 years
Entrapped ducts
Smaller ducts can be seen entrapped in dense fibrous stroma within central region of lesion
Ducts may become distorted and appear angulated giving rise to a pseudoinfiltrative appearance
Appearance may mimic tubular carcinoma, especially on core needle biopsy
Entrapped ducts retain myoepithelial layer of cells
Surrounding stroma typically paucicellular, absence of loose desmoplastic reaction
Immunohistochemistry for myoepithelial cells may be helpful to exclude invasive carcinoma
ANCILLARY TESTS
Immunohistochemistry
Studies to identify myoepithelial cells may be helpful in difficult cases
However, results of myoepithelial cell studies to rule out malignancy must be interpreted with caution
Myoepithelial cells may only be identified around some of entrapped glands
Myoepithelial cells may not be present in plane of section used for the study
Use of more than 1 myoepithelial cell marker is recommended to improve likelihood of detection
p63, calponin, and smooth muscle myosin heavy chain have good sensitivity and specificity
Cocktails of > 1 myoepithelial marker may be helpful
p63 is nuclear marker; nuclei may not be present on all levels
Cytoplasmic myoepithelial markers are more likely to detect cell body of myoepithelial cells
DIFFERENTIAL DIAGNOSIS
Tubular Carcinoma (TC)
May be significant mammographic, macroscopic, and histologic overlap between large RSLs and TC
Proliferating neoplastic ducts of TC are associated with desmoplastic stroma (increased cellularity)
Entrapped ducts of RSL are surrounded by hyalinized fibroelastotic stroma (low cellularity)
Proliferating neoplastic ducts of TC show haphazard arrangement, usually throughout lesion
Entrapped ducts of RSL limited to central fibroelastotic region of lesion
Proliferating neoplastic ducts of TC show single cell layer and lack surrounding myoepithelial cells
Entrapped ducts of RSL demonstrate surrounding myoepithelial cells
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree