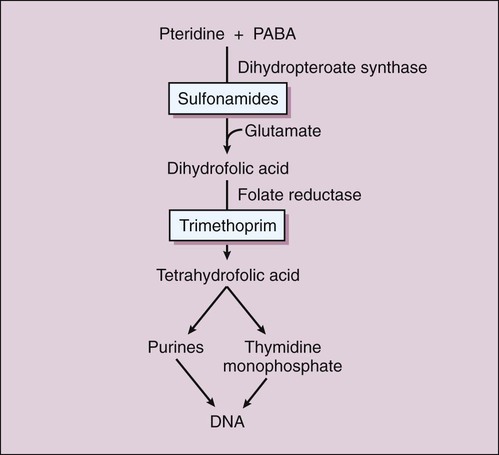
There are two types of antifolate drugs used in chemotherapy. Members of the first group, the sulfonamides, inhibit the synthesis of dihydrofolate in some bacteria and parasites. Members of the second group, the folate reductase inhibitors, block the action of dihydrofolate reductase and the formation of tetrahydrofolate in various organisms. This second group includes the following: pyrimethamine, which inhibits folate reduction in some protozoa and is primarily used to treat toxoplasmosis and malaria (see Chapter 44); methotrexate, which inhibits folate reduction in mammalian cells and is used in the treatment of neoplastic and autoimmune diseases (see Chapter 45); and trimethoprim, which selectively blocks folate reduction in bacteria as described later in this chapter (see section on trimethoprim). In bacteria, the sulfonamides and trimethoprim inhibit sequential steps in the synthesis of folate (Fig. 40-1). The sulfonamides are structural analogues of PABA and competitively inhibit dihydropteroate synthase. Hence their effects can be counteracted by the administration of PABA. Trimethoprim inhibits bacterial folate reductase. Mammals must obtain folic acid in their diet because they are unable to synthesize dihydrofolate. Once absorbed, dihydrofolate is converted to tetrahydrofolate and active folate derivatives (methyl, formyl, and methylene tetrahydrofolate) that donate single-carbon atoms during the synthesis of purine bases and other components of DNA (see Chapter 17). Although folate reductase is found in both microbial and mammalian cells, the affinity of trimethoprim for the enzyme in bacteria is about 100,000 times greater than its affinity for the mammalian enzyme. The sulfonamides are benzene sulfonic acid amide derivatives. Most sulfonamides are adequately absorbed from the gut and are widely distributed to tissues and fluids throughout the body, including the cerebrospinal fluid. The half-lives of sulfonamides vary greatly (Table 40-1), but the most widely used compounds for treating human infections, such as sulfacetamide and sulfamethoxazole, have half-lives ranging from 6 to 10 hours. TABLE 40-1 Pharmacokinetic Properties of Selected Antifolate Drugs, Fluoroquinolones, and Other Agents* IV, Intravenous; NA, not applicable. *Values shown are the mean of values reported in the literature. The sulfonamides were the first drugs used in the treatment of systemic bacterial infections. They were once active against a wide variety of organisms, including streptococci, gonococci, meningococci, many gram-negative bacilli, and chlamydiae. Over the years, however, significant resistance to sulfonamides has developed in many bacterial species, and the antimicrobial spectrum of these drugs has been greatly reduced. Today, sulfonamides are primarily used to prevent or treat urinary tract infections (Table 40-2). TABLE 40-2 Major Clinical Uses of Selected Fluoroquinolones, Antifolate Drugs, and Other Antibacterial Drugs
Quinolones, Antifolate Drugs, and Other Antimicrobial Agents
Antifolate Drugs
Mechanisms of Action
Sulfonamides
Chemistry and Pharmacokinetics
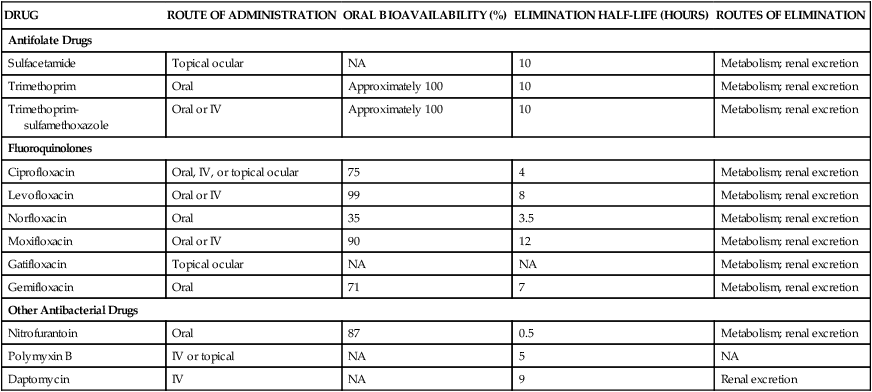
DRUG
ROUTE OF ADMINISTRATION
ORAL BIOAVAILABILITY (%)
ELIMINATION HALF-LIFE (HOURS)
ROUTES OF ELIMINATION
Antifolate Drugs
Sulfacetamide
Topical ocular
NA
10
Metabolism; renal excretion
Trimethoprim
Oral
Approximately 100
10
Metabolism; renal excretion
Trimethoprim-sulfamethoxazole
Oral or IV
Approximately 100
10
Metabolism; renal excretion
Fluoroquinolones
Ciprofloxacin
Oral, IV, or topical ocular
75
4
Metabolism; renal excretion
Levofloxacin
Oral or IV
99
8
Metabolism; renal excretion
Norfloxacin
Oral
35
3.5
Metabolism; renal excretion
Moxifloxacin
Oral or IV
90
12
Metabolism; renal excretion
Gatifloxacin
Topical ocular
NA
NA
Metabolism; renal excretion
Gemifloxacin
Oral
71
7
Metabolism, renal excretion
Other Antibacterial Drugs
Nitrofurantoin
Oral
87
0.5
Metabolism; renal excretion
Polymyxin B
IV or topical
NA
5
NA
Daptomycin
IV
NA
9
Renal excretion
Spectrum, Indications, and Bacterial Resistance
DRUG
MAJOR CLINICAL USES
Fluoroquinolones
Ciprofloxacin
Bacterial diarrhea; intra-abdominal infections; infections of the urinary tract, prostate, bone and joints, skin, and eye; anthrax exposure
Levofloxacin
Bronchitis and community-acquired pneumonia; infections of the urinary tract, prostate, skin, and eye
Gatifloxacin
Bacterial conjunctivitis
Gemifloxacin, moxifloxacin
Community-acquired pneumonia, sinusitis, bronchitis, tuberculosis
Norfloxacin
Urinary tract infections
Antifolate Drugs
Trimethoprim-sulfamethoxazole
Urinary tract and prostatic infections; pulmonary infections caused by Pneumocystis jiroveci (carinii) and Nocardia species
Silver sulfadiazine
Burn infections, other skin infections
Sulfacetamide
Ocular infections
Other Antibacterial Drugs
Nitrofurantoin
Lower urinary tract (bladder) infections
Polymyxin B
Superficial infections of skin and mucous membranes
Daptomycin
Infections caused by methicillin- or vancomycin-resistant staphylococci or vancomycin-sensitive enterococci
Rifaximin
Traveler’s diarrhea, hepatic encephalopathy, irritable bowel syndrome ![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Quinolones, Antifolate Drugs, and Other Antimicrobial Agents
Only gold members can continue reading. Log In or Register to continue