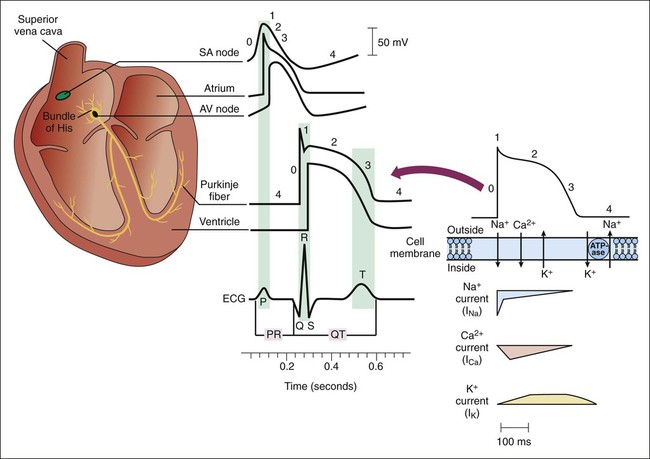
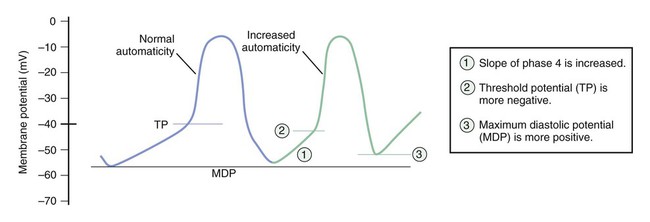
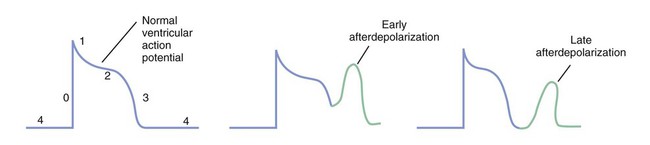
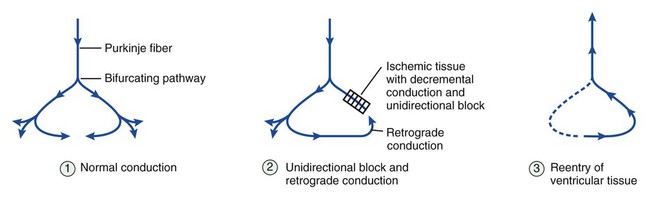
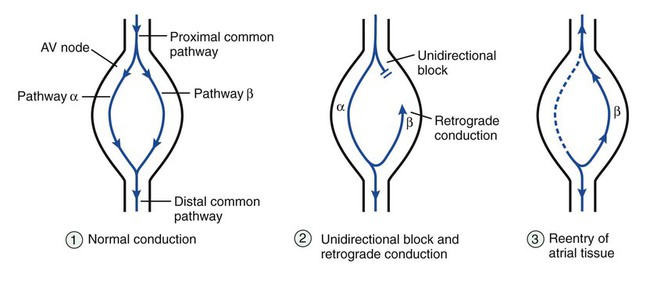
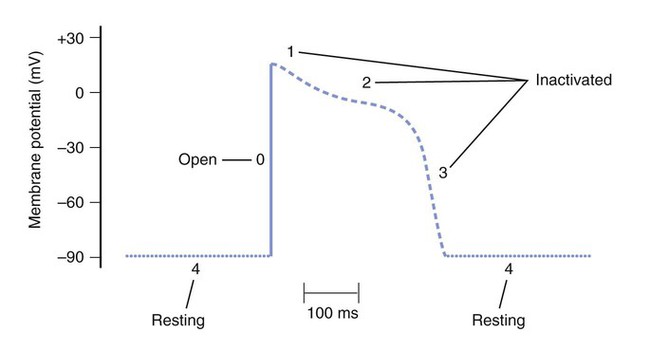
Figure 14-1 depicts the relationships among ion currents, cardiac action potentials in phases 0 through 4, and findings on the surface electrocardiogram (ECG). As shown in Figure 14-1, the surface ECG is a summation of action potentials generated by the heart during the cardiac cycle. The P wave represents atrial depolarization, whereas the PR interval corresponds to the time required to conduct the action potential through both the atria and the AV node. The QRS complex and the T wave represent ventricular depolarization and ventricular repolarization, respectively. Hence, the QT interval represents the duration of the ventricular action potential. Abnormal impulse formation can generate extrasystoles and result in tachycardia. The two mechanisms that are primarily responsible for abnormal impulse formation are increased automaticity and the occurrence of afterdepolarizations. These are depicted in Box 14-1. Abnormal impulse conduction is the basis for the formation of arrhythmias by the process of reentry, a process that involves reexcitation of a particular zone of cardiac tissue by the same impulse. Reentry is believed to be the most common mechanism responsible for the genesis of arrhythmias. It is usually caused by the presence of a unidirectional conduction block in a bifurcating conduction pathway (see Box 14-1). Class I, the largest group of antiarrhythmic drugs, consists of sodium channel blockers. These drugs bind to sodium channels when the channels are in the open and inactivated states, and they dissociate from the channels during the resting state (Box 14-2). The sodium channel blockers have the most pronounced effect on cardiac tissue that is firing rapidly, because sodium channels in this tissue spend more time in the open and inactivated states than in the resting state. This is called use-dependent blockade. Because of use-dependent blockade, sodium channel blockers suppress cardiac conduction more in a person with tachycardia than in a person with a normal heart rate. The drugs in Class I have been subdivided into three groups (IA, IB, and IC), based on whether they have greater affinity for the open state or the inactivated state and based on their rate of dissociation from sodium channels (rate of recovery). As shown in Box 14-2, Class IA drugs have greater affinity for the open state and have a slow recovery; Class IB drugs have greater affinity for the inactivated state and have a rapid recovery; and Class IC drugs have greater affinity for the open state and a very slow recovery. The Class IA drugs block the fast sodium channel and delayed potassium channels. Therefore they slow phase 0 depolarization and phase 3 repolarization in ventricular tissue (Fig. 14-2). These actions decrease the ventricular conduction velocity and prolong the ventricular action potential duration and refractory period (Table 14-1). On the ECG, this increases the QRS duration and prolongs the QT interval. Class IA drugs suppress abnormal (ectopic) automaticity, but they usually do not significantly affect SA node automaticity and the heart rate. TABLE 14-1 Electrophysiologic Properties of Selected Antiarrhythmic Drugs*
Antiarrhythmic Drugs
Overview
Cardiac Action Potentials and Electrocardiographic Findings
Pathophysiology of Arrhythmias
Abnormal Impulse Formation
Abnormal Impulse Conduction
Sodium Channel Blockers
Class IA Drugs
Drug Properties
ATRIOVENTRICULAR (AV) NODE
HIS-PURKINJE SYSTEM AND VENTRICLE
ELECTROCARDIOGRAM
Drug
Ectopic automaticity
Conduction velocity
Refractory period
Conduction velocity
Refractory period
Heart rate
PR interval
QRS duration
QT interval
Class I Drugs
Quinidine
↓
↑ or ↓
→ or ↑
↓↓
↑↑
↑ or ↓
↑ or ↓
↑↑
↑↑
Lidocaine
↓
→
→
→ or ↓
↑ or ↓
→
→
→
→ or ↓
Flecainide
↓
↓
→ or ↑
↓↓
↑
→
↑
↑↑
→ or ↑
Class II Drugs
Propranolol
↓
↓↓
↑↑
→
→
↓↓
↑↑
→
→ or ↓
Class III Drugs
Amiodarone
↓
↓
↑
↓
↑↑
↓
↑
↑
↑↑
Dofetilide
→
→ or ↓
↑
→
↑↑
→
→
→
↑↑
Sotalol
↓
↓
↑
→
↑↑
↓
↑
→
↑↑
Class IV Drugs
Verapamil, diltiazem
↓
↓↓
↑↑
→
→
↓↓
↑↑
→
→
Other Drugs
Adenosine
↓
↓↓
↑↑
→
→
↑
↑↑
→
→ ![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Antiarrhythmic Drugs
Only gold members can continue reading. Log In or Register to continue