Figure 40–1 Mechanisms of action of antifolate drugs. Sulfonamides inhibit the action of dihydropteroate synthase and thereby block the synthesis of dihydrofolate. Trimethoprim inhibits the action of dihydrofolate reductase and thereby blocks the formation of tetrahydrofolate. PABA = p-aminobenzoic acid.
Mammals must obtain folic acid in their diet because they are unable to synthesize dihydrofolate. Once absorbed, dihydrofolate is converted to tetrahydrofolate and active folate derivatives (methyl, formyl, and methylene tetrahydrofolate) that donate single-carbon atoms during the synthesis of purine bases and other components of DNA (see Chapter 17).Although folate reductase is found in both microbial and mammalian cells, the affinity of trimethoprim for the enzyme in bacteria is about 100,000 times greater than the affinity of the drug for the enzyme in mammalian cells.
Sulfonamides
In the 1930s, sulfanilamide was found to be the active metabolite of Prontosil, a dye that had been developed in the search for bacterial stains with antimicrobial properties. This discovery led to the synthesis and development of a large number of sulfonamide compounds to treat bacterial infections. Only a few of these are still used today.
CHEMISTRY AND PHARMACOKINETICS
The sulfonamides are benzene sulfonic acid amide derivatives. Most sulfonamides are adequately absorbed from the gut and are widely distributed to tissues and fluids throughout the body, including the cerebrospinal fluid. The half-lives of sulfonamides vary greatly (Table 40–1), but the most widely used compounds for treating human infections, such as sulfisoxazole and sulfamethoxazole, have half-lives ranging from 6 to 10 hours.
TABLE 40–1 Pharmacokinetic Properties of Selected Antifolate Drugs, Fluoroquinolones, and Other Agents∗
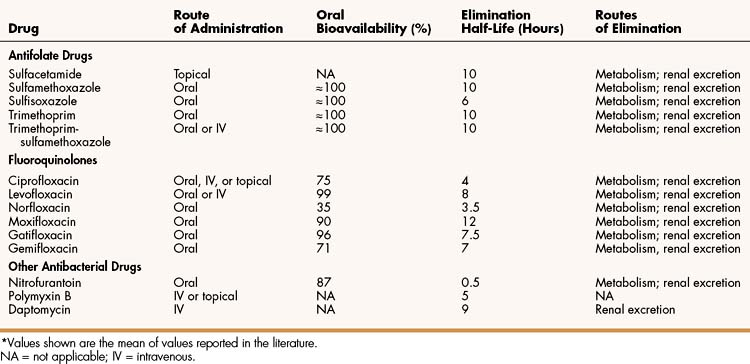
Sulfonamides are converted to inactive compounds by N-acetylation, and the parent drug and its metabolites are excreted in the urine. The acetylated metabolites are less soluble than the parent compound in urine, and they can precipitate in the renal tubules, causing crystalluria. Therefore, it is important for patients who are being treated with a sulfonamide to consume adequate quantities of water.
SPECTRUM, INDICATIONS, AND BACTERIAL RESISTANCE
The sulfonamides were the first drugs used in the treatment of systemic bacterial infections. They were once active against a wide variety of organisms, including streptococci, gonococci, meningococci, many gram-negative bacilli, and chlamydiae. Over the years, however, significant resistance to sulfonamides has developed in many bacterial species, and the antimicrobial spectrum of these drugs has been greatly reduced. Today, sulfonamides are primarily used to prevent or treat urinary tract infections (Table 40–2).
TABLE 40–2 Major Clinical Uses of Selected Fluoroquinolones, Antifolate Drugs, and Other Antibacterial Drugs
| Drug | Major Clinical Uses |
|---|---|
| Fluoroquinolones | |
| Ciprofloxacin | Bacterial diarrhea; intra-abdominal infections; infections of the urinary tract, prostate, bone and joints, skin, and eye; anthrax exposure |
| Levofloxacin | Bronchitis and community-acquired pneumonia; infections of the urinary tract, prostate, skin, and eye |
| Gatifloxacin, gemifloxacin,moxifloxacin | Community-acquired pneumonia, sinusitis, bronchitis, tuberculosis |
| Norfloxacin | Urinary tract infections |
| Antifolate Drugs | |
| Trimethoprim-sulfamethoxazole | Urinary tract and prostatic infections; pulmonary infections caused by Pneumocystis jiroveci (carinii) and Nocardia species |
| Silver sulfadiazine | Burn infections, other skin infections |
| Sulfacetamide | Ocular infections |
| Other Antibacterial Drugs | |
| Nitrofurantoin | Lower urinary tract (bladder) infections |
| Polymyxin B | Superficial infections of skin and mucous membranes |
| Daptomycin | Infections due to methicillin- or vancomycin-resistant staphylococci or vancomycin-resistant enterococci |
Sulfisoxazole or sulfamethoxazole can be used to prevent urinary tract infections or to treat uncomplicated infections of the urinary tract caused by susceptible organisms. Sulfamethoxazole is usually administered in combination with trimethoprim (see “Trimethoprim-Sulfamethoxazole”).
Sulfadiazine is available in the form of silver sulfadiazine ointment to prevent or treat burn infections and other superficial skin infections. The silver ions in this preparation have antibacterial activity and contribute to its utility in these infections.
Sulfacetamide is administered topically to treat blepharitis and conjunctivitis, ocular infections that are common throughout the world. It is also effective in treating trachoma, a highly contagious ocular infection that is caused by Chlamydia trachomatis and is prevalent in Asia and the Middle East.
ADVERSE EFFECTS
In some patients, sulfonamides cause skin rashes, which are hypersensitivity reactions that can remain mild or progress to a serious or life-threatening form, such as erythema multiforme or Stevens-Johnson syndrome. Other adverse effects of sulfonamides include crystalluria (discussed previously), gastrointestinal reactions, headaches, hepatitis, and hematopoietic toxicity. In persons with glucose-6-phosphate dehydrogenase deficiency, sulfonamides can cause hemolytic anemia.
Trimethoprim
Trimethoprim is a synthetic amino-pyrimidine drug. It is well absorbed from the gut and is widely distributed to tissues. After extensive hepatic metabolism, the remaining parent compound and metabolites are excreted in the urine.
Trimethoprim is a weak base and is concentrated in acidic prostate tissues and vaginal fluids via ion trapping (see Chapter 2). This makes trimethoprim useful in the treatment of bacterial prostatitis and vaginitis.
Trimethoprim is active against many aerobic gram-negative bacilli and a few gram-positive organisms. It is usually administered in combination with sulfamethoxazole to prevent or treat urinary tract infections (see “Trimethoprim-Sulfamethoxazole”), but it is occasionally used alone for these purposes. The adverse effects of trimethoprim include nausea, vomiting, and epigastric distress; rashes and other hypersensitivity reactions; hepatitis; and thrombocytopenia, leukopenia, and other hematologic disorders.
Trimethoprim-Sulfamethoxazole
PHARMACOKINETICS
Sulfamethoxazole and trimethoprim have synergistic activity against susceptible organisms and are available in fixed-dose combinations to treat bacterial infections. Sulfamethoxazole has been combined with trimethoprim because it has a similar half-life (10 hours). In vitro tests show that maximal synergistic activity occurs when the concentration of sulfamethoxazole is 20 times greater than the concentration of trimethoprim. To obtain plasma drug concentrations in a ratio of 20:1, the drugs are administered in a ratio of 5 parts of sulfamethoxazole to 1 part of trimethoprim. The 5:1 dose ratio produces a 20:1 plasma concentration ratio because trimethoprim has a greater volume of distribution than does sulfamethoxazole.
SPECTRUM AND INDICATIONS
Trimethoprim-sulfamethoxazole (TMP-SMX) exhibits bactericidal activity against some organisms that are not susceptible to either drug given alone. TMP-SMX is active against some members of the family Enterobacteriaceae, including strains of Escherichia coli, Klebsiella pneumoniae, Proteus species, and Enterobacter species. TMP-SMX is often used to prevent or treat urinary tract and prostate infections caused by these organisms. Because of increased bacterial resistance to TMP-SMX, it is not recommended for the empiric treatment of urinary tract infections in locations where greater than 30% of E. coli isolates are resistant to TMP-SMX. In these locations, fosfomycin, nitrofurantoin, or a fluoroquinolone drug can be used to treat these infections. TMP-SMX is not active against Pseudomonas aeruginosa, a common cause of urinary tract infections in hospitalized and nursing home patients.
TMP-SMX is also the drug of choice for treating pulmonary infections caused by Pneumocystis jiroveci (carinii) and Nocardia asteroides, which most often occur in immunocompromised patients. TMP-SMX is active against Burkholderia cepacia and some strains of Haemophilus influenzae and Moraxella catarrhalis.
TMP-SMX is active against some strains of Salmonella and Shigella, but other strains are resistant. Currently, a fluoroquinolone (see “Fluoroquinolones”) is usually preferred to treat most infections caused by these organisms.
FLUOROQUINOLONES
Fluoroquinolones have become increasingly important in the treatment of a wide range of infections because of their broad-spectrum bactericidal activity and attractive pharmacokinetic properties. The original fluoroquinolones, such as ciprofloxacin, are primarily active against gram-negative bacteria. Newer agents, such as levofloxacin, have good activity against both gram-positive and gram-negative organisms.
CHEMISTRY AND MECHANISMS
The original quinolone, nalidixic acid, had limited antimicrobial activity. Successive modification of its structure by the addition of a fluorine atom and other moieties resulted in a group of fluoroquinolones with increased affinity for DNA gyrase and improved antibacterial activity.
Fluoroquinolones inhibit two types of bacterial DNA topoisomerase: Type II topoisomerase, which is also called DNA gyrase, and Type IV topoisomerase. The topoisomerase enzymes are essential for maintaining DNA in a stable and biologically active form. DNA gyrase introduces negative supercoils (superhelical twists) into closed circular bacterial DNA. These negative supercoils eliminate the positive supercoils that occur ahead of the DNA replication fork during DNA replication. DNA replication cannot proceed without DNA gyrase activity. As shown in Figure 40–2, DNA gyrase produces supercoiling by breaking doubled-stranded DNA, moving another section of double-stranded DNA through the break, and then resealing the broken strands of DNA. DNA gyrase is a tetramer composed of two A and two B subunits. Fluoroquinolones selectively bind to the A subunits, which contain the catalytic site of the breaking and resealing reactions.
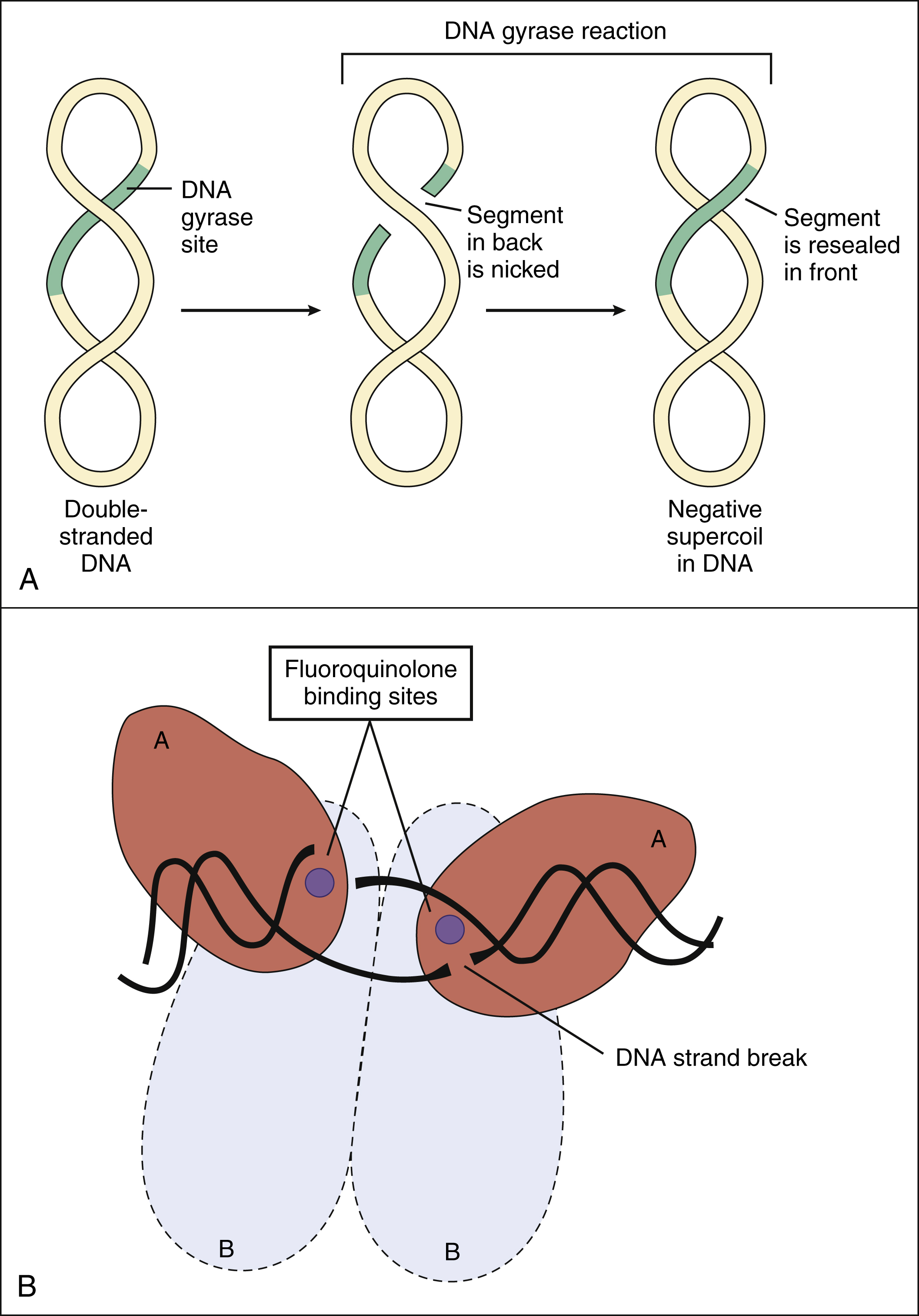
Figure 40–2 Effect of fluoroquinolones on DNA gyrase. (A) In the absence of fluoroquinolones, DNA gyrase catalyzes the formation of negative supercoils in the double-stranded DNA of bacteria. After both strands of one segment are nicked, the broken strands are passed across the other strands of DNA and then are resealed. This reaction requires energy in the form of adenosine triphosphate (ATP). (B) DNA gyrase is a tetramer composed of two A subunits and two B subunits. Fluoroquinolones inhibit DNA gyrase by binding to the catalytic sites on the A subunits. ATP binds to the B subunits.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


