CHAPTER 18
Quinidine
EDGAR R. GONZALEZ, PharmD, FASHP, FASCP
REBECCA B. GONZALEZ, PharmD
OVERVIEW
The medicinal effects of the bark of the cinchona tree have been known for over 350 years. The tree is indigenous to South America and is known as Peruvian, Jesuit’s, or Cardinal Bark. During the 1600s and 1700s, Jesuit priests imported cinchona bark from South America to Europe where it was used as a powder, extract, or infusion to treat fevers as well as “rebellious palpitation.”1,2 In the early 1800s, Pelletier and Caventou worked to isolate the more than 20 structurally related alkaloids found in the bark of the cinchona tree, quinine and quinidine being the most important ones.1-3 Pelletier and Caventou successfully isolated quinine in 1820 (see Figure 18-1).2,3
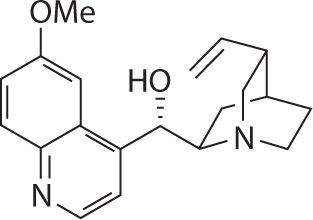
FIGURE 18-1. Chemical structure of quinine.
In 1918, Walter von Frey of Berlin reported that quinidine was the most effective of the four principal cinchona alkaloids in controlling atrial arrhythmias.2-4 Quinidine, the d-isomer of quinine, is both more potent and more toxic than quinine (see Figure 18-2).5 By the 1920s, quinidine became the drug of choice for maintaining normal sinus rhythm (NSR) in patient with atrial fibrillation or atrial flutter (Afib/flutter) and to prevent the recurrence of ventricular tachycardia (VT) or ventricular fibrillation; albeit, quinidine was known to produce a potentially lethal idiosyncratic pro-arrhythmic effect.4 In 1998, quinidine was the most frequently prescribed anti-arrhythmic agent to maintain NSR in after conversion from Afib/flutter.4 Today, quinidine is used infrequently because studies show that quinidine therapy is associated with a threefold increase in the risk of sudden cardiac death when compared with placebo or other antiarrhythmic agents, especially in patients with structural heart disease, including left ventricular dysfunction.6,7
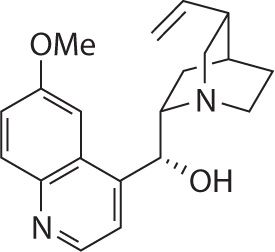
FIGURE 18-2. Structure of quinidine.
CLINICAL PHARMACOLOGY9,10
ANTIMALARIAL ACTIVITY
Quinidine is an intraerythrocytic schizonticide; it is gametocidal to Plasmodium vivax and P. malariae, but not to P. falciparum. Quinidine has minimal effects on sporozites or preerythrocytic parasites.
ANTIARRHYTHMIC ACTIVITY
Quinidine is a Class 1A antiarrhythmic agent that is devoid of negative inotropism. In cardiac muscle and in Purkinje fibers, quinidine depresses the rapid inward depolarizing sodium current, thereby slowing phase-0 depolarization and reducing the amplitude of the action potential without affecting the resting potential.10 In normal Purkinje fibers, it reduces the slope of phase-4 depolarization, shifting the threshold voltage upward toward zero. The result is slowed conduction and reduced automaticity in all parts of the heart, with increase of the effective refractory period relative to the duration of the action potential in the atria, ventricles, and Purkinje tissues. Quinidine also raises the fibrillation thresholds of the atria and ventricles, and it raises the ventricular defibrillation threshold as well.
By slowing conduction and prolonging the effective refractory period, quinidine can interrupt or prevent reentrant arrhythmias and arrhythmias due to increased automaticity, including atrial flutter, atrial fibrillation, and paroxysmal supraventricular tachycardia. In patients with the sick sinus syndrome, quinidine can cause marked sinus node depression and bradycardia. In most patients, however, use of quinidine is associated with an increase in the sinus rate secondary to quinidine’s vagolytic effects.
Quinidine prolongs the QT interval in a dose-related fashion, which may lead to increased ventricular automaticity and polymorphic ventricular tachycardias, including torsades de pointes (TdP).4-11 Intravenous quinidine is a potent α-adrenergic blocker that can precipitously lower blood pressure. These three effects can adversely affect clinical outcome in patient with cardiac arrhythmias requiring treatment with quinidine.
INDICATIONS
Today, the range of indications for quinidine is curtailed by the increased reliance on radiofrequency ablative surgery and implantable devices to correct or control cardiac rhythm disturbances; by the awareness that treatment with quinidine can increase paradoxically the risk of sudden cardiac death; and by the evolution of more effective and potentially safer antiarrhythmic compounds (e.g., sotalol and amiodarone).4,11 Quinidine may still play a useful role for patients in whom more advanced therapeutic modalities are not acceptable or contraindicated (Table 18-1). However, the use of quinidine must follow clear instruction to the patient that quinidine therapy is associated with a threefold increase in the risk of sudden cardiac death when compared with placebo or other antiarrhythmic agents, especially in patients with structural heart disease including left ventricular dysfunction as described in the FDA boxed warning (Table 18-2).6-11
| TABLE 18-1 | Use of Quinidine in Clinical Practice |
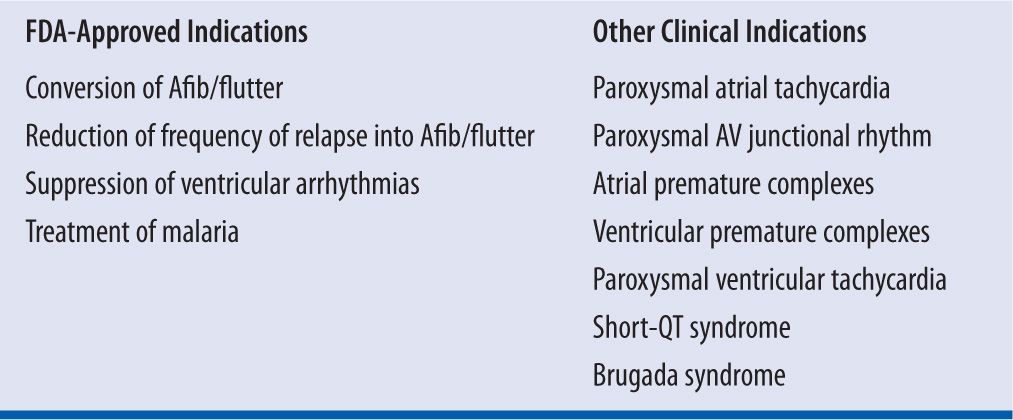
| TABLE 18-2 | FDA Boxed Warning for Quinidine |
Trials of antiarrhythmic therapy for non-life-threatening arrhythmias, active antiarrhythmic therapy resulted in increased mortality; the risk of active therapy is probably greatest in patients with structural heart disease.
In the case of quinidine used to prevent or defer recurrence of atrial flutter/fibrillation, the best available data come from meta-analysis that show mortality associated with the use of quinidine was more than three times greater than the mortality associated with the use of placebo.
Meta-analysis of data from patients with non-life-threatening ventricular arrhythmias show mortality associated with quinidine was consistently greater than that associated with the use of any of a variety of alternative antiarrhythmics.
CURRENTLY AVAILABLE DOSAGE FORMULATIONS8,9
Quinidine is formulated as either the gluconate salt or the sulfate salt. On a molar basis, 267 mg of quinidine gluconate (QG) is equivalent to 200 mg of quinidine sulfate (QS). The gluconate salt is available for oral or parenteral administration; whereas, the sulfate salt comes only in oral formulations (Table 18-3).
| TABLE 18-3 | Commercially Available Formulations of Quinidine |
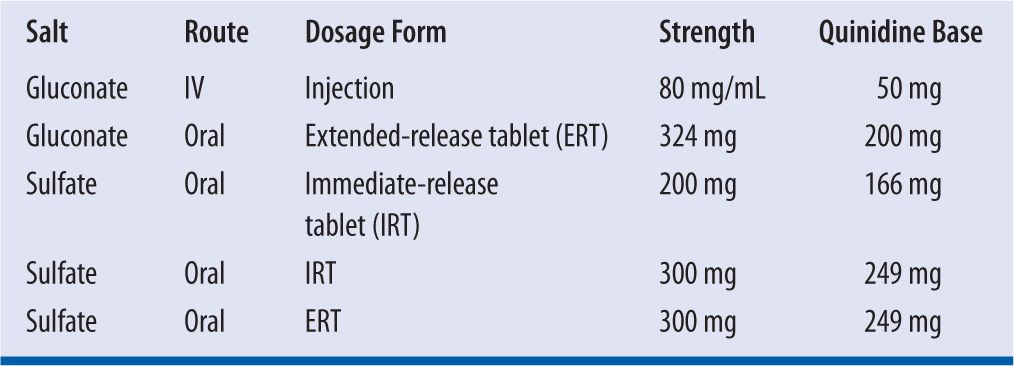
Quinidine formulations must be dispensed in well-closed, light-resistant container with child-resistant closure. Formulations of quinidine should be stored at ambient temperature (i.e., 20° to 25°C or 68° to 77°F) and should be protected from light and moisture. Parenteral quinidine gluconate is stable for 24 hours at ambient temperature and for 48 hours under refrigeration at 4°C. Approximately 3 percent of quinidine is lost to adsorption with a 12-inch polyvinyl chloride (PVC) catheter, and 30 percent of drug is lost to adsorption with a 112-inch PVC catheter.
DOSING AND ADMINISTRATION
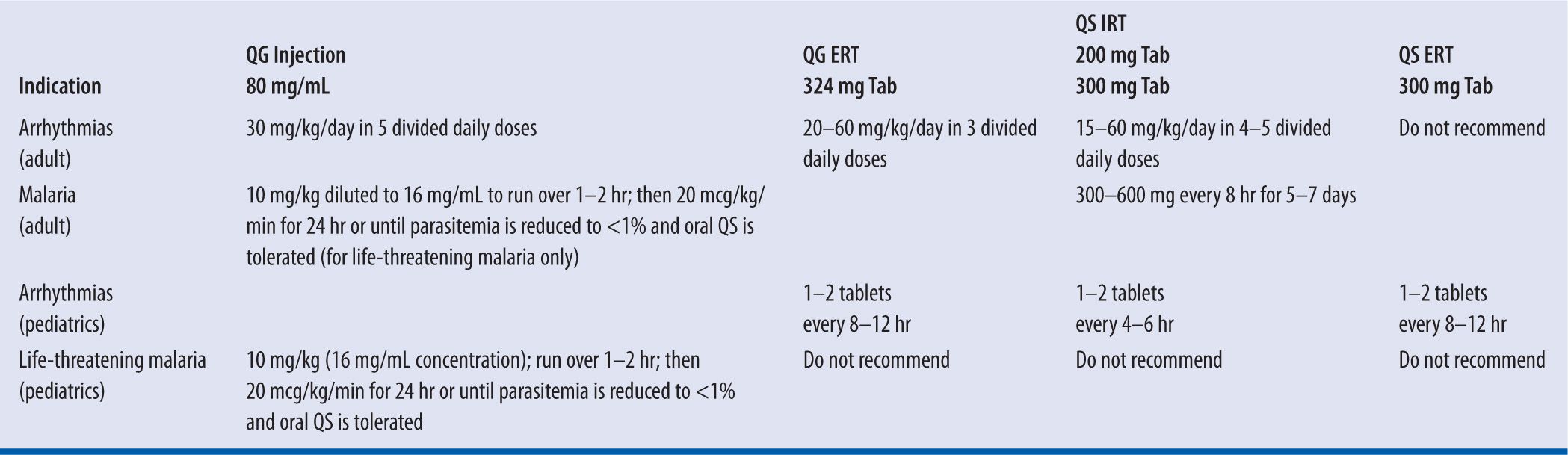
The initial dosing and administration of quinidine require careful monitoring to safeguard against the potential for an idiosyncratic fall in blood pressure and/or pro-arrhythmic effect (i.e., quinidine syncope).12 Therefore, therapy should be initiated only when the patient can be hospitalized and adequate measures are in place for continuous monitoring of vital signs and the electrocardiogram.4,13 The response to therapy should be guided by electrophysiologic testing, evaluation of the serum quinidine concentration, and assessment of the patient’s tolerance to quinidine (e.g., acute gastric distress, diarrhea). Table 18-4 lists the recommended initial dosing regimens for the various commercially available formulations of quinidine. Dosage adjustment for renal failure, liver failure, advanced age, or obesity will be discussed elsewhere in this chapter.
| TABLE 18-4 | Recommended Initial Dosing Regimens for Quinidine |
SAFETY
Cinchonism
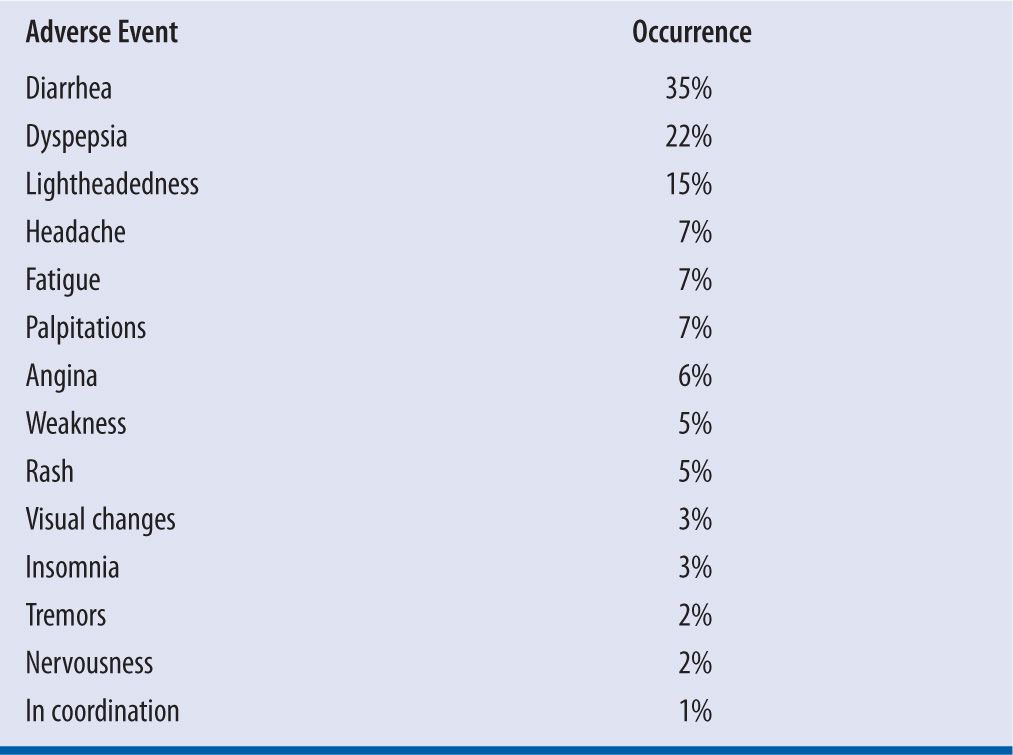
The most common side effects of quinidine are gastrointestinal complaints or the occurrence of “cinchonism” (e.g., tinnitus, headache, vertigo, fever, visual disturbances, or tremor).4 These effects occur in susceptible patients and result from localized gastric irritation and central nervous system effects of cinchona alkaloids. Table 18-5 lists side effects of quinidine that may be encountered in the clinical setting.
| TABLE 18-5 | Adverse Effects of Quinidine |
Pro-arrhythmogenicity
Quinidine-induced, concentration-dependent prolongation of the QT interval and alpha-adrenergic blockade, especially with intravenous administration, can precipitate syncope and sudden cardiac death. These effects are commonly referred to as “quinidine syncope” or quinidine associated TdP. The frequency of TdP is approximately 4.5 percent per patient year.4,11 While initially thought to be an idiosyncratic reaction, we now know that the occurrence of quinidine syncope is highest among patients who meet any of the following criteria: (1) impaired left ventricular function; (2) preexisting cardiac conduction abnormalities, such as familial QT prolongation syndromes; (3) female gender; (4) hypokalemia; or (5) underlying Afib.4,11-14 In these patients, quinidine should be used only if the benefits outweigh the risks.
PHARMACOKINETICS8,9
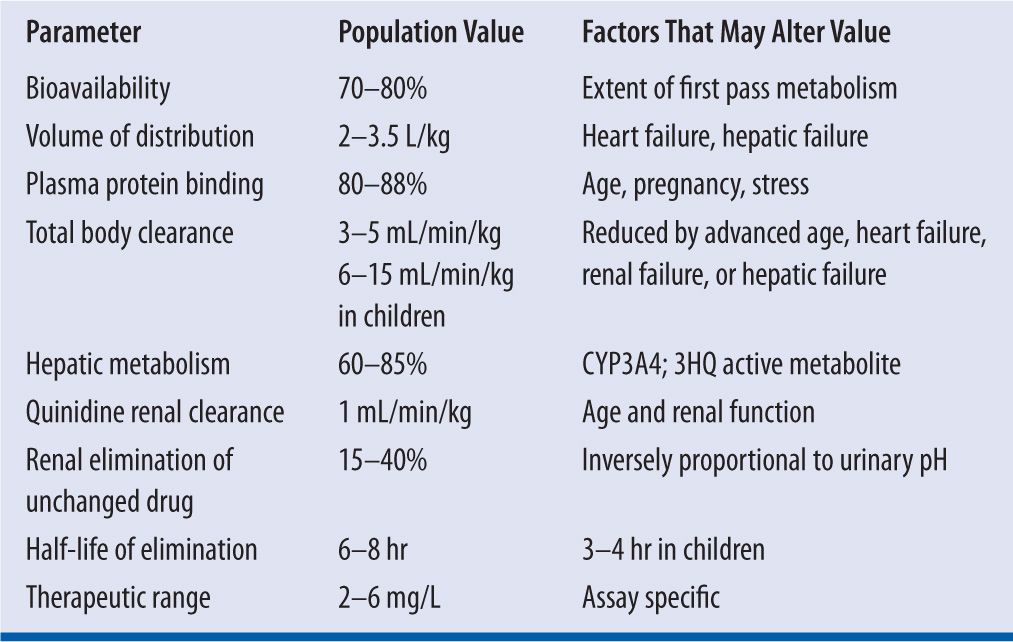
The pharmacokinetics and pharmacodynamics of quinidine have been studies extensively over the past 30 years.4,10,15 Quinidine exhibits linear pharmacokinetics in most patients.4,15,16 Table 18-6 lists the pertinent pharmacokinetic parameter for quinidine
| TABLE 18-6 | Quinidine Pharmacokinetics |
BIOAVAILABILITY
The absolute bioavailability (F) of quinidine ranges from 70 percent to 80 percent because the drug undergoes considerable first pass extraction in the liver.15 The presence of a strong first pass effect explains the wide-ranging (i.e., 45%–100%) interindividual variability in F observed with quinidine.4 The F value is unaffected by the formulation of quinidine with respect to either the salt or the use of IRT or EXT formulations. Oral absorption of quinidine following administration of IRTs is complete within two hours after administration. Serum quinidine concentrations peak approximately two hours after administration of IRTs; whereas, it takes approximately three to six hours to reach peak serum quinidine concentrations following the administration of ERT formulations. Food decreases the rate of absorption for quinidine without altering the amount of drug absorbed over time. Most importantly, F is unchanged by the presence of congestive heart failure (CHF).17
VOLUME OF DISTRIBUTION
Quinidine is distributed rapidly to most organs except the brain. The volume of distribution of quinidine (VdQ) ranges from 2 to 3.5 L/kg and is unchanged by age.16,17 However, the presence of CHF can reduce VdQ to 1.8 L/kg because of decreased perfusion of body tissues, with an associated increase in the amount of drug remaining in plasma.17 Renal disease may also decrease VdQ because of an increase in plasma protein binding (PPB) that keeps quinidine in the intravascular space.16 In contrast, liver failure and hypoalbuminemia reduce PPB and increase VdQ up to 5 L/kg. Smoking does not appear to alter VdQ.
PLASMA PROTEIN BINDING
Quinidine binds avidly to α1-acid glycoprotein and to albumin. Within the serum quinidine concentration range of 2–6 mg/L, quinidine is 80–88 percent plasma protein bound in adults and older children. Quinidine PPB falls to between 50 percent and 70 percent in neonates and infants as well as during pregnancy. Because α1-acid glycoprotein levels are increased in response to stress, the total amount of quinidine in the intravascular space will increase, but the free fraction or active fraction of quinidine (i.e., which exerts its pharmacologic effect may fall in acute stress states such a circulatory shock or acute myocardial infarction. The PPB of quinidine is increased in chronic renal failure, but binding abruptly falls toward or below normal when heparin is administered for hemodialysis because of displacement of quinidine at PPB sites by heparin. Quinidine readily crosses the placenta and is found in breast milk.18 The administration of quinidine during pregnancy can cause damage to the eighth cranial nerve in utero as well as posing a risk for transient neonatal thrombocytopenia.19
TOTAL BODY CLEARANCE AND PLASMA HALF-LIFE
Hepatic Metabolism
Approximately 60–85 percent of an administered dose of quinidine undergoes biotransformation via hepatic CYP3A4.15,16 Total body clearance for quinidine (ClQ) is 3–5 mL/min/kg in adults, and 6–15 mL/min/kg in children. The elimination half-life for quinidine (T½Q) is 6–8 hours in adults and 3–4 hours in children. Quinidine clearance is not reduced by hepatic cirrhosis.16
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


