MATERIALS FOR DIAGNOSIS
Pathologists must deal with a variety of different materials, including cytologic preparations, biopsies, surgical specimens, and frozen sections in approaching the diagnosis of pulmonary neoplasia.
Cytologic Materials
Cytologic specimens may be the only source of tumor cells in patients with inoperable disease (14). The materials for cytologic diagnosis include the following: (a) sputum smears; (b) smears prepared from bronchial washing, scraping, or brushing of the lesion; (c) smears prepared from fine-needle aspiration (FNA), either through a fiberoptic bronchoscope (transbronchial FNA) or through the chest wall (transthoracic FNA); (d) pleural effusion; and (e) pleural washing after surgical resection of the tumor (15). Materials obtained by curettage or brushing are often scant and must be fixed immediately to avoid drying artifacts, which may result in a false-positive diagnosis. Diagnostic material obtained from FNA may be superior to tissue obtained from biopsy, both in quality and quantity, particularly when obtained using a rapid on-site evaluation (ROSE) or under endobronchial ultrasonography (EBUS). Supporting this view, in several institutions, FNA represents 60% to 70% of tumor material obtained from advanced lung cancer (16). Immunohistochemistry and molecular techniques (e.g., fluorescence in situ hybridization [FISH], DNA sequencing, reverse transcriptase polymerase chain reaction [RT-PCR]) are being used increasingly as an adjunct to conventional morphology in the diagnosis as well as in the determination of predictive factors (e.g., epidermal growth factor receptor [EGFR] mutations and anaplastic lymphoma kinase [ALK] FISH testing) even on cytologic preparations (17). Cell blocks prepared from tissue fragments or with the syringe and needle rinses of aspirated material can be a useful adjunct to smears (18).
Biopsy Specimens
Biopsies (bronchial, transbronchial, or transthoracic) are usually obtained under fiberoptic bronchoscopic guidance, transthoracic ultrasonography, or CT-guided procedures. Tissue fragments should be immediately placed in fixative solution (generally 10% buffered formalin). Forceps used for transbronchial lung biopsy and needles may be washed to obtain cytologic preparations and cell blocks.
Surgical Materials
Specimens obtained either by wedge, segmental, or partial resection lobectomy or pneumonectomy should be placed in saline solution under negative pressure after the removal of staples and then in fixative. Fresh samples of tumor and normal tissue can be obtained for biobanking purposes or when tumor cell RNA is required for determination of some diagnostic or predictive biomarkers (e.g., gene expression profiling analysis or next generation sequencing). Surgical specimens should be carefully examined for tumor involvement of resection margins, vessels at the hilum, lymph nodes, extrapulmonary peribronchial soft tissues, and surfaces covering the tumor, such as pleura, thoracic wall, or diaphragm, according to standard recommendations (19). In the case of sleeve lobectomy, both resected ends of the bronchus should be carefully identified and examined. Resected lymph nodes from different locations should be submitted separately by the surgeon.
Frozen Section Materials
The correct diagnosis of a pulmonary specimen at the time of intraoperative frozen section can be very difficult and is based on experience, correct sampling, and careful correlation with the clinical, radiologic, and surgical information (20). Well-known problems include the differential diagnosis between carcinoma versus benign/reactive conditions, primary versus metastatic carcinoma, in situ versus invasive adenocarcinoma, adenocarcinoma versus carcinoid tumors versus benign/low-grade tumors such as sclerosing hemangioma, and carcinoma in situ versus reactive/dysplastic changes at the bronchial margin. Sampling errors are sometimes encountered in extensively cavitated cancers.
BENIGN EPITHELIAL TUMORS
Although rare, these lesions are encountered by pathologists more frequently than in the past due to the increased frequency of pulmonary radiographic procedures. They comprise a variety of different entities, some of which are illustrated in Figure 26.1.
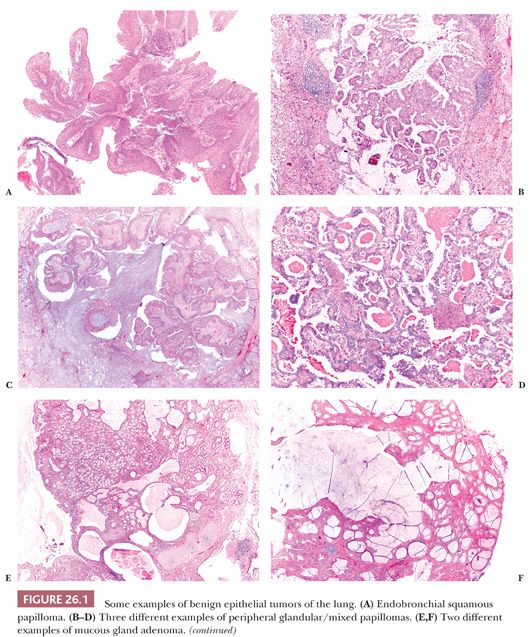
Endobronchial papillomas can be squamous, glandular, or mixed (squamous and glandular) (21). They generally exhibit exophytic growth, although rare inverted squamous papillomas are reported, and these should not be misinterpreted as invasive squamous cell carcinomas. Squamous papillomas can be multiple (papillomatosis) and may have koilocytosis related to human papillomavirus (HPV) infection and/or dysplasia. Endobronchial papillomas are benign, but rare cases of squamous cell carcinoma arising in squamous papillomas and particularly in papillomatosis have been reported (22). Glandular (but also mixed) papillomas can occasionally arise in the peripheral parenchyma, and these cases have been described under different names, probably synonyms (23–25). These peripheral glandular papillomas have a sometimes complex papillary architecture, and they are composed of ciliated, mucous, and basal cells in variable proportion. They can exhibit lepidic growth into the surrounding alveoli, simulating an adenocarcinoma. The scattered ciliated cells and the presence of p63-positive basal cells are the clues of the correct diagnosis. All reported cases pursued a benign clinical course, but until more cases are available, it is probably wise to consider peripheral glandular papillomas as low-grade malignancies and to suggest the complete surgical excision with follow-up.
Mucous gland adenoma (26) is a well-circumscribed endobronchial nodule with a variable multicystic, glandular, or papillary configuration composed of bland mucus-secreting cells. Clear, ciliated, and oncocytic cells can be seen as well. The distinction with low-grade mucoepidermoid carcinoma can be difficult and is based on the absence of intermediate cells.
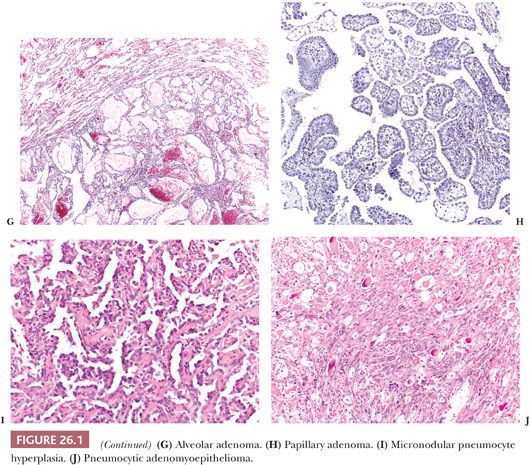
Alveolar adenoma (27,28) is a peripheral nodule composed of irregular cystic spaces containing eosinophilic material. The spongy aspect at low magnification and the well-circumscribed margins are very characteristic. The cysts are lined by bland flat to cuboidal pneumocytes, whereas the interstitium contains bland mesenchymal cells. It is not clear if the neoplastic cells are the pneumocytes, the mesenchymal elements, or both.
Papillary adenoma (29) is a peripheral neoplasm, well-circumscribed but occasionally with infiltrative margins, composed of papillae lined by bland cuboidal to columnar epithelium. The differential diagnosis includes papillary carcinoid, sclerosing hemangioma (30), and particularly well-differentiated papillary adenocarcinoma. The presence of any cytologic atypia should be considered indicative of adenocarcinoma. A peculiar papillary cystadenoma with multiple microcysts has been reported in a patient with von Hippel-Lindau disease (31).
Micronodular pneumocyte hyperplasia (32) consists of multiple small nodules, frequently arising in patients with tuberous sclerosis complex and composed of bland pneumocytes with mild interstitial thickening. Rarely, it can present as a “coin lesion” (33). The lack of cellular atypia and lepidic growth distinguishes micronodular pneumocyte hyperplasia from atypical adenomatous hyperplasia, but sometimes the limits are blurred. Recent molecular data suggest the benign neoplastic nature of the lesion (34).
Several benign salivary gland tumors can exceptionally arise as primary lung lesions, including pleomorphic adenoma, myoepithelioma, oncocytoma, and sialadenoma papilliferum (35–37). Five cases of a peculiar tumor called pneumocytic adenomyoepithelioma have been described (38). It is a peripheral, well-circumscribed nodule composed of glands lined by mildly atypical pneumocytes and filled by colloid-like secretion intermixed with bland spindle cells immunoreactive with myoepithelial markers. As a note of caution, we have seen a metastasis from an epithelial-myoepithelial carcinoma of salivary gland origin growing in the interstitium and simulating pneumocytic adenomyoepithelioma.
PREINVASIVE LESIONS
With the increasing efforts of clinicians to find lung cancer in its early stages, the chance of observing preinvasive lesions has correspondingly increased. These lesions may cause a diagnostic dilemma, particularly on frozen section and on small biopsy (39).
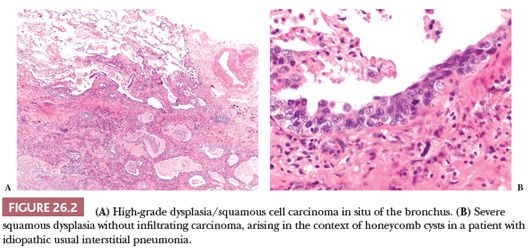
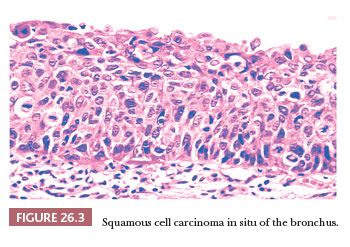
Squamous dysplasia and carcinoma in situ generally occur as single or multifocal lesions in the tracheobronchial tree. Rarely, they arise in the peripheral lung, isolated (40) or in the context of fibrosing interstitial lung diseases, particularly usual interstitial pneumonia (UIP), a condition that increases the risk of developing lung cancer (41) (Fig. 26.2). Squamous dysplasia and carcinoma in situ of the bronchus can be seen as flat or nodular lesions at bronchoscopy, are asymptomatic, and generally occur in heavy smokers. The diagnostic criteria (both on cytologic and histologic specimens) are similar to those of lesions occurring in the uterine cervix and head and neck (Fig. 26.3), with an acceptable interobserver agreement among pathologists (42). There are few data to predict the progression to invasive squamous carcinoma (43,44), and the prognostic significance of finding these lesions in isolation is currently unknown. The presence of squamous carcinoma in situ at the bronchial resection margin is an infrequent event, and it does not seem to negatively affect the recurrence and survival (45). In a small surgical series, all stump recurrences arose when carcinoma in situ extended to the bronchial gland acini, whereas no recurrences occurred when carcinoma in situ was limited to the surface epithelium or involved the glandular ducts but not the acini (46).
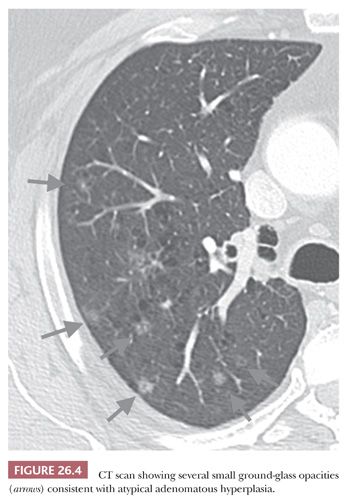
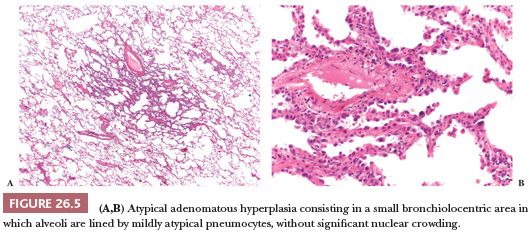
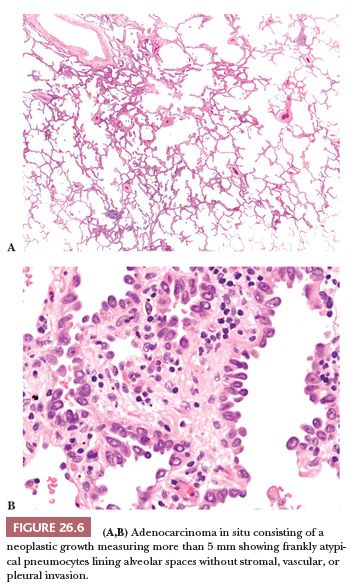
Atypical adenomatous hyperplasia (AAH) and adenocarcinoma in situ are preinvasive lesions of peripheral pulmonary adenocarcinoma (39,44,47). AAH is generally found in lungs bearing a carcinoma, particularly an adenocarcinoma, and in these cases, it has no prognostic significance (48). Rarely, it is seen in lungs without carcinoma. Grossly, AAH is difficult to localize but sometimes can be seen as small, ill-defined areas of slight discoloration, frequently multifocal, corresponding to small ground-glass opacities at high-resolution CT scan (Fig. 26.4). Histologically, AAH is a localized lesion, generally centriacinar and less than 5 mm in diameter, consisting in a proliferation of mildly to moderately atypical pneumocytes frequently associated with mild interstitial thickening (Fig. 26.5). Ciliated and mucinous cells are absent, and the background lung generally lacks significant fibrosis or inflammation. The main differential diagnosis is with adenocarcinoma in situ (Fig. 26.6). The latter is generally larger, with more abrupt transition to surrounding alveoli. Compared with AAH, the cells of adenocarcinoma in situ are more columnar with overlapping nuclei and more pronounced cytologic atypia, although atypia can be mild and is never marked. AAH and adenocarcinoma in situ are a spectrum of the same disease, and not surprisingly in some cases, the limits are blurred. Both diagnoses cannot be made on cytologic or small biopsy specimens but require a surgical specimen with complete sampling of the lesion. Adenocarcinoma in situ is further discussed in the section “Adenocarcinoma.” Interestingly, AAH and adenocarcinoma in situ are probably the precursor lesions of just a subset of infiltrating pulmonary adenocarcinomas, those having a distal alveolar phenotype. There is another subset of adenocarcinomas, frequently with a mucinous morphology and without immunohistochemical features of alveolar differentiation (negative for thyroid transcription factor-1 [TTF-1] and napsin A), that seems to arise through dysplastic changes of the bronchiolar epithelium (48–50).
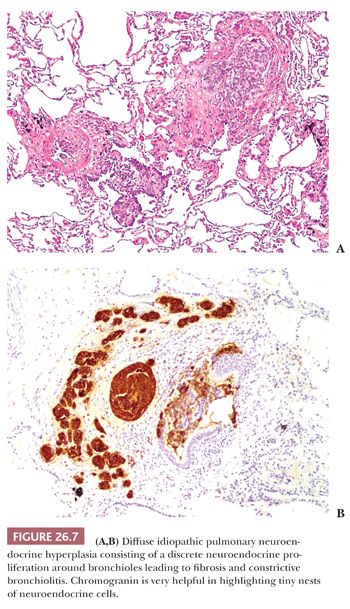
Diffuse idiopathic pulmonary neuroendocrine cell hyperplasia (DIPNECH) is an infrequent but probably under recognized entity, representing a precursor of peripheral carcinoid tumors (39,44,51). It is characterized by an increased number of isolated or small groups of neuroendocrine cells confined to the bronchioles, frequently associated with tumorlets (Fig. 26.7). DIPNECH is not infrequent in the background lung of peripheral carcinoids (52,53). In some cases, the neuroendocrine proliferation causes a fibrous obliteration of the bronchioles, and some of these patients (generally nonsmoker, middle-aged women) have clinical, functional, and radiologic evidence of small airway disease. The CT scan can be characteristic, showing the combination of mosaic attenuation and small nodules. Most patients remain stable, but progression to severe airflow obstruction may occur. The diagnosis generally requires a surgical lung biopsy, but a fortunate transbronchial biopsy showing a bronchiole with a neuroendocrine proliferation may be sufficient if the clinical context is appropriate. DIPNECH must be distinguished from secondary neuroendocrine hyperplasia frequently seen in the context of bronchiectasis or other fibroinflammatory conditions.
MALIGNANT EPITHELIAL TUMORS
SQUAMOUS CELL CARCINOMA
Epidemiology
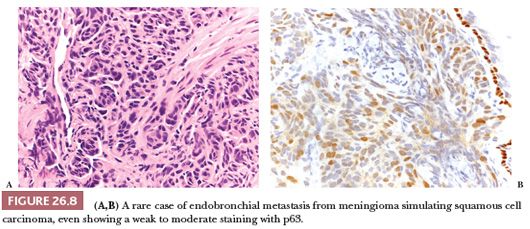
Although its frequency is declining, probably due to changes in cigarette smoking habits, squamous cell carcinoma (SCC) remains the second most frequent histotype of lung cancer after adenocarcinoma (2,13). A marked male predominance is seen, and over 90% of SCC occur in cigarette smokers: In a nonsmoker, the diagnosis of SCC should be made with extreme caution, particularly if the patient is young and/or female. Alternative possibilities such as mucoepidermoid carcinoma, poorly differentiated adenocarcinoma, or metastasis should be considered (Fig. 26.8). Rarely, SCC arise in the setting of respiratory papillomatosis, and these cases occur in the young and are associated with HPV (54). Apart from this rare condition, primary SCC of the lung is generally unrelated to HPV, and the detection of the viral genome by in situ hybridization or polymerase chain reaction favors a metastasis, particularly from uterine cervix or head and neck. It should be noted that, in the lung, p16 is not a surrogate immunomarker for the presence of HPV, being positive in 35% to 40% of lung cancers (54).
Prognosis
Staging and performance status are the main prognostic factors for pulmonary SCC, with approximately 80% 5-year survival for patients with resected stage 1 disease; nevertheless, some histologic findings have independent prognostic significance (see the following texts).
Macroscopic Features
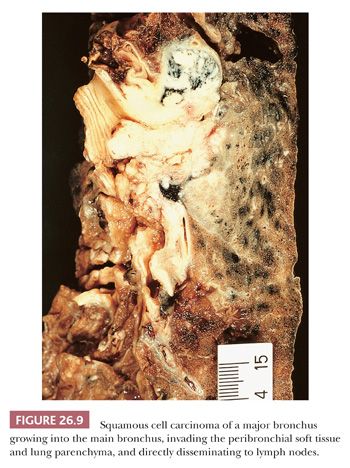
About two-thirds of SCC arise centrally in the main to segmental bronchi. In its early phase, central SCC is confined to the bronchial wall, either growing laterally along the bronchial mucosa or forming polypoid lesions, with variable submucosal infiltration (13). The prognosis for these early SCCs is excellent. With time, central SCC invades into the peribronchial soft tissue, lung parenchyma, and nearby lymph nodes. This is often visible grossly as white neoplastic tissue, frequently with associated anthracotic pigmentation (Fig. 26.9). Endobronchial tumor growth often results in secondary changes in the distal lung, including bronchiectasis, obstructive pneumonia, and atelectasis. SCC of peripheral origin has a nodular growth with well-defined borders that often show extensive coagulation necrosis and, at times, cavitation. Macroscopic cavitation is more frequent in SCC than in other histotypes of lung carcinoma. In advanced cases, peripheral SCC may involve chest wall, mediastinum, and diaphragm, but pleural carcinomatosis is quite rare.
Cytologic Features
SCC is the primary lung neoplasm most likely to be diagnosed on exfoliative cytology (sputum, brushing, washing) and FNA. Cellular features depend on the grade of differentiation, but in general, the tumor consists of keratinized malignant cells with moderate to abundant bright orange refractile cytoplasm and hyperchromatic or pyknotic nuclei, dispersed or closely packed in rigid sheets or clusters. A background of necrotic keratinized ghost cells without nuclei along with apoptotic debris may be present.
Histologic Features and Differential Diagnosis
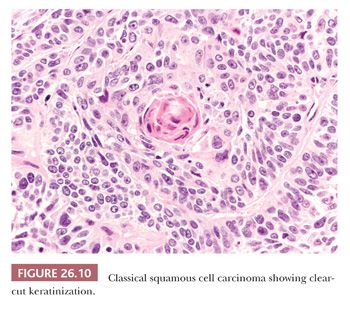
On hematoxylin and eosin (H&E)–stained sections, the defining features of SCC are keratinization with or without pearl formation and intercellular bridges (Fig. 26.10). These findings vary with the degree of differentiation, being obvious in well-differentiated tumors and focal or absent in poorly differentiated ones. We find the identification of intercellular bridges sometimes quite subjective, and we use keratinization as a more reliable criterion. In the absence of clear-cut keratinization, the diagnosis of SCC should be confirmed by immunohistochemistry, because neoplastic sheets with a “squamoid hint” can be found (and are indeed quite frequent) in poorly differentiated adenocarcinoma.
Some variants of SCC are recognized by the WHO classification (13), in particular (Fig. 26.11):
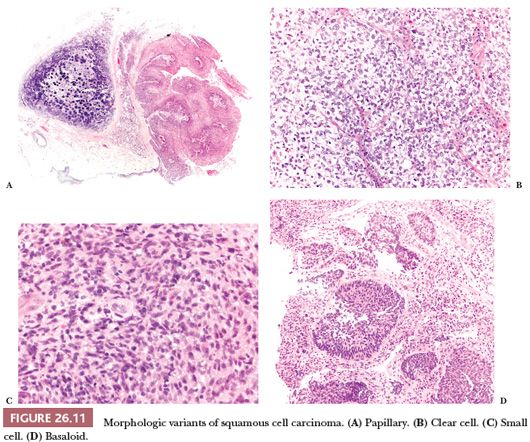
1. Papillary, a rare form of SCC presenting as an exophytic endobronchial tumor, frequently with just a minimal infiltration and a favorable prognosis (55). When infiltration is absent, the distinction with squamous papilloma is based on cytologic atypia but can be difficult and occasionally impossible, particularly on small biopsy specimens.
2. Clear cell, composed predominantly of cells with clear cytoplasm, a nonspecific finding that can be found in many different tumors and has no prognostic significance.
3. Small cell, a poorly differentiated variant consisting in small cells that retain the morphologic characteristic of SCC. In difficult cases, the differential diagnosis with small cell carcinoma requires immunohistochemistry (see “Diagnostic Immunohistochemistry”).
4. Basaloid, a poorly differentiated SCC composed of small cells arranged in a lobular pattern with peripheral palisading (56). Basaloid SCC is associated with poor prognosis (13).
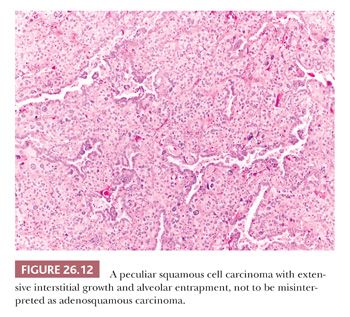
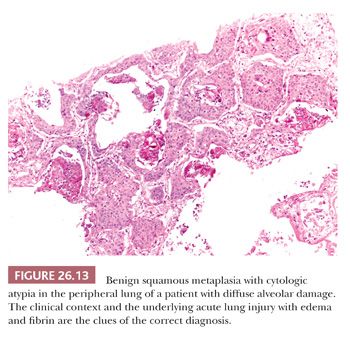
Besides these variants, others have been reported in the literature as single-case reports or small series, including acantholitic (sometimes simulating angiosarcoma) (57), microcystic (similar to microcystic adnexal carcinoma of the skin) (58), with pilomatricoma-like features (59), with sebaceous features (60), and with a lymphoid-rich background (frequently positive for Epstein-Barr virus [EBV] and sometimes simulating lymphoma) (61). Pulmonary SCC can massively infiltrate the mediastinum, raising the differential diagnosis with a primary SCC of the thymus. Although thymic SCC tend to maintain an organoid configuration and express CD5 and CD117 more frequently than SCC of the lung (62), the final diagnosis requires a careful correlation with surgical and radiologic findings. Peripheral SCC may entrap alveolar pneumocytes, and sometimes this phenomenon is extensive enough to simulate an adenosquamous carcinoma (Fig. 26.12). Occasionally in peripheral SCC, the neoplastic cells fill the alveolar spaces without destruction of the parenchymal framework: This “airspace-filling pattern” seems to be associated with a favorable prognosis (63,64). Finally, some rare reactive conditions can closely simulate SCC, including squamous metaplasia with cytologic atypia arising in diffuse alveolar damage (Fig. 26.13) (65), squamous hyperplasia overlying a granular cell tumor of the bronchus, and necrotizing sialometaplasia of the bronchial mucosa (66).
Immunohistochemistry
SCC is always strongly positive for p40, p63, and high–molecular-weight cytokeratins (CKs), and it is always negative for TTF-1 and napsin A. CK7 is positive in about 30% of SCC of the lung (11).
ADENOCARCINOMA
Epidemiology
Adenocarcinoma is the most common histotype of lung cancer worldwide. It predominates in male smokers but not infrequently occurs in women, often relatively young, and in individuals who have never smoked. A pulmonary carcinoma in a never smoker is more likely an adenocarcinoma than a squamous or a small cell carcinoma, and the probability is even higher if the patient is young, a woman, or the tumor is peripheral. Rare pulmonary adenocarcinomas developing in young people treated for pediatric malignancies are reported (67).
Prognosis
Staging and performance status are the main prognostic factors for pulmonary adenocarcinoma, with approximately 70% 5-year survival for patients with resected stage 1 disease; nevertheless, some histologic findings have independent prognostic significance (see the following texts).
Macroscopic Features
The majority of pulmonary adenocarcinomas present as a single, peripheral nodule of variable dimensions, white in color with associated anthracotic pigmentation. The borders can be lobulated or stellate, and the overlying pleura is frequently puckered. A variable amount of parenchyma with preserved alveolar architecture can be seen at the periphery, corresponding to areas of lepidic growth microscopically and to areas of ground-glass opacity on CT scan. Other macroscopic patterns include multiple nodules, areas of pneumonia-like consolidation (more frequent in mucinous adenocarcinomas), and diffuse interstitial thickening due to lymphatic spread (13). Rare pulmonary adenocarcinomas arise in central bronchi (68) or disseminate along the pleura, mimicking radiologically and macroscopically a mesothelioma (so-called pseudomesotheliomatous adenocarcinoma) (69). Finally, pulmonary adenocarcinoma may occasionally arise in the context of underlying lesions, particularly fibrosing interstitial lung diseases (41) and pulmonary airway malformations (70).
Cytologic Features
Tumor cells are often arranged in three-dimensional clusters and have a moderate amount of delicate vacuolated cytoplasm with nuclei with prominent red nucleoli. Discohesive single cells may be seen. Papillae formation and tall columnar or mucin-secreting cells sometimes arranged in glandular structures may be noted, depending on the variant and differentiation of adenocarcinoma. The finding of cilia at the top of columnar or goblet cells rules out malignancy, and the presence of ciliated cells in an otherwise atypical epithelial cluster is a helpful clue of benignancy.
Histologic Features
In the last few years, a revolution has occurred in the field of pulmonary adenocarcinoma, and the reasons are outlined earlier (see “General Comments”). The main changes proposed by the International Association for the Study of Lung Cancer (IASLC)/American Thoracic Society (ATS)/European Respiratory Society (ERS) classification of lung adenocarcinoma (12), and the rationale underlying these changes, are presented in Table 26.2.
Adenocarcinoma in situ (Fig. 26.6) is a single lesion, 3 cm or less, in which the neoplastic cells grow exclusively along preexisting alveolar structures (lepidic pattern) without stromal, vascular, or pleural invasion. Papillae or micropapillae are considered features of invasion and must be absent. Adenocarcinoma in situ is generally composed of nonmucinous cells and corresponds to the 1999 and 2004 WHO definitions of nonmucinous bronchioloalveolar carcinoma (BAC) (13,71–73). Only exceptionally it is composed of mucinous cells, and the vast majority of tumors previously classified as mucinous BAC have invasive foci and correspond now to the mucinous variant of invasive adenocarcinoma (see the following texts). Adenocarcinoma in situ is considered a precursor lesion of infiltrating adenocarcinoma, together with AAH (see “Preinvasive Lesions”). On CT scan, it produces a purely ground-glass opacity (72,73).
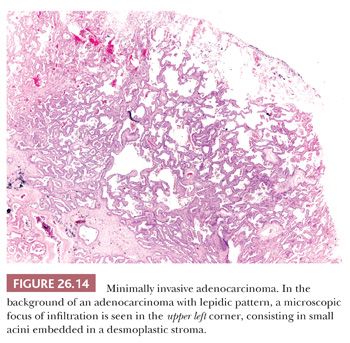
Minimally invasive adenocarcinoma is a single lesion, 3 cm or less, with a predominant lepidic pattern and with foci of invasion 5 mm or less (Fig. 26.14). If multiple foci of invasion are present, only the size of the largest invasive area should be measured. Vascular and pleural invasion and tumor necrosis must be absent. On CT scan, it is a predominantly ground-glass opacity with small solid areas, corresponding to foci of infiltration (74). As with adenocarcinoma in situ, minimally invasive adenocarcinoma is almost invariably nonmucinous.
Adenocarcinoma in situ and minimally invasive adenocarcinoma are quite infrequent, particularly in Western countries, but their frequency is rising due to the increased use of CT. Their importance lies in the fact they have an almost 100% cure rate if completely excised (74–81). Prior studies have dealt with small tumors (≤2 or ≤3 cm); there are not enough scientific data on noninvasive or minimally invasive adenocarcinomas larger than 3 cm. For these larger lesions, the term lepidic-predominant adenocarcinoma, suspect adenocarcinoma in situ, or minimally invasive adenocarcinoma, followed by a comment, is suggested (73). Of note, neither adenocarcinoma in situ nor minimally invasive adenocarcinoma can be diagnosed on cytologic preparations or small biopsy specimens. A surgical specimen with complete sampling of the lesion to rule out more significant infiltration is required. If a small biopsy specimen shows an adenocarcinoma with a purely lepidic pattern, the diagnosis “adenocarcinoma with lepidic pattern in the sampled material” is suggested, and correlation with CT scan can be helpful: If CT scan shows a purely or prevalent ground-glass opacity, the probability of adenocarcinoma in situ or minimally invasive adenocarcinoma is high; if it shows a solid nodule, the biopsy most likely sampled just the periphery of an invasive adenocarcinoma.
Invasive adenocarcinoma of the lung has an extremely varied histologic appearance, and not surprisingly in the 2004 WHO classification (13), the “mixed subtype” comprised about 90% of the cases. In the IASLC/ATS/ERS classification (12), the category mixed subtype is deleted and invasive adenocarcinoma is classified according to the predominant pattern, with reporting of the percentage of all the patterns present (in 5% increments). This better histologic stratification provides not only important prognostic information, as we will see, but is also useful when dealing with multiple tumors because their morphologic comparison helps to differentiate multiple primaries from metastases (82). Moreover, some morphologic features tend to be associated with specific molecular alterations (see “Molecular Biology in Routine Practice”).
The IASLC/ATS/ERS classification recognizes the following categories (Fig. 26.15):
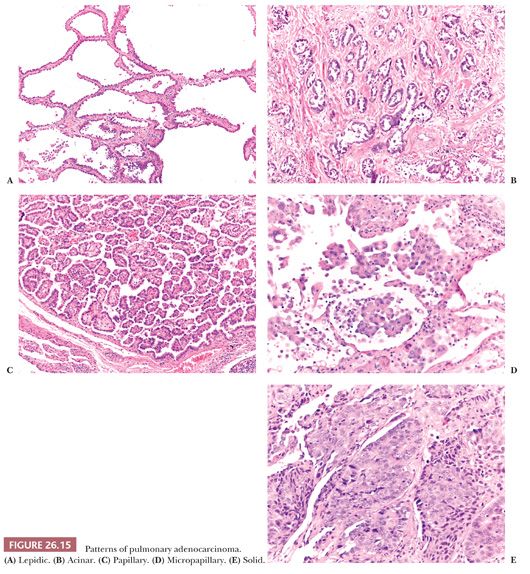
1. Lepidic-predominant adenocarcinoma is a nonmucinous adenocarcinoma with a predominant lepidic pattern and with invasive foci measuring more than 5 mm. Invasive foci are easily recognized at low power when the stroma assumes a desmoplastic character and contains irregular glands or when papillae or micropapillae are present. More subtle features of invasion are gland shapes inconsistent with alveoli (angulated, branched, or of small dimensions) and an increase in cytologic atypia. In some cases, the decision regarding the presence or absence of invasion, and consequently its quantification, can be difficult. In particular, alveolar collapse can mimic infiltrating glands, and lepidic growth along preexisting emphysematous blebs can mimic papillae. In addition, reactive changes in adenocarcinoma due to prior biopsy should not be misinterpreted as invasive foci. Several studies on small adenocarcinomas have shown the favorable prognostic impact of the lepidic pattern. More precisely, the smaller the invasive component that is seen microscopically, the better the prognosis (83–86). As a correlate, the smaller the solid component and the more the lesion is purely ground glass in appearance on CT scan, the better the prognosis (85). Invasive size evaluated microscopically seems to correlate better with prognosis than gross size (83,86), so in lepidic-predominant adenocarcinoma, the histologic report should include the dimension of the largest focus of invasion as evaluated histologically.
2. Acinar-predominant adenocarcinoma is predominantly composed of neoplastic glands. Cribriform structures are generally considered part of the spectrum of acinar adenocarcinoma, although in a recent study, cribriform arrangement was associated with a more aggressive behavior (85).
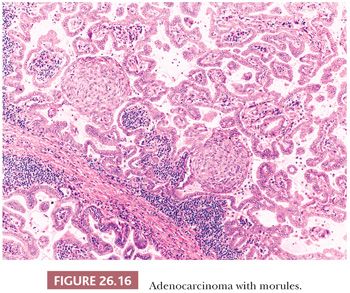
3. Papillary-predominant adenocarcinoma is mostly composed of neoplastic cells disposed along fibrovascular cores (87). Rare papillary-predominant (but also lepidic-predominant) adenocarcinomas show morules (Fig. 26.16) (88).
4. Micropapillary-predominant adenocarcinoma is mostly composed of papillary tufts. The absence of fibrovascular cores distinguishes micropapillae from papillae. Micropapillae may be connected to alveolar lumina or may “float” within alveoli and sometimes infiltrate the stroma as small clusters reminiscent of the “budding” of colonic adenocarcinoma. Many studies demonstrate an adverse prognostic effect of micropapillary foci, even when they represent as low as 1% of the tumor (89).
5. Solid-predominant adenocarcinoma is mostly composed of solid sheets, sometimes with a deceptively “squamoid hint,” and is associated with an aggressive behavior.
Several recent studies have validated the prognostic significance of this classification (90–93), with an acceptable interobserver agreement among pathologists, and have provided the basis for several grading systems (94,95). At the moment, no grading system is recommended over the others. We use the one proposed by Sica et al. (94), in which lepidic pattern is considered grade 1, acinar and papillary patterns are grade 2, and solid and micropapillary patterns are grade 3; a score is obtained summing the two prevalent grades, and this score was able to stratify patients with stage I adenocarcinoma into different risk categories.
Besides the different growth patterns, the following variants of invasive lung adenocarcinoma are recognized (Fig. 26.17):
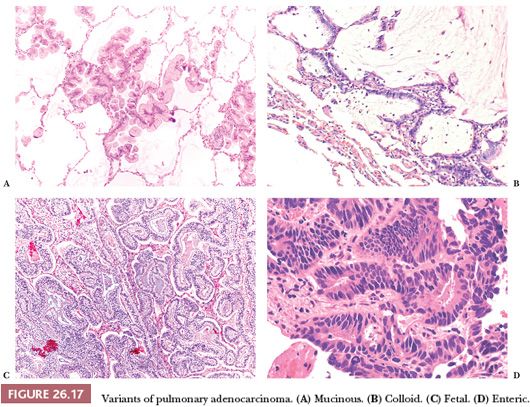
1. Invasive mucinous adenocarcinoma corresponds to the vast majority of the tumors previously classified as mucinous BAC. It is composed of columnar cells, sometimes with a goblet cell appearance. Intracytoplasmic mucin is generally abundant, but it is our impression that some acinar-prevalent adenocarcinomas composed of mucin-poor columnar cells may be part of the spectrum of mucinous adenocarcinomas, and this impression has been corroborated by a recent study (96). Cytologic atypia is minimal, and alveolar spaces are often filled with mucin. The tumor is frequently multicentric, probably due to a high frequency of aerogenous spread. By immunohistochemistry, invasive mucinous adenocarcinoma frequently coexpresses CK7 and CK20, is positive for TTF-1 and napsin A in about 30% of the cases (sometimes focally), and is negative for CDX2 (97,98). Metastases, particularly from pancreas and biliary tract, can be morphologically identical to primary mucinous adenocarcinoma (although they are always negative for TTF-1 and napsin A) (97), and the differential diagnosis requires clinical correlation.
2. Colloid adenocarcinoma (98) shows large pools of extracellular mucus filling and disrupting the alveolar spaces, containing groups of goblet cells floating in the mucus or rimming the fibrous septa. Neoplastic cells are generally few, and nuclear atypia is minimal. The colloid variant can be combined with other histotypes of lung adenocarcinoma. By immunohistochemistry, colloid adenocarcinomas frequently coexpress CDX2, TTF-1, CK7, and CK20. In our experience, the prognosis is good, although a recent study documented quite aggressive behavior (85).
3. Fetal adenocarcinoma is a rare tumor predominantly occurring in women in their fourth decade of life. It is composed of glands with clear cytoplasm and subnuclear vacuoles, resembling fetal lung tubules or endometrial glands. Squamoid morules with optically clear nuclei can be found, adding to the resemblance with endometrioid adenocarcinoma. Immunohistochemically, the neoplastic cells express nuclear and cytoplasmic staining for beta-catenin. This variant is generally low grade, but a high-grade form has been reported (99).
4. Enteric adenocarcinoma refers to those primary lung tumors that share some histologic and immunohistochemical features with colorectal adenocarcinoma (100). The resemblance to a colonic cancer varies from case to case, and it is always wise to exclude clinically a metastasis. By definition, these tumors are positive for at least one immunohistochemical marker of enteric differentiation (CDX2, CK20, or MUC2): In our experience, they frequently express CDX2 and CK7, whereas TTF-1 and CK20 are often negative, but any combination is possible.
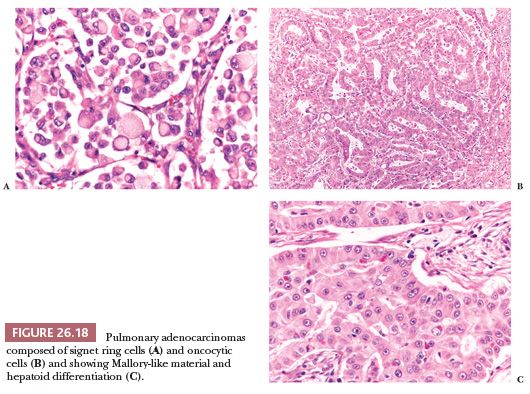
Other morphologic variations (Fig. 26.18) include lung adenocarcinomas composed (totally or in part) of signet ring cells (101), clear cells, and oncocytic cells (102) and containing eosinophilic intracytoplasmic globules (103), sometimes with a clear-cut hepatoid differentiation (104). Primary signet ring cell adenocarcinomas generally express a “lung immunophenotype” (positivity for TTF-1 and CK7, negativity for CDX2 and CK20) (98,101) and are associated with an aggressive behavior (13), although the exact percentage of signet ring cells that should be present to be clinically significant has not been definitively established. The presence of large collections of tumor cells within alveolar spaces isolated from the main mass (“tumor islands”) is another histologic feature recently associated with poor prognosis (105).
Immunohistochemistry
The vast majority of pulmonary adenocarcinomas are diffusely positive for CK7, and about 80% express TTF-1 and napsin A. p63 is occasionally positive, whereas p40 is always negative. As previously pointed out, a subset of pulmonary adenocarcinomas can express CK20 and/or CDX2.
Differential Diagnosis
In the lung, the differential diagnosis between adenocarcinoma and some reactive lesions can be very challenging, particularly at frozen section and in small biopsy specimens but occasionally on surgical resection specimens as well (106–110). The main reasons are the following: in adenocarcinoma, cytologic atypia can be minimal (and tends to fade toward the periphery of the lesion); the neoplastic cells can be few and can be obscured by fibrosis or inflammation; and some benign conditions, particularly pneumocyte hyperplasia and peribronchiolar metaplasia (111) but also entrapped alveolar epithelium and crushed macrophages or mesothelial cells in bronchoscopic biopsies (112), can be so exuberant or atypical as to mimic an adenocarcinoma. In difficult cases, the differential diagnosis is based on a constellation of findings, as illustrated in Fig. 26.19. As a first step, it is important to consider the clinical and radiologic scenario because it can favor either a reactive or a neoplastic process. At low magnification, the neoplastic glands tend to be crowded and irregularly angulated or branched. This architectural complexity contrasts with most benign processes, in which bronchioloalveolar spaces are generally more round and uniform. Papillae, cribriform structures, and cellular buds are other architectural features favoring an adenocarcinoma. At higher magnification, the cells of well-differentiated adenocarcinoma are generally columnar with overlapping nuclei and without cilia. Some atypia is frequently present but can be minimal. Importantly, atypia tends to be uniform from one cell to the other, whereas in pneumocyte hyperplasia, atypia can be marked but is not uniform, and the cells tend to be more flat/cuboidal and less crowded; the cells of peribronchiolar metaplasia are bland and at least focally ciliated. A monotonous proliferation of crowded columnar cells, without cilia, is the clue to recognize well-differentiated adenocarcinoma. The degree of atypia is particularly inconspicuous in mucinous adenocarcinoma; however, the simple presence of a row of mucinous cells without intercalated ciliated cells is diagnostic of malignancy, no matter how bland these cells are.
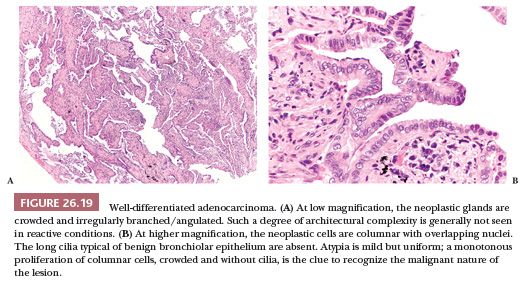
Finally, it is important to consider the histologic background. In case of an associated acute lung injury, the diagnosis of adenocarcinoma should be made very cautiously and only if the alternative possibility of a reactive process has been excluded. In difficult cases, a few immunostains can be useful. p63 or CK5/6 can assist in distinguishing adenocarcinoma from peribronchiolar metaplasia because they highlight a rim of basal cells, present in the latter but absent in the former (and also absent in reactive pneumocytes). As we have seen, some primary adenocarcinomas of the lung variably express CK20 and/or CDX2. In the lung, both antibodies are negative in reactive conditions but are also negative in the majority of primary adenocarcinomas, so they are useful only when positive.
Primary versus metastatic adenocarcinoma and adenocarcinoma versus SCC or epithelioid mesothelioma will be discussed in the following paragraphs of this chapter and in Chapter 27, respectively.
ADENOSQUAMOUS CARCINOMA
Epidemiology
Adenosquamous carcinoma comprises about 0.5% to 3.5% of surgically resected lung cancers. It may arise from the main bronchi, but most are peripheral and clinicoradiologic, and macroscopic presentation resembles that of adenocarcinoma. Patients are commonly current or former smokers (13,113).
Prognosis
The 5-year overall survival is 20% to 30% and is generally poorer than stage-matched conventional SCC or adenocarcinoma (13). The adenocarcinomatous component tends to metastasize more frequently, unless the SCC component is predominant in the primary tumor.
Histologic Features and Differential Diagnosis
The neoplasm shows clear-cut areas of SCC (keratinization, intercellular bridges) and adenocarcinoma (acini, papillae, tubules), separated or closely merging one into the other (Fig. 26.20). By convention, at least 10% of each component must be present to call a carcinoma “adenosquamous” (13).
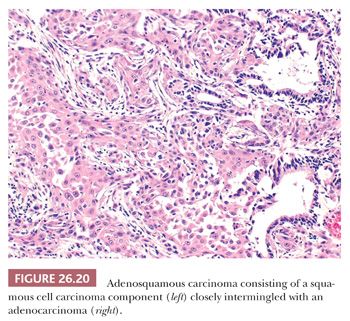
The differential diagnosis includes SCC entrapping hyperplastic alveoli or bronchiolar structures (Fig. 26.12), solid adenocarcinoma (lacking squamous differentiation), adenocarcinoma with morules, and particularly mucoepidermoid carcinoma. The latter arises from seromucinous glands of the main bronchi in younger nonsmokers and consists in a mixture of mucinous and intermediate to squamoid cells, typically without an overt squamous component. The presence of severe cellular atypia with mitotic figures should be sufficient to rule out peripherally located mixed glandular-squamous papillomas.
Immunohistochemistry and Molecular Biology
Both components express pan-CKs, but a mutually exclusive expression for markers of adenocarcinoma (e.g., TTF-1, napsin A) and SCC (e.g., p63, p40) is observed in the relevant components. The tumor is generally wild type, but in some cases, identical EGFR and KRAS mutations in both components have been found by molecular analysis (114).
NEUROENDOCRINE TUMORS
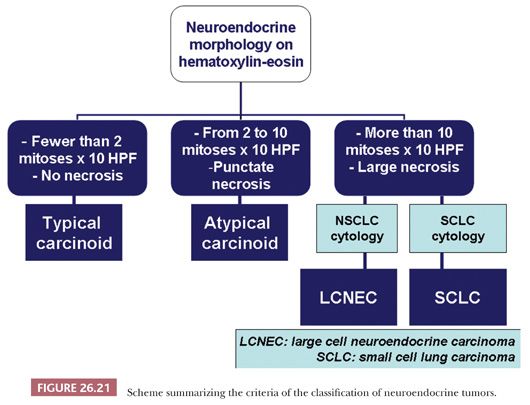
Neuroendocrine tumors of the lung are defined as having both a neuroendocrine morphology on H&E-stained sections (organoid, trabecular, insular patterns, nuclear palisading, ribbons, rosette-like formations) and neuroendocrine differentiation demonstrated by electron microscopy (membrane-bound, electron-dense cytoplasmic granules 100 to 400 nm in diameter) or most often by immunohistochemistry (expression of chromogranin A, synaptophysin, and/or CD56/neural cell adhesion molecule [NCAM]) (13). Depending on mitotic rate and necrosis, these tumors are stratified into low-grade typical carcinoid, intermediate-grade atypical carcinoid, and high-grade neoplasms, namely large cell neuroendocrine carcinoma (LCNEC) and small cell lung carcinoma (SCLC) (Fig. 26.21). This scheme has been originally proposed in 1998 by Travis et al. (115) and was subsequently accepted into the 2004 WHO classification (13). Other pathologists have proposed classifying pulmonary neuroendocrine tumors as grade 1 or well-differentiated neuroendocrine carcinoma (corresponding to typical carcinoid), grade 2 or moderately differentiated neuroendocrine carcinoma (corresponding to atypical carcinoid), and grade 3 neuroendocrine carcinoma, further subdivided into LCNEC or SCLC (116).
CARCINOID TUMORS
Epidemiology and Etiology
Carcinoid tumors account for about 2% of all lung malignancies (13,117). Epidemiologic studies have shown an increased incidence of pulmonary neuroendocrine tumors in the last decades, particularly typical carcinoids, possibly secondary to improved diagnostic skills (118). Typical carcinoid usually arises in the fourth to fifth decade with an equal sex distribution. The majority of the patients are nonsmokers (119). Atypical carcinoid is uncommon, accounting for 10% of all pulmonary neuroendocrine tumors (13,117,120). The percentage of typical versus atypical carcinoids is 85% versus 15% (121). A smoking history has been recorded in at least half of patients with atypical carcinoid. Most carcinoids are symptomatic (cough, hemoptysis, and recurrent pulmonary infections) when arising in the main to segmental bronchi, but peripheral tumors are often incidentally disclosed (13,119). Carcinoid syndrome occurs in only 1% to 5% of bronchial carcinoids and is caused by the systemic release of vasoactive substances (e.g., serotonin, adrenocorticotrophic hormone [ACTH]). Sporadic mutations of MEN1 gene have been detected in a subset of typical carcinoids (13,119–121).
Prognosis
Overall 5- and 10-year survival of patients with typical carcinoid range from 90% to 100% and from 80% to 90%, respectively, and prognosis mainly depends on tumor stage (13,115,119). Typical carcinoids with regional lymph node metastasis still have an excellent outcome (122), and vascular or lymphatic invasion does not modify classification and staging. The 5- and 10-year overall survivals for patients with atypical carcinoids range from 61% to 88% and from 35% to 67%, respectively (13,123). Carcinoids should be staged according to the seventh edition of Union for International Cancer Control (UICC)/American Joint Committee on Cancer (AJCC) staging system (124). Surgery with nodal dissection is the mainstay of the treatment. In advanced/metastatic disease, different chemotherapeutic agents (doxorubicin, 5-fluorouracil, dacarbazine, cisplatin, etoposide, streptozotocin, and carboplatin) have been investigated, reporting 20% to 30% of objective response rate (125). Somatostatin analogues, peptide receptor radionuclide therapy with radiolabeled somatostatin analogues, and mammalian target of rapamycin (mTOR) inhibitors are promising single agents or combined therapies (125).
Macroscopic Features
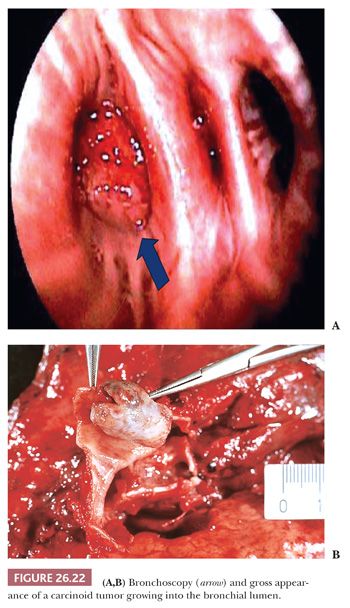
Grossly, the tumor is polypoid and endobronchial when arising in the major bronchi (Fig. 26.22), bicameral or iceberg-shaped in the intermediate bronchi, and nodular in the periphery of the lung. Tumors are generally well defined, with a smooth, sometimes lobulated or granular, ivory to pink, glistening cut surface. Necrotic foci are rarely visible at gross examination.
Cytologic Features
Typical carcinoids are characterized by hemorrhagic smears showing uniform, bland-looking, rounded-to-oval tumor cells isolated or aggregated in cohesive sheets. The centrally located type tends to show plasmacytoid features, whereas the peripheral type has spindle-shaped cells. Nuclear chromatin is finely dispersed with a “salt and pepper” appearance. Nucleoli are inconspicuous, round, and small. The background often shows fragments of delicately vascularized connective tissue with loosely attached tumor cells. The differential diagnosis between typical and atypical carcinoid may be challenging or even impossible on cytology. Greater pleomorphism, coarser chromatin with more prominent nucleoli, mitotic figures, and a necrotic background favor an atypical form.
Histologic Features and Differential Diagnosis
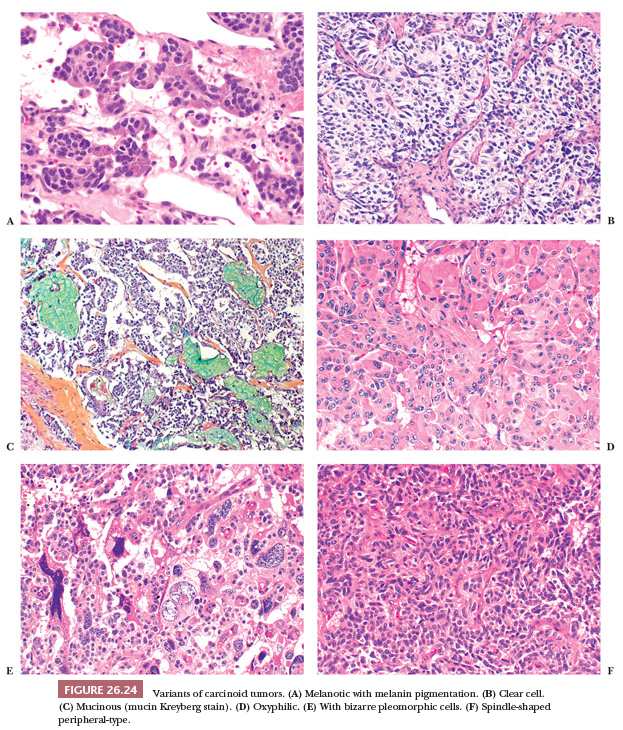
Central carcinoids may show a larger spectrum of growth patterns than the peripheral ones but usually appear as a proliferation of polygonal or round cells with abundant granular and eosinophilic cytoplasm; a central to eccentric round-shaped nucleus with finely granular chromatin; and a single, inconspicuous nucleolus (Fig. 26.23) (13,126). Endobronchial polypoid carcinoid tumors are often covered by bronchial epithelium, which may show squamous metaplasia. The lung distal to the tumor may show secondary changes of atelectasis, obstructive pneumonia, and bronchiectasis. A mixture of growth patterns including nests, solid sheets, trabeculae, ribbons, insular configurations, and rosettes (13,115,126) may be appreciated. The tumor nests are generally dissected by a delicate fibrovascular stroma with dense hyaline areas that may contain calcifications, amyloid deposits, and metaplastic bone and/or cartilage. Several histologic changes without clinical significance have been described in carcinoids: papillary, oncocytic, melanocytic type, psammoma-rich, signet ring, acinic-like, mucin-producing, and clear cell (Fig. 26.24) (126–130). Even cytologic pleomorphism does not have diagnostic or prognostic value (130).
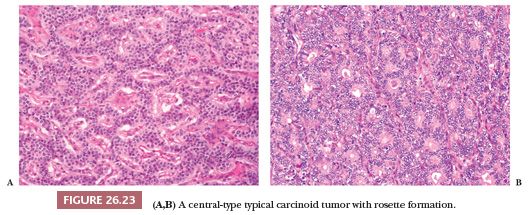
Peripheral carcinoids tend to show a prominent spindle cell growth pattern with some whorl formation and are usually associated with foci of neuroendocrine cell hyperplasia and tumorlets in the adjacent lung parenchyma (13,53). Intraoperative frozen section examination of carcinoids may be problematic when cytologic atypia and/or prominent spindle-shaped cells are present. The finding of an organoid pattern, stromal hyalinization, spindle-to-ovoid cell proliferation and finely dispersed nuclear chromatin, and the absence of extensive necrosis and high mitotic activity support the diagnosis of carcinoid (131). Distinction between typical and atypical carcinoid may prove very problematic at intraoperative consultation, and a diagnosis of pulmonary carcinoid tumor not otherwise specified (NOS) is generally sufficient for therapeutic purposes (Fig. 26.25). Distinction from metastatic tumors (mainly breast cancer and lymphomas) can be difficult as well. Only a few anedoctal cases of combined carcinoids and non–small cell lung carcinoma (NSCLC) have been reported to date, possibly deriving from different neoplastic clones (132,133).
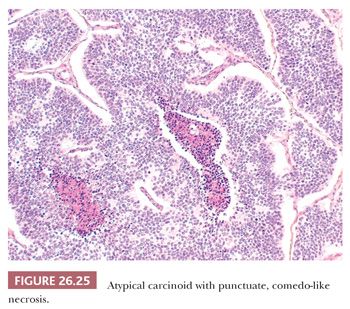
The histologic diagnosis of carcinoid tumor on surgical specimens is rarely difficult, but small bronchial biopsy specimens with crush artifacts mimicking SCLC may be challenging. The presence of mitotic figures, apoptotic debris, or a high Ki-67 index are helpful findings pointing to a high-grade neuroendocrine carcinoma (134). Central carcinoids could also be mistaken for salivary gland–type tumors, such as mucoepidermoid and adenoid cystic carcinomas. Immunohistochemistry can be useful, because the latter are negative for neuroendocrine markers. The differential diagnosis also includes pulmonary paraganglioma (expressing neuroendocrine markers but not CKs), glomus tumor (negative for CKs and neuroendocrine markers, positive for smooth muscle actin), and various metastatic tumors (in particular breast and prostate carcinoma) (135–137). Carcinoid may mimic sclerosing hemangioma, but this latter tumor has a dual cell component lacking neuroendocrine differentiation. The clinical history, the frequent presentation as multiple nodules, and some immunostains like calcitonin and CDX2 are useful to distinguish pulmonary carcinoid from metastatic neuroendocrine tumors, including gastrointestinal (GI), pancreatic, and thyroid (medullary) carcinomas. Finally, peripheral spindle cell carcinoids may be misdiagnosed as any of several spindle-shaped neoplasms, including smooth muscle tumors, spindle cell carcinoma, metastatic melanoma, and primary or metastatic meningioma. Organoid growth pattern and coexpression of CKs and neuroendocrine markers confirm the diagnosis of carcinoid tumor.
Immunohistochemistry
Chromogranin A, synaptophysin, and CD56/NCAM are the most specific and sensitive neuroendocrine markers (13,120,138). PGP9.5, CD57, and neuron-specific enolase (NSE) are additional markers of neuroendocrine differentiation, but with a very low specificity, and their use should be avoided in the diagnostic practice. Carcinoids are usually reactive for pan-CKs and CK7 but not for high–molecular-weight CKs (34betaE12, CK903, CK5/6), napsin A, p40, and p63 (120,138,139). S100 protein–positive sustentacular cells have been described in peripheral carcinoids and are generally absent in central carcinoids (140). TTF-1 expression is limited to a subset of peripheral carcinoids (141). The proliferative activity assessed by Ki-67/MIB-1 index is generally low (<5%) in typical and significantly higher in atypical forms (ranging from 5% to 20%) (120,134). Typical carcinoids tend to be negative for PAX5 as opposed to atypical carcinoids and high-grade neuroendocrine carcinomas (142). Focal to diffuse expression of estrogen and/or progesterone receptors may be detected in typical carcinoids but less frequently in other lung neuroendocrine tumor subtypes (143). Human ASH1 expression was reported in all neuroendocrine lung proliferations (144). Carcinoids generally express high levels of somatostatin receptors, particularly type 2, also displaying an activated mTOR pathway with expression of phosphorylated forms of mTOR and the downstream molecules S6K and 4EBP1 (145,146). The bcl-2 protein is infrequently expressed in carcinoid tumors.
LARGE CELL NEUROENDOCRINE CARCINOMA
Epidemiology
LCNEC is a high-grade neuroendocrine tumor sharing epidemiologic, prognostic, and genetic features with SCLC (13,147). The tumor was first described in 1991 by Travis et al. (147) and then included in the 2004 WHO classification of lung tumors as a subtype of large cell carcinoma (13). LCNEC accounts for less than 3% of all lung tumors and is closely related to cigarette smoking. Male-to-female ratio is about 4 to 5:1 and mean age at diagnosis is 60 to 65 years (13,147).
Prognosis
The outcome of LCNEC is generally dismal, with a 5-year survival ranging from 15% to 57% (mean 35%) according to different series (13,147–149). LCNEC should be staged in agreement with the seventh edition of the AJCC/UICC pTNM manual. Advanced or metastatic LCNEC should be managed as SCLC (12,13).
Macroscopic Features
LCNECs present as large peripheral masses in 80% of cases, with a mean size of approximately 4 cm. LCNEC has elevated metabolic uptake for 18F-fluorodeoxyglucose (FDG) uptake on positron emission tomography (PET) scan (13). The tumor is often lobulated with a gray-white cut surface. Necrosis and hemorrhagic areas are often seen.
Cytologic Features
The diagnosis of LCNEC is almost impossible and not recommended on cytologic preparations. Reproducible morphologic criteria are lacking. Tumor cells are usually larger than those observed in carcinoid tumors and possess prominent nucleoli, resulting in an appearance that is often indistinguishable from poorly differentiated NSCLC. Neuroendocrine differentiation should be demonstrated even on cytologic specimens (150). A well-prepared cell block may permit a better morphologic recognition of neuroendocrine features as well as reliable demonstration or neuroendocrine differentiation (Fig. 26.26).
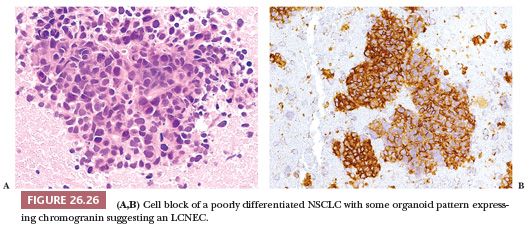
Histologic Features and Differential Diagnosis
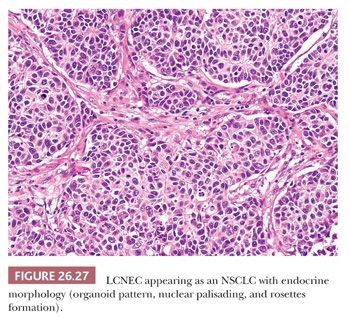
LCNEC is characterized on H&E-stained sections by organoid, nested, or trabecular growth, with nuclear palisading or rosette-like structures (Fig. 26.27). By definition, these tumors exhibit a mitotic rate higher than 10 mitotic figures per 2 mm2 and NSCLC cytologic features. However, some LCNEC contain a prominent number of smaller cells reminiscent of SCLC (151). Tumor cells have medium-to-large amounts of amphophilic cytoplasm and correspondingly low nuclear-to-cytoplasmic ratios. Nucleoli should be prominent, and foci of comedo-like or extensive confluent necrosis are commonly present. All of these morphologic features are difficult to demonstrate on small biopsy samples, and a diagnosis of LCNEC is recommended only on surgical resection specimen or a generous biopsy specimen (12,13).
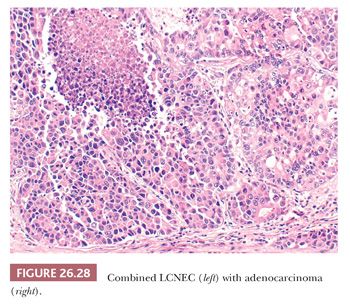
A significant number (up to 30%) of LCNEC are combined with conventional NSCLC or SCLC (Fig. 26.28) (13). The diagnosis of LCNEC needs a strict application of the recommended criteria and can be posed only on high-grade carcinomas showing neuroendocrine features on H&E-stained sections, associated with the immunohistochemical expression of neuroendocrine markers or neuroendocrine granules by electron microscopy. To complicate matters, it is important to know that there are large cell carcinomas with apparent neuroendocrine features by light microscopy that lack neuroendocrine differentiation when tested by immunohistochemistry or ultrastructural examination. Conversely, large cell carcinomas may have neuroendocrine differentiation by immunohistochemistry or ultrastructure but lack neuroendocrine morphologic features. At present, there is no consensus on how to classify these tumors, and they should be treated as conventional NSCLC. Agreement on LCNEC diagnosis is low, even in the hands of expert pulmonary pathologists (151). The differential diagnosis for LCNEC mainly includes atypical carcinoid and SCLC, and this distinction may be impossible on small biopsies or cytology specimens. In these cases, the proliferative index by Ki-67/MIB-1 may be very helpful, and values exceeding 25% generally favor LCNEC over carcinoids. Distinction between LCNEC and SCLC is sometimes impossible even on surgical specimens, and the term high-grade neuroendocrine carcinoma or combined SCLC–LCNEC is recommended in controversial cases. Different non-SCLCs (adenocarcinomas, SCC, and basaloid carcinoma) may show a solid or cribriform growth pattern with some palisading or pseudorosettes and some neuroendocrine differentiation. The correct diagnosis is based on the strict application of morphologic criteria with the use of neuroendocrine markers only in tumors with overt neuroendocrine morphologic features. Distinguishing primary LCNEC of the lung from identical extrapulmonary tumors may be nearly impossible because TTF-1 stains only half of LCNEC and its expression has been reported in high-grade neuroendocrine carcinomas from other sites. CDX2 expression may be noted in some pulmonary high-grade neuroendocrine carcinomas (152). Clinical correlation is highly recommended in such cases.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


