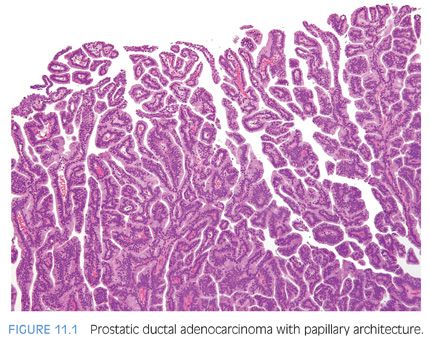
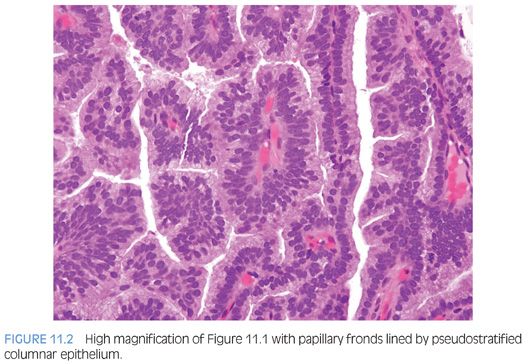
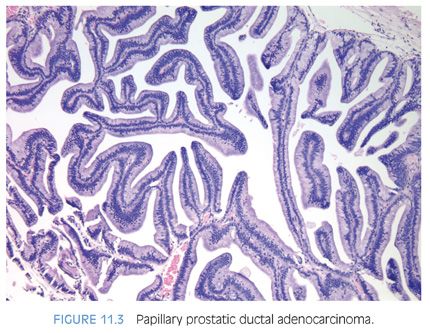
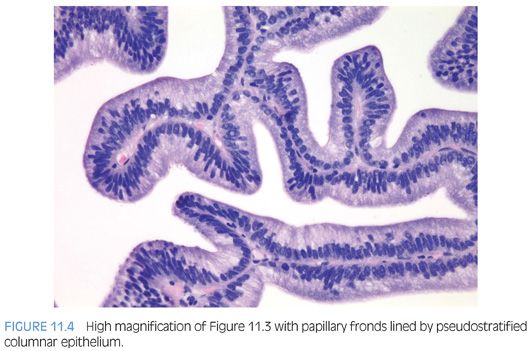
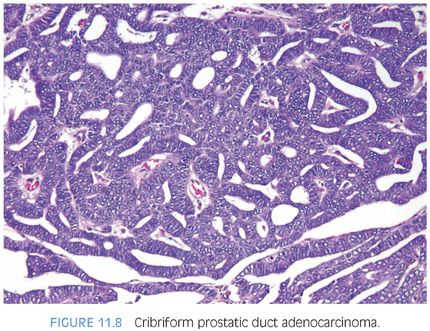
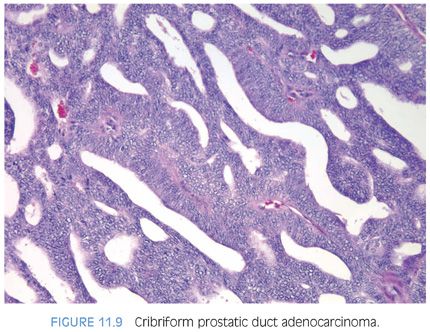
The cribriform pattern of prostatic duct adenocarcinomas is more commonly seen deeper within the tissue, although it may also be noted in the exophytic urethral component of the lesion (Figs. 11.8 and 11.9). The cribriform pattern is formed by back-to-back large glands with intraglandular epithelial bridging resulting in the formation of slit-like lumens. The epithelial lining is composed of pseudostratified tall columnar epithelium often with amphophilic cytoplasm. The pattern is somewhat reminiscent of endometrial adenocarcinoma within the female. This pattern of prostatic adenocarcinoma differs from the cribriform pattern of prostatic acinar adenocarcinoma, which is composed of cuboidal epithelium and punched-out round lumina. It is not uncommon to find areas of papillary formation admixed with cribriform patterns.
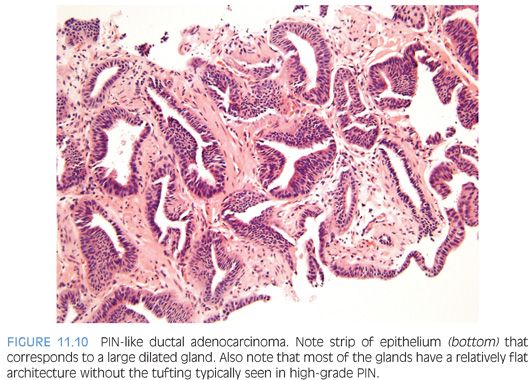
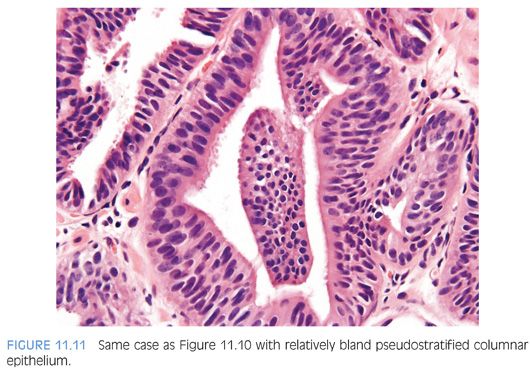
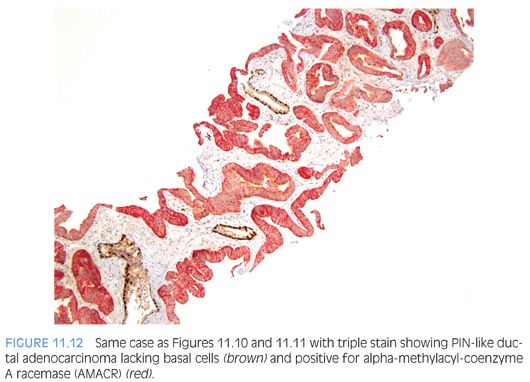
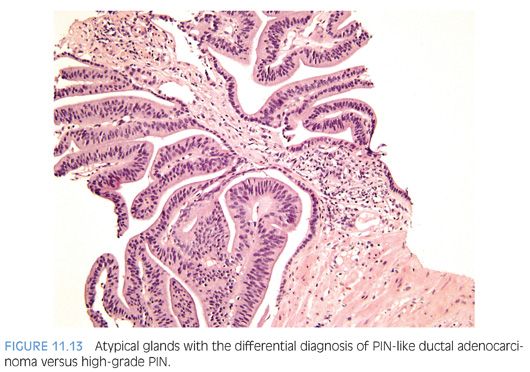
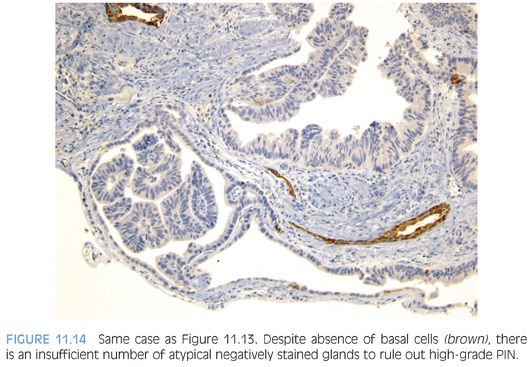
The most recently described common pattern is prostatic intraepithelial neoplasia (PIN)–like ductal adenocarcinoma (also see Chapter 5).14,15 This variant consists of simple, often cystically dilated glands lined by pseudostratified columnar epithelium, definition of ductal adenocarcinoma (Figs. 11.10 to 11.12). On needle biopsy, the cystic nature of the glands can be discerned by strips of columnar epithelium lining the edge of the cores. The glandular lining is often flat, lacking the papillary or cribriform morphology initially described in ductal adenocarcinoma. As with other patterns of ductal adenocarcinoma, there can be a spectrum of cytologic atypia, although typically, PIN-like ductal adenocarcinoma lacks diffuse prominent nucleoli. Glands of PIN-like ductal adenocarcinoma can be more crowded than high-grade PIN or can be more spaced apart, more closely mimicking high-grade PIN. As a few glands of high-grade PIN can be negative for basal cell markers, one needs many negative glands in order to diagnose PIN-like ductal adenocarcinoma. Cases with only a few glands morphologically suggestive of PIN-like ductal adenocarcinoma are diagnosed as “atypical glands with the differential diagnosis of PIN-like ductal adenocarcinoma versus high-grade PIN. Repeat biopsy is recommended” (Figs. 11.13 and 11.14).
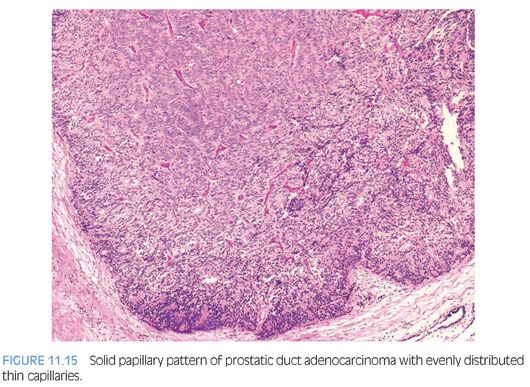
Other patterns of prostatic duct adenocarcinoma, which by themselves may be difficult to identify as being of prostatic duct origin, may be seen in association with either the papillary or cribriform pattern. Occasionally, solid tumor masses with numerous thin-walled vessels distend prostatic ducts (Fig. 11.15). This pattern is a compact papillary form of prostatic duct adenocarcinoma, because areas can be seen where the solid pattern containing these thin fibrovascular cores open up into more recognizable papillary structures. Prostatic duct adenocarcinomas may also grow as solid nests of tumors with central necrosis. Without seeing this solid pattern in association with more recognizable prostatic duct adenocarcinoma, this pattern cannot be distinguished from poorly differentiated prostatic acinar adenocarcinoma. Prostatic duct adenocarcinomas may resemble infiltrating colonic adenocarcinoma. The differentiation between prostatic duct adenocarcinoma and secondary involvement of the prostate by colonic adenocarcinoma can be made by finding more typical prostatic duct adenocarcinoma elsewhere within the biopsy as well as by immunohistochemical demonstration of PSA and other prostate markers in ductal adenocarcinoma. Adding B-catenin, CDX-2, and villin for colon cancer to the immunohistochemistry panel can be of further use in such a differential.16,17 However, one must be aware that rare cases of prostatic ductal adenocarcinoma can diffusely express CDX2.18 TMPRSS2-ERG gene fusions are seen to a lesser frequency than with acinar carcinoma.19 Prostatic duct adenocarcinoma on TUR specimens can also mimic papillary urothelial carcinoma. Nuclear features can be helpful in such differential; nuclei in urothelial carcinoma tend to be more pleomorphic and angulated. Immunohistochemical demonstration of PSA and other prostate markers and negative thrombomodulin and GATA3 staining in prostatic duct adenocarcinoma can also be of help.17,20–22 Rarely, prostatic ductal adenocarcinoma can be focally lined by mucinous epithelium, although typically, this finding suggests extension from an intestinal primary (Fig. 11.16). Rare variations of ductal adenocarcinoma include those with goblet cells; foamy; and containing Paneth cell-like, micropapillary, and cystic features.23
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


