Microscopically, the prostate is composed of glandular epithelium and fibromuscular stroma. The duct and glandular system is arranged in a complex architectural pattern. The ducts consist of elongated branching tubular structures and end blindly in rounded acini. Ducts cut in cross section are indistinguishable from acini. The luminal surfaces of benign prostate glands often have undulating contours and papillary infolding. These glands are lined by three distinct epithelial cell populations: secretory, basal, and neuroendocrine cells. The luminal secretory cells are terminally differentiated and stain positively with PSA, prostatic acid phosphatase, and other enzymes. Nonneoplastic secretory cells may also contain acid and neutral mucins, lipofuscin, and melanin (6–8). Basal cells are peripherally located in the gland between the secretory cells and basement membrane. They are cigar-shaped with the long axis parallel to the basement membrane. The basal cells may have either a finely granular, uniformly distributed chromatin pattern or prominent nucleoli and show cytoplasmic staining with high–molecular-weight keratin or nuclear reactivity for p63 (clone 4A4) (9–12). Basal cells are thought to represent the stem cell compartment within the prostate (13). Neuroendocrine cells are irregularly distributed throughout the ducts and acini and are difficult to recognize without the use of special staining techniques. Their exact function is unknown, but it is thought that they function in a paracrine fashion to regulate adjacent cells.
BENIGN PROSTATIC HYPERPLASIA
The prostate slowly enlarges from birth to puberty. Thereafter, the prostate rapidly increases in size until the age of 21 to 30 years, at which point the prostate weighs 20 g on average (14). The frequency of benign prostatic hyperplasia (BPH) is extremely low in men younger than 40 years, after which its development increases steadily to the point at which BPH is the most common urologic disorder in men. Symptomatic BPH increases proportionately with age.
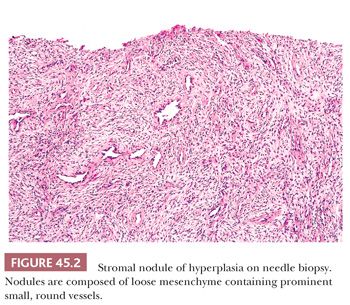
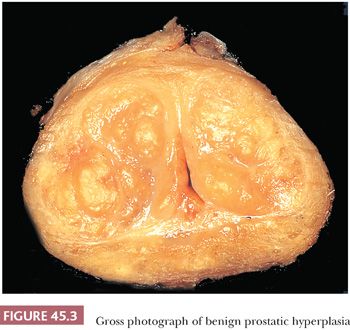
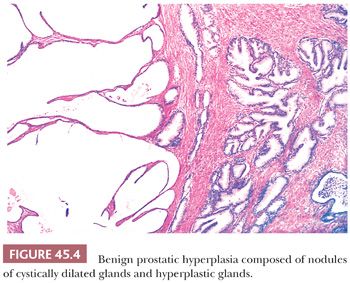
The earliest BPH nodules are predominantly stromal and are composed mainly of fibrous tissue admixed with some smooth muscle (Fig. 45.2) (5,15). In contrast to the myxoid variant of stromal tumor of uncertain malignant potential (STUMP), which consists of diffuse sheets stroma without glands, stromal-predominant BPH shows nodules of stroma with characteristic cross sections of thick-walled arterioles. These stromal nodules are located submucosally in the periurethral region and seldom reach large size except near the bladder neck, where they may protrude as a solitary midline mass into the bladder lumen. The larger and more numerous BPH nodules are typically more laterally situated within the transition zone (Fig. 45.3). Less commonly, BPH nodules may be found within the peripheral zone. These laterally placed nodules are predominantly glandular from inception and are the cause of most clinically evident BPH. Histologically, the glandular component is made up of nodules of small and large acini lined by basal and secretory cells. Some glands show papillary infolding and projections containing central fibrovascular cores and others that are dilated and cystic (Fig. 45.4). Crowded epithelial nodules have been labeled adenosis (atypical adenomatous hyperplasia). The stromal component often shows both fibrous and smooth muscle elements. Benign needle biopsy specimens of the prostate should be diagnosed as “benign prostate tissue,” not as “benign prostatic hyperplasia.” First, many needle biopsy specimens do not even sample the transition zone. Second, histologic findings on needle biopsy, with the exception of stromal nodules, do not correlate with size of the prostate or urinary obstructive symptoms (16). Finally, signing out a specimen as benign prostatic hyperplasia may falsely reassure the urologist that a palpable or hypoechoic lesion of concern has been sampled.
Within areas of BPH, nodules or a diffuse stromal infiltrate of lymphocytes and plasma cells are commonly seen in a periglandular distribution. However, in the majority of cases, these findings have not been identified with an infectious process or clinical symptoms of prostatitis (17,18). Although acute inflammation appears to correlate with serum PSA increases, there are conflicting studies as to the effect of chronic inflammation (19,20). Because the histologic finding of inflammation does not correlate with clinical prostatitis, we do not sign out surgical specimens as showing “acute prostatitis” or “chronic prostatitis” but, rather, as showing “acute inflammation” or “chronic inflammation.”
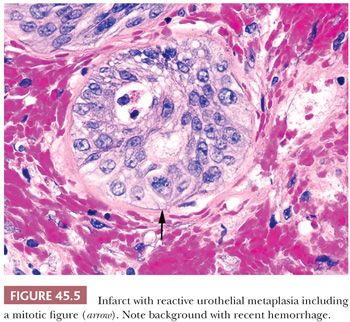
Prostatic infarcts can be found in approximately 20% to 25% of specimens removed for BPH and, more rarely, on needle biopsy, ranging in size from a few millimeters to 5 cm (15,21,22). In the acute phase, discrete foci of coagulative necrosis involve the epithelium and connective tissue. Healed infarcts can be identified by areas of dense scar formation. Patients with acute prostatic infarcts have glands twice as large as those in patients without infarcts and are more prone to have acute urinary retention or histories of gross hematuria. Because the infarcts often are small and not close to the urethra, the symptoms may not result from the infarcts but, instead, may result from the larger size of the glands containing them. Immediately adjacent to the infarcts, reactive epithelial nests with prominent nucleoli, some pleomorphism, and even atypical mitotic figures can be seen, mimicking urothelial or squamous cell carcinoma (Fig. 45.5). Progressing away from the center of the infarct, more mature squamous metaplasia is seen. These nests are distinguished from carcinoma by the localized nature of the process immediately adjacent to the infarct. On needle biopsy, it is harder to appreciate the zonation and recognize the background stromal fibrosis, hemosiderin deposition, and extravasated red blood cells.
PROSTATIC INFECTIONS
BACTERIAL PROSTATITIS
Although acute and chronic prostatitis comprises a significant portion of urologic practice, they are usually diagnosed clinically and treated with antibiotics, without the need for surgical intervention. Consequently, the histologic examination of specimens removed for symptomatic prostatitis is uncommon. It is difficult to distinguish histologically chronic infectious prostatitis from nonspecific chronic inflammation seen in hyperplasia, but acute bacterial prostatitis is characterized by sheets of neutrophils within and around acini, intraductal desquamated cellular debris, and stromal edema and hyperemia. Although microabscess formation is not uncommon, effective antibiotics have led to a vast decline in the incidence of symptomatic prostatic abscesses (23).
MYCOTIC PROSTATITIS
Of the deep mycoses affecting the prostate, the most common are blastomycosis, coccidioidomycosis, and cryptococcosis. Cases also have been reported of histoplasmosis, paracoccidioidomycosis, aspergillosis, and candidiasis of the prostate (24). Most, if not all, cases of mycotic prostatitis occur in the setting of systemic hematogenous dissemination in immunocompromised hosts.
TUBERCULOUS PROSTATITIS
The incidence of prostatic involvement in systemic tuberculosis ranges from 3% to 12%, with more than 90% of these cases also showing lung involvement (25,26). Systemic tuberculous prostatitis is rarely seen in today’s surgical pathology practice as a result of (a) the declining incidence of the disease and (b) the presence of other nonprostatic signs and symptoms of infection leading to diagnosis and treatment. More commonly, tuberculous involvement of the prostate is seen following bacillus Calmette-Guérin (BCG) immunotherapy for superficial urothelial cancer of the bladder. The granulomas may be small, noncaseating, and close to the urethral surface, or large, caseating, and throughout the prostate. The latter can result in an abnormal rectal examination or increased serum PSA levels (27,28). Regardless of the histologic pattern of BCG-related granulomatous prostatitis or the presence or absence of acid-fast bacilli on special stains, most patients have remained asymptomatic and require no specific therapy. It is not necessary in a man with a history of BCG therapy to perform stains for acid-fast organisms to evaluate prostatic granulomas; it is debatable whether stains for fungi should be done for completeness. Patients are monitored for systemic signs and symptoms because disseminated tuberculosis after BCG has been reported on rare occasions.
MISCELLANEOUS INFECTIONS
Other rare infections involving the prostate, some of which are more commonly seen in developing countries, include brucellosis, schistosomiasis, amebic prostatitis, syphilis, actinomycosis, infection by atypical mycobacteria, echinococcosis, cytomegalovirus infection, and herpes zoster infection (29–37).
NONINFECTIOUS INFLAMMATORY CONDITIONS
NONSPECIFIC GRANULOMATOUS PROSTATITIS
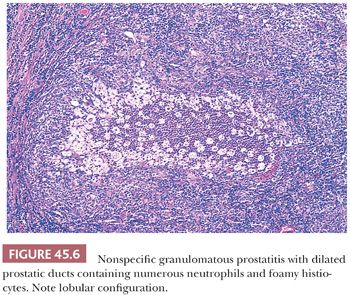
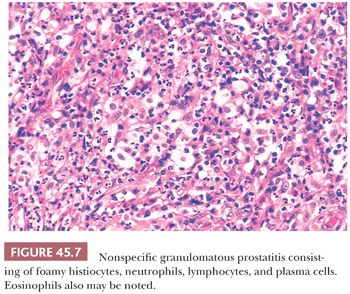
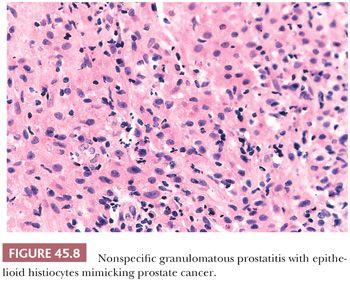
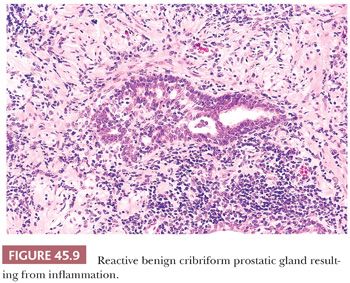
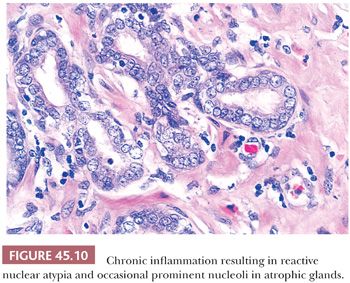
The most commonly diagnosed noninfectious granulomatous process within the prostate is termed nonspecific granulomatous prostatitis (38,39). The lesion consists of a lobular, dense infiltrate of lymphocytes, plasma cells, and histiocytes (Fig. 45.6). Many of the histiocytes have a foamy appearance, and some are multinucleated. Neutrophils and eosinophils make up a smaller component of the inflammatory infiltrate (Fig. 45.7). These dense nodules of inflammation tend to obscure and efface ductal and acinar elements. The earliest lesion of nonspecific granulomatous prostatitis consists of dilated ducts and acini filled with neutrophils, debris, and desquamated epithelial cells (Fig. 45.6). Focal rupture of these ducts and acini results in a localized granulomatous and chronic inflammatory reaction. The extension of the infiltrate into surrounding ductal and acinar units accounts for the characteristic lobular pattern of fully developed nonspecific granulomatous prostatitis. In most cases, there is little histologic similarity between nonspecific granulomatous prostatitis and infectious granulomatous inflammation of the prostate, and special stains for organisms are not necessary in these examples. Although discrete small granulomas can be seen with nonspecific granulomatous prostatitis, they are always seen with the early lesion surrounding a ruptured, dilated duct or acinus. In contrast, early infectious noncaseating granulomas surround intact acini. Areas of necrosis within the center of small lesions of nonspecific granulomatous prostatitis contain numerous neutrophils rather than caseation. Older lesions of nonspecific granulomatous prostatitis show a more prominent fibrous component. Nonspecific granulomatous prostatitis is thought to result from a local foreign body reaction to ruptured prostatic secretions and contents and tends to resolve spontaneously. Clinically, the symptoms are those of either urinary obstruction or a severe lower urinary tract infection. The major significance of this lesion is that the rectal examination in nonspecific granulomatous prostatitis commonly reveals nodularity or induration and serum PSA levels may be elevated such that carcinoma of the prostate is often suspected. Nonspecific granulomatous prostatitis, in particular cases with prominent epithelioid histiocytes, may be misdiagnosed as adenocarcinoma of the prostate on needle biopsy (Fig. 45.8) (40). The finding of plasma cells, neutrophils, and eosinophils helps to identify the lesion as prostatitis. The clustering of the epithelioid histiocytes around partially ruptured acini, which sometimes may be appreciated on needle biopsy, contrasts with the diffuse nature of high-grade prostate cancer. If difficulty persists, immunohistochemical (IHC) stains for histiocytic and epithelial markers can differentiate the two entities (41). As a result of the inflammation, reactive architectural and cytologic atypia may occur (Figs. 45.9 and 45.10).
POSTBIOPSY GRANULOMAS
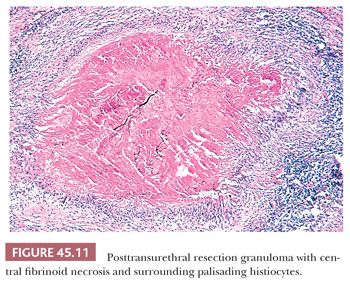
Prostatic granulomas are frequent sequelae after transurethral resection. Postbiopsy granulomas are composed of a central region of fibrinoid necrosis surrounded by palisading epithelioid histiocytes (Fig. 45.11) (38). Although lesions can assume a multitude of round, ovoid, triangular, and rectangular shapes, a somewhat characteristic configuration is that of long, tortuous granulomas dissecting the tissue. In the tissue surrounding the necrobiotic granulomas, multinucleated giant cells and granulomas without central necrosis are common findings. Inflammation surrounding the granulomas is usually minimal, consisting predominantly of lymphocytes and plasma cells with scattered eosinophils. The posttransurethral resection (post-TUR) intervals with which these granulomas may be identified range from 9 days to 52 months. When the interval between TUR resections is approximately 1 month or less, abundant eosinophils can be identified. The post-TUR granuloma appears to be a reaction to altered epithelium and stroma from the trauma of previous cautery. Although there is only amorphous fibrinoid material within the center of most post-TUR granulomas, in some cases, there may be necrotic epithelial and stromal components. The recognition of similar granulomas after cautery in other sites argues against the process resulting solely from altered epithelium or secretions unique to the prostate. Rarely, similar linear granulomas may be seen after needle biopsy. Post-TUR granulomas appear to be incidental findings and are commonly seen in radical prostatectomy specimens removed for carcinoma when a prior TUR resection was performed. The lesion is so characteristic and histologically distinct from infectious granulomas that stains for organisms are not necessary.
ALLERGIC GRANULOMATOUS PROSTATITIS
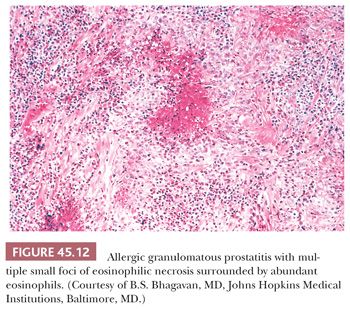
Allergic granulomatous prostatitis, as a reflection of a more generalized allergic reaction, is an exceedingly uncommon condition (42). It is characterized by multiple small, ovoid, necrobiotic granulomas surrounded by numerous eosinophils (Fig. 45.12). The regularity of the size and shape of these granulomas and the extensive infiltration of eosinophils throughout the stroma, not just surrounding the granulomas, separates this entity from that of post-TUR granulomas with eosinophils. Of the 12 patients with allergic granulomatous prostatitis reported in the literature, all had either asthma or evidence of systemic allergic reactions at the time of diagnosis of the prostatic lesions, and the majority of affected individuals had increased blood eosinophil counts. Most important, in a few cases, similar granulomas were found systemically and in one individual were a contributing factor in his death. Consequently, recognition of this disease, as well as distinguishing it from other granulomatous prostatic lesions with eosinophils, is important if prompt and aggressive treatment with steroids is to be instituted.
MALACOPLAKIA
As in the bladder, the majority of men with prostatic malacoplakia have urinary tract infections, most frequently with Escherichia coli (43). Malacoplakia may mimic cancer clinically, resulting in prostatic induration and a hypoechoic lesion seen on transrectal ultrasound.
BENIGN NONNEOPLASTIC CONDITIONS
AMYLOID
Some 2% to 10% of prostates with BPH or carcinoma contain vascular amyloid deposits (44,45). A higher incidence of prostatic amyloidosis is seen in patients with multiple myeloma, primary amyloidosis of the kidney, or chronic debilitating diseases. The location of amyloid in these cases is in vessels and subepithelial areas. Corpora amylacea also often nonspecifically stain for amyloid.
CALCULI
Prostatic calculi are found within acini of the gland and should be distinguished from urinary calculi found within the prostatic urethra (46). Although prostatic calculi are common, they are usually small, multiple, asymptomatic, and are discovered incidentally during diagnostic procedures performed for other conditions. Prostatic calculi are present in 70% to 100% of the glands studied at autopsy—the majority found in men older than 50 years. Histologically, the calculi appear stratified in concentric layers resembling calcified corpora amylacea. Prostatic calculi appear to form by the consolidation and calcification of corpora amylacea or by calcification of precipitated prostatic secretions or both. An uncommon complication is abscess formation from infected prostatic calculi in patients with antibiotic-resistant urinary tract infections.
CYSTS
Prostatic cysts can be subdivided into utricle and retention cysts (47,48). Most utricle cysts lie outside the prostate between the bladder and rectum, with the orifice of the cyst located at the prostatic utricle. The average age of patients with utricle cysts is 26 years. In approximately 25% of cases, abnormalities of the external genitalia can be identified, and in about 10% of cases, there is unilateral renal dysgenesis or agenesis. Histologically, the cyst walls may lack an epithelial lining or may be composed of columnar, cuboidal, urothelial, or, less frequently, squamous epithelium. In approximately 10% of cases, calculi are formed within the cyst. Cystadenoma, squamous cell carcinoma, and adenocarcinoma rarely may be associated with these cysts. Retention cysts arise when prostatic acini become distended with clear fluid. Because small, asymptomatic, dilated prostatic acini frequently are found, the term retention cyst should be reserved only for those cysts that are symptomatic. Defined accordingly, the cysts range in size from 1 to 2 cm, are usually unilocular, and are found adjacent to the urethra. Histologically, the cysts are lined with flattened prostatic glandular epithelium or urothelial epithelium.
MELANOCYTIC LESIONS
In general, blue nevus has been used to denote melanin confined to ovoid or elongated melanocytes in the prostatic stroma and melanosis for prostatic lesions with melanin present in both the stroma and glandular epithelium (7). Rarely, adenocarcinoma of the prostate has also been noted to contain melanin. Nonneoplastic and neoplastic prostatic epithelial cells with melanin contain only mature stage IV melanosomes, suggesting that epithelial melanin results from a transfer of pigment from the stromal melanocytes. There has only been one report of a malignant melanoma that presented in the prostate gland (49,50).
MUCOUS GLAND METAPLASIA
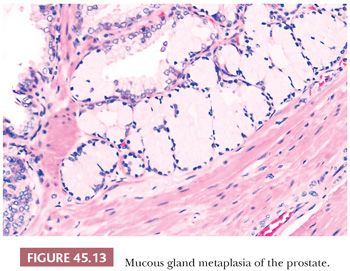
Mucous gland metaplasia, which is found in approximately 1% of prostates, consists of tall, mucin-filled goblet cells with tiny, dark, basally located nuclei (Fig. 45.13) (51). The cells are positive for mucicarmine and Alcian blue and are negative for PSA and prostate-specific acid phosphatase (PSAP). These may occur as randomly scattered individual cells or in groups of 5 to 10 cells. Mucous gland metaplasia may be found in normal and hyperplastic prostate glands and in areas of urothelial metaplasia, basal cell hyperplasia, or atrophy. In contrast to foamy gland hyperplasia, where the lumen typically contains dense pink secretions, extensive mucous gland metaplasia contains globoid cells distended with mucin that occludes the lumen.
MISCELLANEOUS BENIGN LESIONS
In a study of prostates from a medical examiner’s office, 9% of prostates contained sperm (52). Rare cases of prostatic endometriosis, hair granuloma, and lymphangiolipomatosis have been diagnosed (53). Vasculitis involving the prostate may occur, including polyarteritis nodosa, Wegener granulomatosis, and giant cell arteritis (54–56). Extramedullary hematopoiesis and ganglioneuroma have been described in the prostate (57,58). Eosinophilic changes to the cytoplasm of benign glands include eosinophilic metaplasia and Paneth cell–like neuroendocrine differentiation (59,60). Prostatic cystadenomas are large, tumorlike lesions with the appearance of BPH. They were reported to arise in the retrovesical space, either attached to the prostate by a pedicle or apparently separate from the prostate (61).
ADENOCARCINOMA OF THE PROSTATE
In 2013, prostate cancer in the United States was the most common cancer, accounting for approximately 28% of cancers in men (62). However, prostate cancer only accounted for 10% of cancer deaths in men. From birth to death, there is a 1 in 6 probability of being diagnosed with prostate cancer. The death rate due to prostate cancer has decreased since 1990. Although the 5-year survival with prostate cancer was 69% in the mid-1970s, virtually no one dies of prostate cancer within 5 years in current times.
The incidence of adenocarcinoma of the prostate found at autopsy increases with age and varies depending on the method of sampling the gland. The carefully done study of medical examiner cases by Sakr et al. (63) highlighted the high incidence (34%) of histologically diagnosable but clinically inapparent prostate cancer in men younger than 50 years who die of trauma.
Men younger than 50 years have a broad spectrum of disease, ranging from small, insignificant tumors to advanced incurable cancer. Young men who are candidates for radical prostatectomy do not have a worse prognosis after surgery than do older men. These men may be diagnosed in the workup of other genitourinary symptoms unrelated to the prostate or because of a family history of prostate cancer (64,65).
Adenocarcinoma of the prostate in childhood and adolescence is rare, with approximately 5 cases in patients younger than 10 years and 21 cases between the ages of 10 and 21 years reported (66). Patients tend to have obstructive symptoms and be at an advanced stage, showing a metastatic pattern similar to that seen in adults. The tumors are almost uniformly poorly differentiated and are not responsive to treatment, with an average survival ranging between 3 and 10 months.
Most studies demonstrate that African American men are first seen with a higher stage of disease than whites and thus have an overall greater mortality rate from prostate cancer. However, the possible etiologic factors and whether prostate cancer has a different biology in black men compared with white men are yet to be determined (67).The incidence of clinically detected adenocarcinoma of the prostate varies significantly among nations. In particular, Scandinavian countries have relatively high rates and Asian nations have very low rates. It is of note that the incidence of incidentally discovered adenocarcinoma of the prostate at autopsy is relatively uniform among different nations. On migration of persons from low- to high-incidence countries, the rate of clinical prostate cancer increases, suggesting a role for environmental factors (68).
STAGING CLASSIFICATION
Stages T1a (≤5% cancer) and T1b (>5%) adenocarcinoma of the prostate are clinically unsuspected tumor that is discovered in either transurethral resection of the prostate (TURP) or enucleation specimens removed for BPH (69). Stage T1c disease refers to nonpalpable prostate cancer found on needle biopsy, usually performed for an abnormal serum PSA level. If a patient undergoes radical prostatectomy, stages T1a to T1c are converted to pT2 or pT3 if the tumor is organ-confined or shows extraprostatic extension, respectively. Pathologic stage T2 is defined as tumor localized to the prostate, which is currently further subcategorized into T2a to c depending on the extent of cancer. However, numerous studies have shown that subdividing pathologic stage T2 disease has no prognostic significance. The reason is that bilateral prostate cancer may represent (a) a dominant tumor nodule with contralateral small, low-grade, clinically insignificant tumor; (b) significant discrete right and left tumor nodules; or (c) a single, large, confluent tumor mass involving both sides. Consequently, the designation “pathologic stage T2c” (bilateral cancer) has no meaning, and it is the expectation that future TNM classifications will be changed to reflect this finding. This author merely denotes “stage T2” without subclassification into “T2a” or “T2b” or “T2c” (67,70). “Stage T2+” (stage T2x) refers to tumor with no identifiable tumor in extraprostatic adipose tissue but with a positive margin due to the surgeon’s cutting into the prostate (intraprostatic incision). Because the edge of the prostate has been left in the patient, the pathologic stage cannot be assessed in the area of intraprostatic incision.
Pathologic stage T3 represents tumor that has extended out of the prostate gland, which is further subclassified into T3a and T3b, depending on whether the extraprostatic tumor is without or with seminal vesicle invasion, respectively.
PRESENTATION OF DISEASE: MEANS OF ESTABLISHING DIAGNOSIS
With the advent of numerous treatment options, including pharmacologic and thermal therapies, the number of TUR resection specimens seen in the surgical pathology laboratory has markedly decreased. Conversely, with the widespread use of serum PSA testing, the number of nonpalpable cancers diagnosed by needle biopsy (stage T1c) has markedly increased (71). In patients with an abnormality noted on rectal examination or an abnormal serum PSA, tissue obtained for histologic examination usually is obtained by core biopsy. Transrectal ultrasonography (TRUS) is not used as a screening device because it has poor sensitivity and specificity. Rather, it is used to localize the position of the needle biopsies to ensure effective sampling of the prostate gland. PSA suffers from issues relating to both sensitivity and specificity. Approximately 50% of organ-confined tumor and 33% with extraprostatic extension are associated with normal serum PSA levels (72–74). Elevated serum PSA levels also may result from nodular hyperplasia, prostatic biopsy, and inflammation. Attempts to factor in the volume of BPH as a contributor to serum PSA levels resulted in a measurement called PSA density (PSAD). The serum PSA level is divided by the volume of the prostate, as calculated by TRUS, yielding PSA per gram of tissue. Because carcinoma produces more PSA per gram of tissue than does BPH, a high PSAD value may be a better indication of carcinoma than the use of the uncorrected PSA value (75). Another technique to enhance screening is PSA velocity, in which the change of PSA per unit of time is measured (75,76). Age-specific PSA reference ranges and percentage of free PSA have become additional tools in the prostate cancer screening armamentarium (77). Age-specific reference ranges try to increase sensitivity and specificity of the test by accounting for the increasing PSA seen with aging. Most prostate cancer is associated with a relative increase of the complex form of PSA in the serum, as opposed to free PSA. Therefore, a low percentage of free PSA is suggestive of cancer (75). Screening by some method is recommended in young (40- to 50-year-old) men with a strong family history of prostate cancer or in African Americans (78). Core biopsy of the prostate may be performed via a transrectal or, rarely, a transperineal route. The core biopsy needle typically is an 18-gauge spring-driven Biopty gun (CR Bard, Inc., Covington, GA). In addition to performing a biopsy of the area of a palpable abnormality, random systematic biopsies should be performed to enhance detection (79). It has been demonstrated that the posterolateral aspects of the prostate should be sampled in particular (80). Extended biopsy schemes with 10 or more cores sampled have become standard because 6 core sextant biopsies miss significant cancers. There still is a false-negative rate with core biopsies of the prostate ranging from 12% to 19% (81). These figures vary depending on the type of biopsy performed, degree of palpable abnormality, and number of biopsies performed. Specimens should be considered unsatisfactory when there is only minimal or no prostatic glandular or stromal tissue. Cores of prostatic tissue containing only stroma are not necessarily unsatisfactory specimens and may represent biopsy of a predominantly stromal nodule of hyperplasia. Negative biopsies of prostates that are clinically suggestive of carcinoma are not uncommon and may not represent a false-negative biopsy because only about 50% of nodular prostates contain carcinoma (82). Tumor seeding along the needle track has not been seen with the thin Biopty gun needles, in contrast to prior reports seen with larger perineal needle biopsies.
Pathologists should be aware of certain issues relating to needle biopsy specimens. When using fixatives such as Bouin, one must judge what are prominent nucleoli relative to the nucleoli seen in adjacent benign glands. If one does not see nucleoli in the majority of prostate cancers sampled on needle biopsy fixed in formalin, it is not necessary to switch to these other alternative fixatives. Rather, careful attention to microtomy and staining can improve the situation; sections that are too thick or overstained result in hyperchromatic nuclei without visible nucleoli. Misdiagnosis of clinically relevant features on prostate biopsy can be minimized with histologic review of three levels per tissue core (83). The use of intervening unstained slides has been shown to be critical in establishing diagnosis in approximately 3% of prostate needle biopsies (84). Each laboratory must decide whether these data justify the cost of preparing extra unstained slides.
The most frequent sites of metastatic prostate carcinoma are lymph nodes and bone. Metastatic prostate cancer to the lung usually takes the form of multiple small nodules or diffuse lymphatic spread rather than large metastatic deposits. Adenocarcinoma of the prostate also may be diagnosed in the workup for metastatic carcinoma of unknown origin. The importance of identifying these tumors as being of prostatic origin is that even widespread metastatic prostate carcinoma may be hormonally responsive, and treatment may lead to dramatic and sometimes long-term symptomatic relief. Because the bones are a common site of presentation for prostate cancer, IHC stains for prostatic markers should be performed on all cases of metastatic adenocarcinoma to the bone in men without known primaries. Prostate carcinoma also shows a tendency to metastasize to left-sided cervical or other supradiaphragmatic lymph nodes, often as the first manifestation of prostate cancer (85). These cancers often are poorly differentiated and may not be suggestive of prostate carcinoma histologically. Furthermore, patients may have normal rectal examinations and an absence of metastatic bone disease. Despite the high grade of these tumors, PSA staining is present in almost all cases. However, because some poorly differentiated prostate adenocarcinomas are negative for PSA yet positive for the newer antibodies NKX3.1 and P501S (prostein), these authors use all three markers in the workup of a poorly differentiated carcinoma with the differential diagnosis of adenocarcinoma of the prostate versus urothelial carcinoma (86,87).
Pathologists also should be attuned to prostate carcinoma biopsied as a rectal mass. In some cases, it is the initial manifestation of prostate cancer. In others, it represents prostate cancer recurring several years after diagnosis, such that a new colonic primary is suspected (88). Endoscopically, the lesion may be indistinguishable from a colonic primary, and in some cases, serum PSA levels may be low. Most cases are poorly differentiated histologically with solid nests, individual cells, microacinar, or cribriform formation. The finding of submucosal tumor in the colon that lacks an in situ component and is not histologically typical of colonic adenocarcinoma should suggest the possibility of an extrinsic tumor. IHC reactivity with prostatic markers is expected. Although typically adenocarcinoma of the prostate is CDX2 negative, uncommonly they can be diffusely positive (89).
GLEASON GRADING OF NEEDLE BIOPSIES
The Gleason system is based on the glandular pattern, where cytology is not factored (Figs. 45.14 through Figs. 45.20) (90–93). Architectural patterns are identified and assigned a grade from 1 to 5, with 1 being the most differentiated and 5 being undifferentiated. Although in the original Gleason system, the most common and second most common grades were combined, in 2005, the Gleason system was updated and modified with one change being that on biopsy, the most common and highest grade patterns on a given core are added to result in the Gleason score (94). If a tumor has only one histologic pattern, then for uniformity, both patterns are given the same grade. Although in theory, the Gleason scores range from 2 (1 + 1 = 2), which represents tumors uniformly composed of Gleason pattern 1 tumor, to 10 (5 + 5 = 10), which represents totally undifferentiated tumors, Gleason patterns 1 and 2 are rarely assigned in current practice (95).
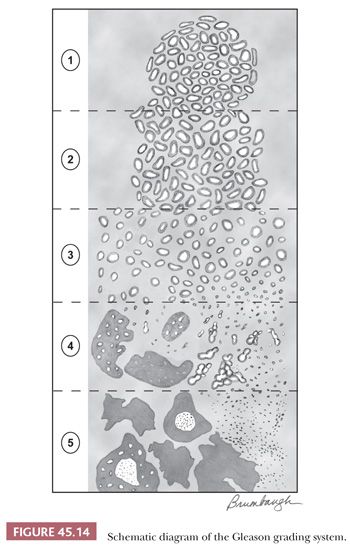
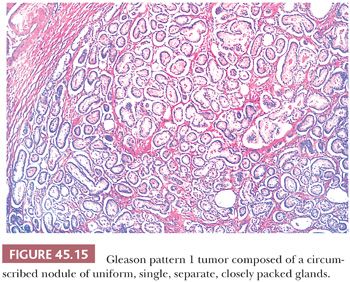
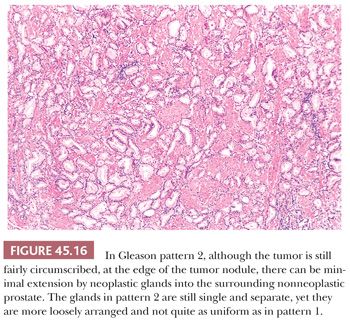
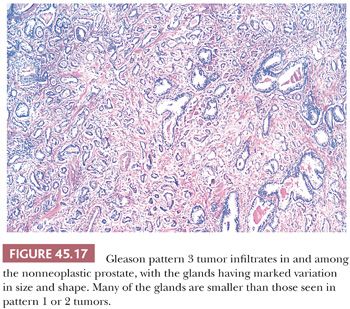
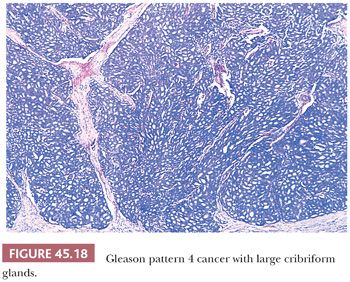
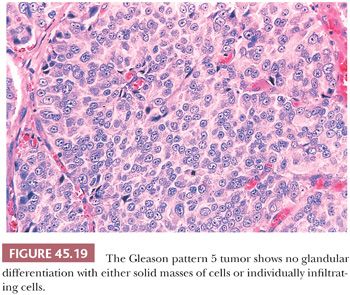
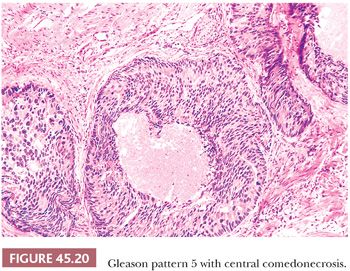
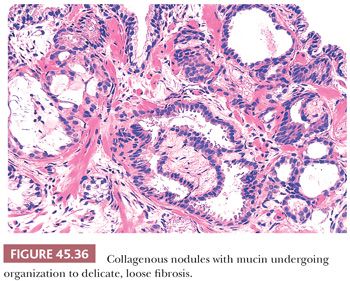
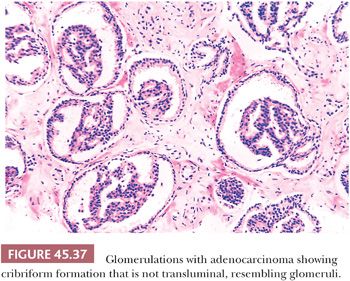
A more contemporary grouping of Gleason scores based on differing prognoses is Gleason scores less than or equal to 6; 3 + 4 = 7; 4 + 3 = 7; 8; 9 to 10, which reflect prognostic grade groups I to V (96). Most cases with divergent patterns, especially on needle biopsy, do not differ by more than one pattern. It is reasonable to assign a full Gleason score even to small foci of cancer on needle biopsy because it has been demonstrated that the grade assigned to these minimal cancers is just as accurate compared with cases with more extensive cancer on biopsy (97). Gleason pattern 3 cancer consists of variably sized individual glands that are well formed. In contrast to Gleason pattern 4, the glands in Gleason pattern 3 are discrete units (98). If one can mentally draw a circle around well-formed individual glands, then it is Gleason pattern 3. One should assign a Gleason grade at relatively low power (i.e., 4× or 10× objective). The presence of a few poorly formed glands at high power, which could represent a tangential section off of small well-formed glands, is still consistent with Gleason pattern 3 tumor. There are certain situations that lead to overgrading of Gleason pattern 3 as pattern 4. Crowded glands at low magnification can have the appearance of fused glands, mimicking Gleason pattern 4 cancer (98). Small glands are acceptable for Gleason pattern 3 as long as they are well formed and not fused with other glands. The delicate ingrowth of fibrous tissue seen with mucinous fibroplasia (collagenous micronodules) can result in glands appearing to be fused resembling cribriform structures, although the underlying architecture is really that of individual discrete rounded glands invested by loose collagen. Gleason pattern 4 is diagnosed in the presence of cribriform glands, fused glands, and ill-defined glands with poorly formed glandular lumina (99). Only when there is a cluster of poorly formed glands, where a tangential section of Gleason pattern 3 glands cannot account for the histology, should the focus be graded as Gleason pattern 4 (98). On needle biopsy, cribriform Gleason pattern 4 tumor often manifests as fragments of cribriform carcinoma as there is little supporting stroma in larger cribriform glands. Glomerulations represent an early stage of cribriform pattern 4 cancer and should be assigned Gleason pattern 4 (100). Gleason pattern 5 consists of sheets of tumor, individual cells, and cords of cells. Less commonly, there are nests of cells. Solid nests of cells with vague microacinar or only occasional gland space formation are still consistent with Gleason pattern 5. A relatively uncommon morphology is comedonecrosis with solid nests. Occasionally, one can see necrosis with cribriform masses that by themselves might be cribriform pattern 4; the consensus is that these patterns should be regarded as Gleason pattern 5. There is a tendency for pathologists to undergrade Gleason pattern 5 (101,102).
One of the most frequent causes of discordant grading is the assessment of tumors that bridge two grades. As shown on Gleason’s schematic diagram (Fig. 45.14), there is a continuum of differentiation between the various Gleason patterns such that the grade assigned at the extremes of a particular Gleason pattern may be somewhat subjective. Interobserver reproducibility studies of Gleason grading have highlighted these problem areas (103,104).
The Gleason grade on biopsy material has been shown to correlate fairly well with that of the subsequent radical prostatectomy (105). In general, a Gleason score less than or equal to 6 on biopsy corresponds to a Gleason score less than or equal to 6 in the radical prostatectomy in about 80% of cases. An unavoidable cause of discrepant grading between the biopsy and subsequent prostatectomy specimen is that caused by sampling error by the needle biopsy. The following factors are associated with upgrading from the needle biopsy to the radical prostatectomy: increased cancer extent on biopsy; increased serum PSA levels, smaller prostates; and fewer cores sampling the prostate (106).
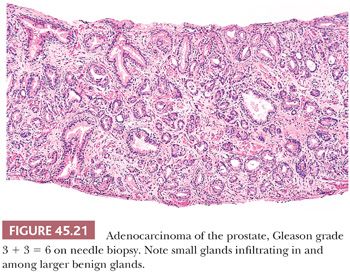
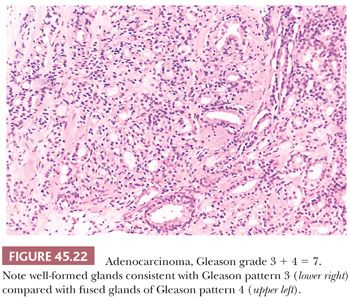
When different cores have different Gleason scores, pathologists should report the grades of each core separately as long as the cores are submitted in separate containers or the cores are in the same container yet specified by the urologist as to their location (i.e., by different color inks). There is no consensus how to grade different cores with different grades when the different cores are present within the same specimen container without a designation as to site. High-grade tumor of any quantity on needle biopsy should be included within the Gleason score. Consequently, a needle biopsy with 98% Gleason pattern 3 and 2% Gleason pattern 4 should be graded as Gleason score 3 + 4 = 7. In all specimens, in the setting of high-grade cancer, one should ignore lower grade patterns if they occupy less than 5% of the area of the tumor. For example, tumor composed of 98% Gleason pattern 4 and 2% Gleason pattern 3 should be graded as Gleason score 4 + 4 = 8. Over a 2- to 3-year period, the grade of prostate cancer on needle biopsy does not tend to change (107). The Gleason grading system is one of the more powerful prognostic indicators in prostate cancer. Gleason score correlates with all of the important pathologic parameters seen in the radical prostatectomy specimen, with prognosis after radical prostatectomy and with outcome following radiotherapy (98). The major shift in terms of the likelihood of having adverse findings in the prostatectomy or with failure following prostatectomy or radiotherapy is between a Gleason score of 6 and 7 (Figs. 45.21 and 45.22). The importance of grade is evidenced by the use of various nomograms using preoperative variables such as Gleason score, clinical stage, serum PSA, and, in a more recent study, the extent of cancer on biopsy to predict pathologic stage, post–radical prostatectomy progression, and postradiotherapy failure (73,108–111). Grade on biopsy can influence whether definitive treatment is given (active surveillance vs. surgery or radiation), what type of treatment (surgery vs. radiation), and even decisions within a specific therapy (brachytherapy vs. external beam therapy; whether to resect the neurovascular bundle[s] at radical prostatectomy; whether to remove lymph nodes at surgery).
EXTENT OF CANCER, PERINEURAL INVASION, AND EXTRAPROSTATIC EXTENSION ON NEEDLE BIOPSY
Multiple techniques of quantifying the amount of cancer found on needle biopsy have been developed and studied, including measurement of (a) the number of positive cores, (b) the percentage of positive cores, (c) total millimeters of cancer among all cores, (d) percentage of each core occupied by cancer, and (e) total percentage of cancer in the entire specimen. There are multiple studies claiming superiority of one technique over the other, with no one method being clearly superior to the others (112). When cancer discontinuously involves a core with intervening benign prostate tissue, in our experience, the method that correlates best with findings at radical prostatectomy is to record the length of the cancer from one end of the cancer to the other, as opposed to mentally collapsing the different foci of cancer into one smaller measurement. For example, in a core with scattered small foci occupying the entire core length, the extent of cancer should be recorded as “small foci of cancer discontinuously involving 100% of the length of the core” rather than “5% of the total area of the core” (113). Pathologists should report the number of cores containing cancer as well as one other system quantifying tumor extent. At our institution, the number of cores containing cancer is reported, along with the percentage of cancer present on each involved core. When cores are fragmented, we record the percentage involvement of the entire specimen part. We advocate the precise labeling of the initial biopsies according to sextant site to localize the sites of an initial atypical diagnosis and to direct the location of repeat biopsies because increased sampling of the initial atypical sextant site and adjacent sextant sites increases the yield of cancer detection on repeat biopsy (114).
Although the majority of stage T1c cancers are significant tumors warranting definitive therapy, approximately 25% of these tumors detected by needle biopsy are thought to be “insignificant” tumors. Although limited cancer on the biopsy by itself is not predictive, a combination of relatively low PSA values and minimal findings of cancer on needle biopsy can help predict preoperatively which patients may have insignificant tumors and which may be candidates for conservative therapy (71). Extensive cancer on biopsy cores on systematic prostate biopsy is a powerful predictor of adverse pathologic findings at radical prostatectomy. In about 5% of radical prostatectomy specimens, minute cancer will be found and, in about 3% of cases, no tumor will initially be seen in the entirely submitted specimen. A methodical, limited, targeted approach to identifying cancer can identify cancer in 73% of the cases with no initial cancer. After reviewing the biopsy, our protocol for looking for tumor in the radical prostatectomy with no initial tumor is:
1. Perform immunostains on any suspicious foci.
2. Perform levels on blocks with high-grade prostatic intraepithelial neoplasia.
3. Perform three levels on the sextant and adjacent sextant region of the prostate where the cancer was identified on biopsy.
4. Flip the blocks in these regions and perform three additional levels.
In only a rare case when no tumor is found in the prostate is it the result of a switch in needle biopsy specimens. Rather, the explanation is minute cancer sampled on needle biopsy that is not identified despite completed submission of the prostate for analysis (115).
Although controversial due to some conflicting data, the cumulative analysis shows a higher incidence of extraprostatic extension in men with perineural invasion on prostate needle biopsy (116). Perineural invasion on biopsy is prognostic in men undergoing external beam radiotherapy but less so with brachytherapy (117). We suggest that perineural invasion should be noted on the biopsy pathology report.
The diagnosis of extraprostatic extension on needle biopsy can be established when tumor is seen admixed with adipose tissue on needle biopsy. Although rarely one can see intraprostatic adipose tissue, the likelihood of cancer involving intraprostatic adipose tissue is so rare that one can assume the adipose tissue in this situation is extraprostatic. Extraprostatic extension on core needle biopsy of the prostate is typically seen with extensive high-grade prostatic adenocarcinoma, such that its usefulness as an isolated prognostic factor is relatively limited (118).
DIAGNOSIS OF LIMITED PROSTATE CANCER
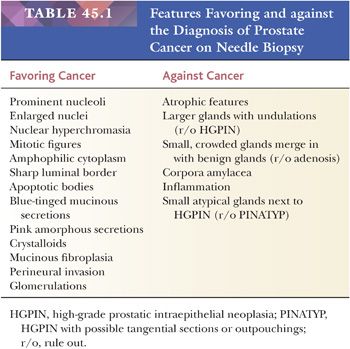
One of the most common problems with the evaluation of needle biopsy material from the prostate is the diagnosis of carcinoma as a result of the limited amount of tumor present (119,120). Commonly, biopsies of adenocarcinoma of the prostate yield several cores of tissue containing benign glands and stroma, with only a few neoplastic glands insinuating themselves within the benign tissue. Evaluating an atypical focus in a needle biopsy of the prostate should be a methodical process. When reviewing needle biopsies, one should develop a mental balance sheet where on one side of the column are features favoring the diagnosis of carcinoma and on the other side of the column are features against the diagnosis of cancer (Table 45.1). A definitive diagnosis can be made if all of the criteria are listed on one side of the column or the other at the end of evaluating a case. The diagnosis of cancer should be based on a constellation of features rather than relying on any one criterion by itself.
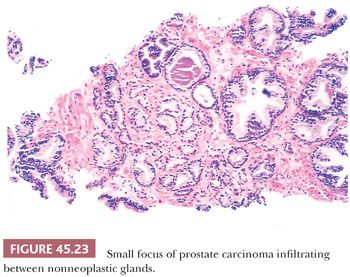
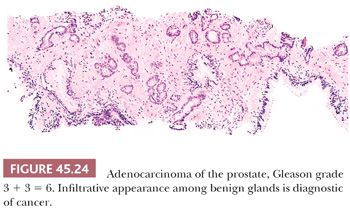
The recognition of prostatic cancer in these cases rests on both the architectural abnormalities resulting from the infiltrating neoplastic glands and the cytologic features of the neoplastic epithelium. In order to identify limited amounts of cancer on needle biopsy material, one must first identify the normal nonneoplastic prostate and then look for glands that do not fit in. Although most prostates are relatively similar in their histologic appearance, some contain numerous small foci of crowded glands similar to adenosis. In such a case, the diagnosis of cancer based on a small focus of crowded glands with minimal cytologic atypia should be performed with caution. Other men’s prostate glands are characterized by widespread atrophy; one should, in these cases, hesitate to diagnose cancer if the atypical glands have scant cytoplasm. Architectural patterns suspicious for carcinoma are (a) crowded glands, (b) small glands situated between larger benign glands, and (c) a row of glands going across the core (Figs. 45.23 and 45.24).
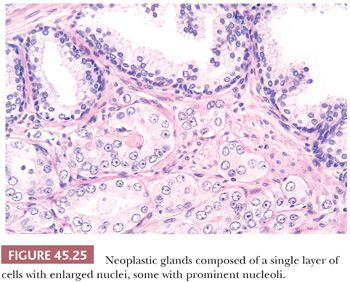
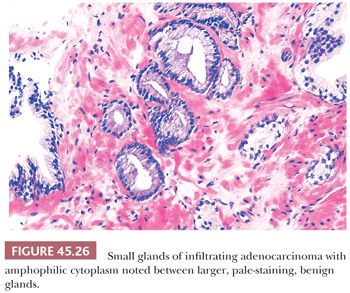
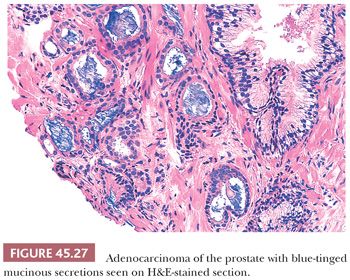
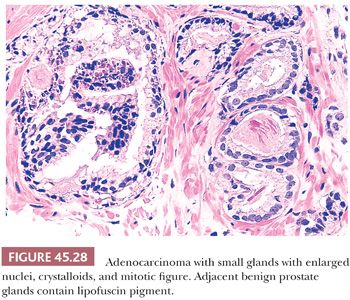
In most cases of limited cancer on needle biopsy, there are cytologic differences in the malignant glands when compared with surrounding benign glands (Fig. 45.25). Although the finding of prominent nucleoli in the small glands is reassuring, it is not necessary to diagnose carcinoma. Often, only significant nuclear enlargement or nuclear hyperchromasia discriminates cancer from the surrounding benign glands. Other nuclear features favoring the diagnosis of cancer are mitotic figures and apoptotic bodies (121). In addition to differentiating nuclear features, neoplastic glands may have amphophilic cytoplasm contrasted with the pale to clear cytoplasm of adjacent benign glands (Fig. 45.26). Intraluminal pink, acellular, dense secretions; blue-tinged mucinous secretions; and crystalloids seen in small atypical glands may be additional features that help to differentiate malignant from benign glands, given their greater frequency in malignancy (Figs. 45.27 and 45.28). Prostatic crystalloids are dense, eosinophilic, crystalloid structures that appear in various geometric shapes (122). If the differential diagnosis is between adenosis (see “Mimickers of Prostate Cancer”) and low-grade adenocarcinoma (i.e., a lobular collection of crowded glands), the presence of crystalloids is not helpful. If the focus consists of small, atypical glands infiltrating between larger, benign glands, in which adenosis is not in the differential, then the findings of crystalloids can help to establish a malignant diagnosis. Crystalloids also may be seen in scattered benign glands on needle biopsy, where there is no increased likelihood of cancer on repeated biopsy (123).
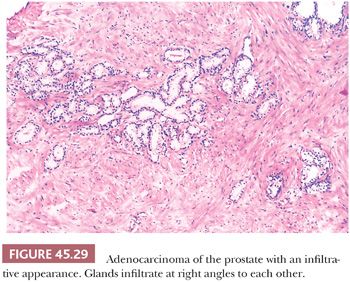
Although nuclear features play a prominent role in the diagnosis of adenocarcinoma of the prostate on needle biopsy material, they may not be as helpful in diagnosing low-grade adenocarcinoma on TURP specimens. Often, low-grade adenocarcinomas of the prostate lack enlarged nuclei and prominent nucleoli, and mitoses are rarely found. Cytoplasmic features are also often not very helpful because they are often pale-clear, similar to benign glands. The most useful feature in diagnosing low-grade adenocarcinoma on TURP is the recognition of cancer’s architectural growth pattern as seen at relatively low magnification (Fig. 45.29). Benign prostatic glands tend to grow either as circumscribed nodules within BPH or radiate in columns out from the urethra in a linear fashion. In contrast, adenocarcinoma of the prostate grows in a haphazard fashion.
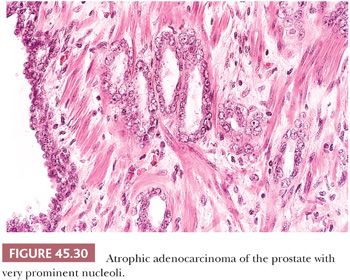
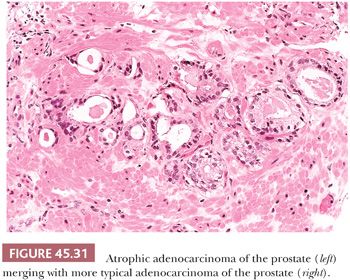
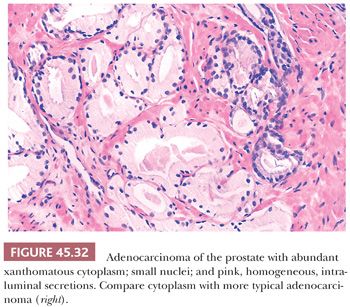
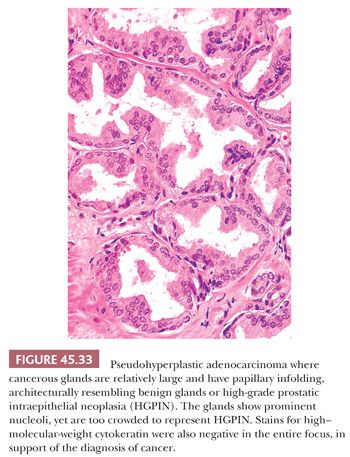
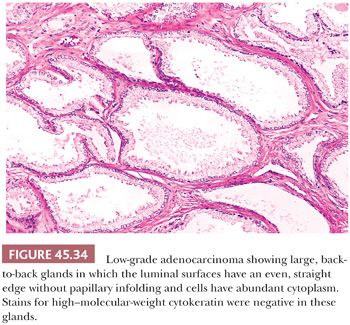
Some cancers closely resemble benign prostate glands in their architectural pattern or cytologic features and may not be recognized as malignant. Atrophic adenocarcinomas mimic benign atrophy and are distinguished by (a) an infiltrative pattern of growth; (b) the presence of macronucleoli (Fig. 45.30); and (c) the presence of adjacent, nonatrophic cancer (Fig. 45.31) (124,125). Another unusual pattern of adenocarcinoma seen on needle biopsy that may be underdiagnosed involves cancers with voluminous xanthomatous cytoplasm in which nuclei are small and often show no or minimal atypia, termed foamy gland carcinoma; intraluminal pink homogeneous secretions are often present (Fig. 45.32) (126). A third pattern of prostatic adenocarcinoma that may resemble benign glands is called pseudohyperplastic adenocarcinoma (127,128). Pseudohyperplastic prostate cancer is characterized by the presence of larger glands, often with branching and papillary infolding (Fig. 45.33). The recognition of cancer with this pattern is based on the architectural pattern of numerous closely packed glands as well as nuclear features more typical of carcinoma. A variant of pseudohyperplastic adenocarcinoma is composed of numerous large glands that are almost back-to-back with straight, even luminal borders and abundant cytoplasm (Fig. 45.34). Comparably sized benign glands have papillary infolding, undulations, or ruffling of their luminal border or atrophic cytoplasm. The use of basal cell–specific keratin antibodies or p63 (clone 4A4) is often essential for the diagnosis of these cancers that mimic benign glands. In rare cases, carcinoma may mimic crushed stroma or inflammation. The identification of this tissue as epithelial and of prostatic origin by the use of prostate-specific markers and keratins may help to establish the diagnosis of prostatic carcinoma.
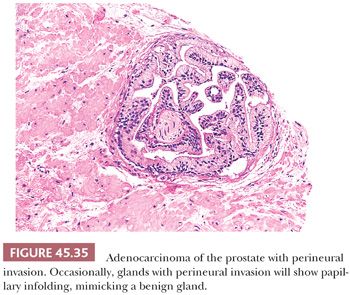
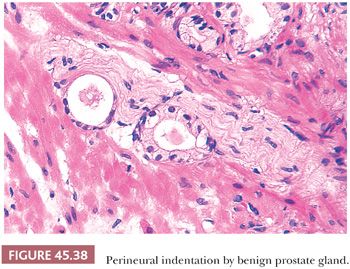
There are three features that have not been identified in benign glands to date and that are in and of themselves diagnostic of cancer. These are perineural invasion, mucinous fibroplasia (collagenous micronodules), and glomerulations (Figs. 45.35 to 45.37) (129). Although perineural indentation by benign prostatic glands has been reported, the glands in these cases appear totally benign and are present at only one edge of the nerve or are intraneural rather than circumferentially involving the perineural space, as can be seen in carcinoma (Fig. 45.38) (130).
FINDINGS OF ATYPICAL GLANDS SUSPICIOUS FOR CANCER: IMMUNOHISTOCHEMICAL ADJUNCTS IN THE DIAGNOSIS OF PROSTATE CANCER
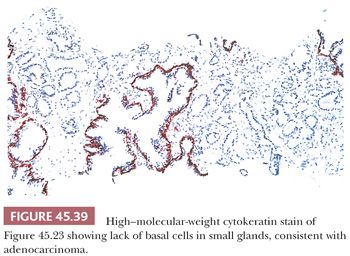
When certain features are present on needle biopsy that favor the diagnosis of prostate cancer but there are insufficient features for a definitive malignant diagnosis, a report of “atypical, suspicious for carcinoma” may be rendered (Table 45.1). The incidence of atypical glands on needle biopsy is approximately 5% (131). Approximately 50% of cases with an atypical diagnosis have cancer on repeat biopsy. A rational approach for the rebiopsy of men with an initial atypical diagnosis is relatively increased sampling of the initial atypical and adjacent sextant sites with usual sampling elsewhere (114). If repeat biopsy is negative, this does not exclude the presence of carcinoma because prostatic needle biopsies are associated with fairly high false-negative rates of up to 25% (81). It is incumbent on the pathologist in these cases to have the initial biopsy sent off for consultation or to try to resolve the initial biopsy with ancillary techniques such as immunohistochemistry with antibodies to basal cell antibodies (i.e., high–molecular-weight cytokeratin or p63). If there are numerous atypical glands that are negative for basal cell–specific markers, a diagnosis of carcinoma may be rendered (Fig. 45.39) (9–12). Because benign prostate glands show some heterogeneity in staining with these antibodies, negative staining for basal cell–specific markers in only a few glands suggestive of cancer is not proof of their malignancy. In cases of prostate cancer mimickers, positive basal cell–specific marker staining may definitively label a focus as benign. Uncommonly, prostate adenocarcinoma tumor cells show cross-reactive staining for high–molecular-weight cytokeratin, yet not in a basal cell distribution. Exceedingly rarely, unequivocal cancer on hematoxylin and eosin (H&E)–stained tissue sections reveal focal basal cell staining (132). Rare carcinomas, often having a distinctive morphology consisting of glands, nests, and cords with atrophic cytoplasm, hyperchromatic nuclei, and visible nucleoli, have diffuse aberrant p63 positivity but negative staining for high–molecular-weight cytokeratin (133–135).
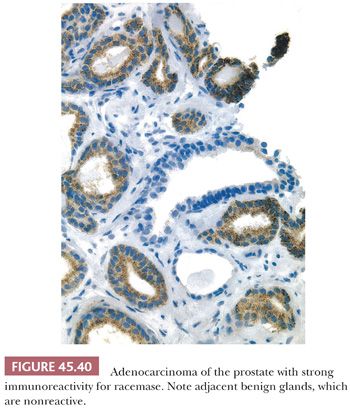
Another IHC marker that is helpful in diagnosing limited adenocarcinoma on prostate needle biopsy is alpha-methylacyl-CoA racemase (AMACR). Approximately 80% of small foci of prostate cancer on biopsy are positive for AMACR, where benign glands are usually negative (136). In addition, a high proportion of high-grade prostatic intraepithelial neoplasia and some foci of adenosis and partial atrophy also are positive for the marker. When the pathologist favors the diagnosis of cancer on routine stained sections and stains for basal cells are negative, and a definitive diagnosis of cancer is still difficult because of the cancer’s deceptively benign appearance or limited amount, positive staining for AMACR can provide the additional confidence to establish a definitive malignant diagnosis (Fig. 45.40) (137–141). Nuclear immunoreactivity for ERG can be used as a surrogate for TMPRSS2-ERG gene fusion, a specific molecular event seen in approximately 50% of prostate carcinomas and approximately 20% of high-grade prostatic intraepithelial neoplasia. The major limitation of this technique is that on limited foci of carcinoma on needle biopsy, the sensitivity may be somewhat lower to around 30% to 40% and positivity does not exclude high-grade prostatic intraepithelial neoplasia (142,143).
MIMICKERS OF PROSTATE CANCER
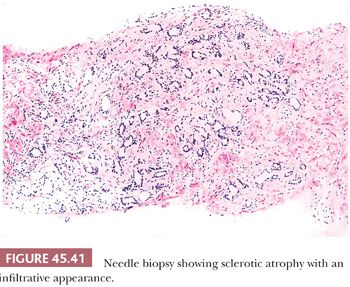
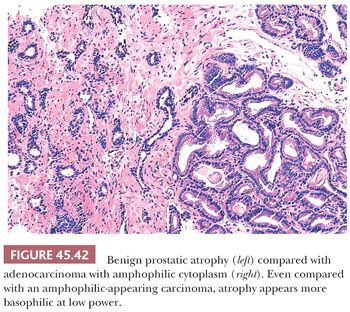
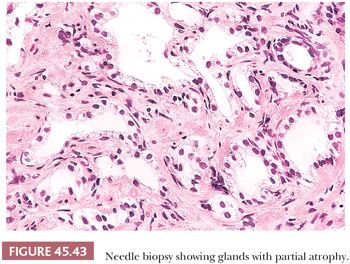
Fully atrophic glands stand out at scanning magnification because of their open lumina lined by cells with crowded nuclei and scant apical and lateral cytoplasm, resulting in a very basophilic appearance to the glands (Fig. 45.41). When there is an increased number of crowded atrophic glands, the term postatrophic hyperplasia (PAH) is used (144,145). Within the center of these small, atrophic glands, there may be seen a dilated gland surrounded by fibrosis. These small glands, despite their atrophic appearance, have an increased proliferation rate (146). In contrast, gland-forming adenocarcinomas at low magnification usually appear pale or amphophilic, with cells showing abundant cytoplasm and basally situated nuclei (Fig. 45.42). Small glands with partial atrophy may be crowded and have a disorganized infiltrative appearance and be misdiagnosed as carcinoma (147,148). In partial atrophy, the small glands have pale to clear attenuated cytoplasm with small, somewhat crinkly nuclei without prominent nucleoli (Fig. 45.43). Although the apical cytoplasm in partial atrophy is attenuated, the nuclei in partial atrophy are more spaced apart with more laterally placed cytoplasm, giving rise to a pale appearance at low magnification in contrast to PAH. Basal cell–specific cytokeratin staining may be positive in a patchy fashion or can be negative in a small focus of partial atrophy. AMACR may also be positive, such that the staining pattern of negative basal cells and positive AMACR can be confused with cancer.
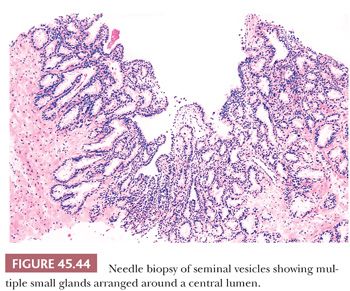
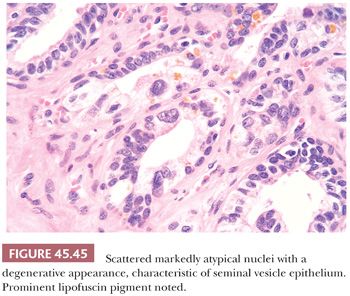
Another potential source of overdiagnosing prostatic adenocarcinoma is the presence of seminal vesicles or histologically similar ejaculatory duct epithelium on needle biopsy. A common finding on needle biopsy of the seminal vesicle is to see at the tip or edge of the core of tissue an irregular row of glandular epithelium that represents the lining of the central dilated seminal vesicle lumen because the core of tissue fractures at this interface (Fig. 45.44). Surrounding this lumen may be numerous small, glandular diverticula of the seminal vesicle, which may be confused with carcinoma. The presence of prominent lipofuscin granules within seminal vesicle epithelium is an important diagnostic aid, although it should be recognized that normal prostate glands also may demonstrate lipofuscin pigment with refractile red-brown granules corresponding to lysosomes (8). In addition, seminal vesicles characteristically have scattered cells showing prominent nuclear atypia, although the atypia appears degenerative in nature (149) (Fig. 45.45). Seminal vesicle–type epithelium is negative for prostate-specific markers and has a basal cell layer identified with high–molecular-weight cytokeratin and p63 staining.
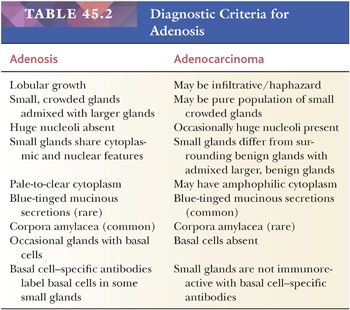
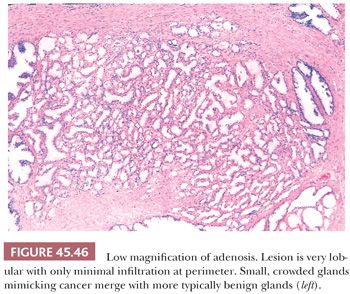
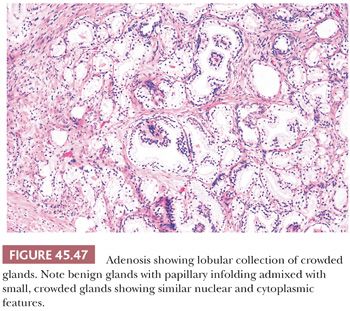
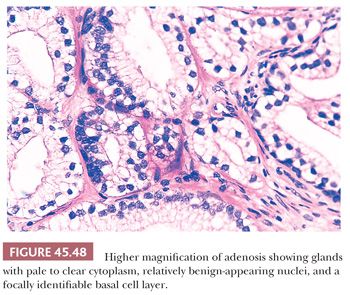
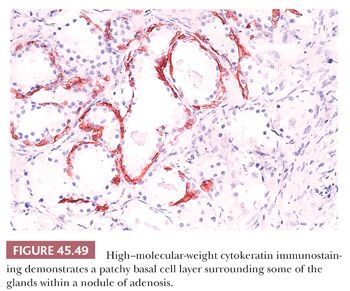
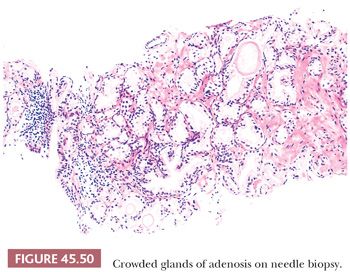
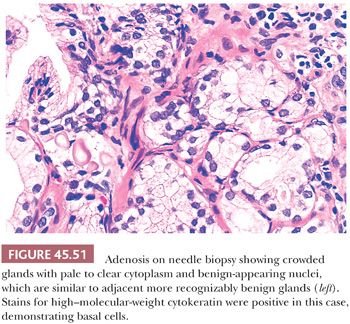
One of the greatest difficulties with TUR material, also seen in approximately 1% of needle biopsy specimens, is the distinction of adenocarcinoma from adenosis (Table 45.2) (atypical adenomatous hyperplasia) (Figs. 45.46 through 45.51) (150–153). Adenosis at low magnification is composed of numerous crowded, small, pale-staining glands that resemble a nodule of low-grade adenocarcinoma of the prostate. Whereas the glands in adenosis have a lobular configuration, the glands in adenocarcinoma are arranged in a haphazard array, with glands often appearing to infiltrate into the stroma at right angles to each other. In adenosis, there is a gradual transition between the small glands with pale to clear cytoplasm and adjacent, more recognizably benign glands; some of the architectural features seen in the benign glands include branching, larger, more irregular shapes, and papillary infolding. Typically, the nucleoli are unapparent in adenosis, although on occasion, they may be focally prominent (152). Features that do not differentiate adenosis from low-grade adenocarcinoma are (a) back-to-back crowded glands; (b) intraluminal crystalloids; (c) medium-sized nucleoli; (d) scattered, poorly formed glands and single cells; and (e) minimal infiltration at the periphery of the nodule. The use of basal cell markers can help diagnose adenosis, especially when there are some atypical features. Within a nodule of adenosis, there are scattered glands, often very few, with positive staining of basal cells with high–molecular-weight cytokeratin or p63. The staining is typically patchy within a positive gland, sometimes consisting of only a few flattened, immunoreactive basal cells. Adenosis tends to be located centrally within the gland, such that it is most commonly seen in TUR specimens. In these specimens, where there is an entire nodule of adenosis to evaluate, it is highly unlikely for the entire focus to show no immunoreactivity with basal cell–specific cytokeratin. Consequently, the lack of basal cell–specific cytokeratin staining in a nodule of glands with a differential diagnosis of adenosis and low-grade adenocarcinoma is highly supportive of the diagnosis of adenocarcinoma. There is no evidence that patients with adenosis have an increased risk of adenocarcinoma, either at the time of diagnosis or in the future (154).
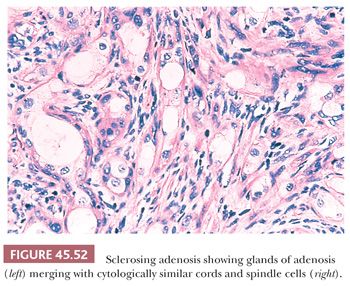
A variant of adenosis that may be confused with intermediate- to high-grade adenocarcinoma of the prostate is sclerosing adenosis of the prostate (155). Sclerosing adenosis consists of a small, relatively localized focus of glands that resemble ordinary adenosis, which merge with cytologically similar cords and individual cells (Fig. 45.52). Nucleoli may be fairly prominent. A thick hyaline basement membrane–like sheath invests some of the glands. Between the glands and individual epithelial cells, there is a cellular spindle cell component, in contrast to prostatic carcinoma, which typically lacks a cellular stromal reaction. Immunohistochemistry with actin and S-100 demonstrate that there is myoepithelial cell differentiation to the basal cells around the glands in sclerosing adenosis and in the spindle cells between the glands, which differs from basal cells in other prostate conditions, which lack myoepithelial cell differentiation (156,157).
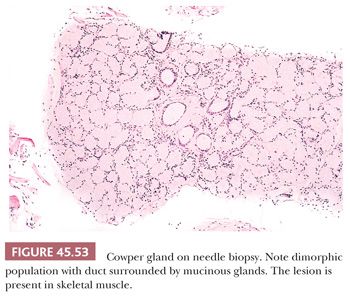
Lesions that may be misdiagnosed as Gleason scores 2 to 6 and Gleason scores ≥7 adenocarcinoma are listed in Tables 45.3 and 45.4, respectively (Figs. 45.53 to 45.55) (40,145,147,149–151,155,158–172).
PROSTATIC INTRAEPITHELIAL NEOPLASIA
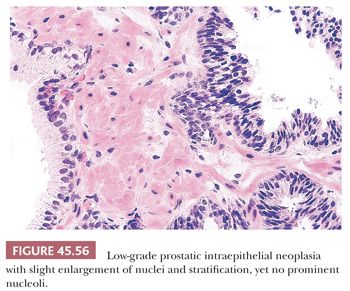
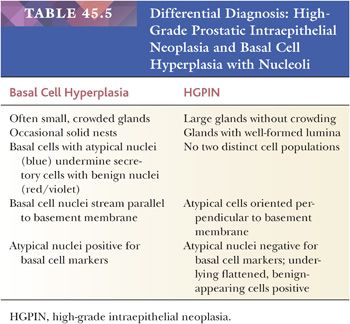
Prostatic intraepithelial neoplasia (PIN) consists of architecturally benign prostatic acini or ducts lined by cytologically atypical cells and is subclassified into low-grade PIN (LGPIN) and high-grade PIN (HGPIN) (Figs. 45.56 to 45.58) (173–175). The distinction between LGPIN and HGPIN is the finding of prominent nucleoli in HGPIN. Diagnostic reports should not comment on LGPIN. First, pathologists cannot reproducibly distinguish between LGPIN and benign prostate tissue. Second, when LGPIN is diagnosed on needle biopsy, these patients are at no greater risk of having carcinoma on repeated biopsy than are men with a benign biopsy finding (131). This author’s threshold for diagnosing HGPIN is whether prominent nucleoli can be visualized at 20× magnification (176). The average incidence of HGPIN reported on needle biopsy is 4% to 5% (131).
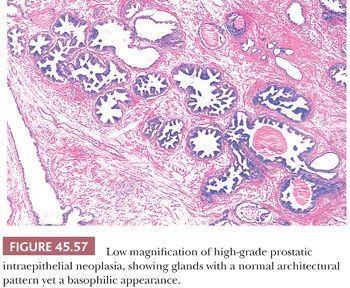
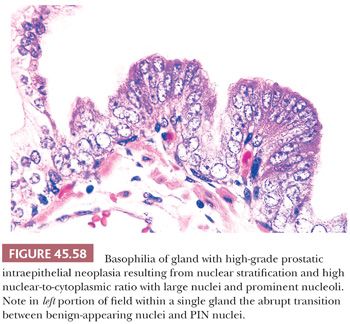
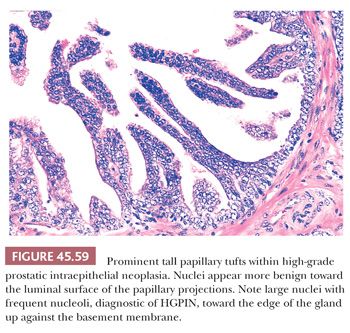
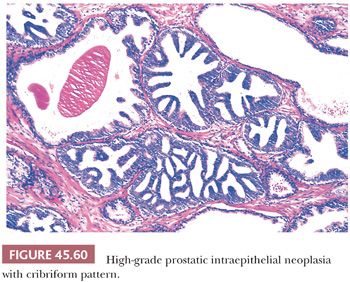
Although HGPIN is characterized by nuclear atypia, often, there are accompanying architectural abnormalities. At low magnification, glands that are separated by a modest amount of stroma and have a normal overall architectural pattern characterize HGPIN. These glands resemble benign glands in that they are large, branch, and have papillary and undulating luminal surfaces. At low magnification, glands with HGPIN have a basophilic appearance resulting from a combination of features, including enlarged nuclei, hyperchromatism, overlapping nuclei, amphophilic cytoplasm, and epithelial hyperplasia (Figs. 45.57 and 45.58). The earliest form of HGPIN is characterized by nuclear atypia within epithelium without epithelial hyperplasia (i.e., flat pattern) (177). Tufting HGPIN is the most common pattern, consisting of slight mounds of hyperplastic, cytologically atypical epithelium in a preexisting benign gland. With more pronounced forms of HGPIN, nuclei project into the lumen as either micropapillary PIN composed of tall epithelial buds lacking fibrovascular cores or as cribriform HGPIN (Figs. 45.59 and 45.60). In HGPIN, the nuclei toward the center of the gland tend to have a blander cytologic appearance, as compared to the nuclei peripherally located up against the basement membrane. The grade of PIN is assigned based on assessment of the nuclei peripherally located up against the basement membrane (Fig. 45.59). Unusual subtypes of HGPIN include PIN with signet ring features, small cell neuroendocrine PIN, PIN with mucinous features, foamy PIN, and PIN with inverted nuclei (178–180).
DIFFERENTIAL DIAGNOSIS
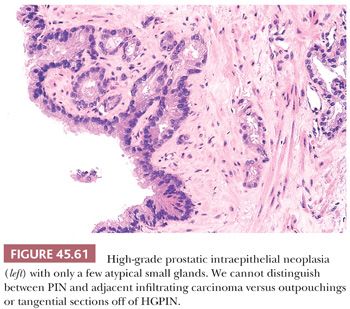
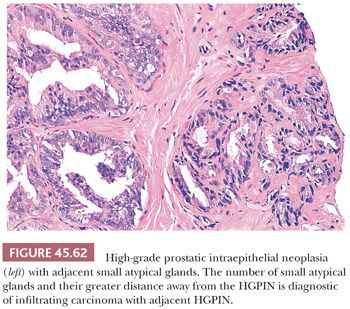
Prostatic intraepithelial neoplasia must be differentiated from variants of infiltrating carcinoma as well as several benign entities. Cytologically, HGPIN may be indistinguishable from infiltrating acinar carcinoma. The diagnosis of infiltrating carcinoma is made when the cytologically atypical glands are too small or crowded, in contrast to HGPIN, which occurs when benign glands are replaced by cytologically atypical cells. In contrast to PIN, ductal adenocarcinomas have papillary formations with fibrovascular cores or show prominent cribriform patterns often with extensive comedonecrosis. A very close mimicker of PIN is PIN-like ductal adenocarcinoma (see “Variants of Prostatic Adenocarcinoma”). Cribriform PIN may be indistinguishable from cribriform acinar carcinoma (176,181). Carcinoma should be diagnosed only when the cribriform glands are back-to-back or when many such glands are negative for basal cell–specific cytokeratin. In some cases, HGPIN is surrounded by a few small atypical glands. It may be difficult to distinguish between outpouchings or tangential sections of HGPIN (PINATYP) as opposed to PIN with associated infiltrating carcinoma. The greater distance of the small atypical glands away from the HGPIN and the greater number of small crowded atypical glands favor the diagnosis of infiltrating carcinoma associated with HGPIN (Figs. 45.61 and 45.62) (182). Most of these cases will require immunohistochemistry for basal cell markers, where cancer should be diagnosed only when there is a large cluster of entirely negative glands. Even a few small glands with a patchy basal cell layer results in a diagnosis of PINATYP.
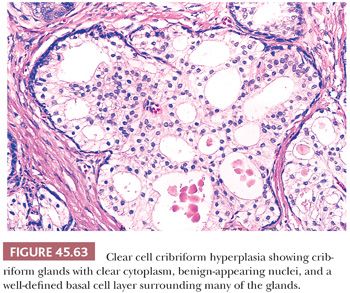
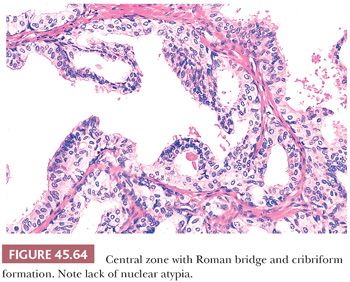
The most common benign lesions mimicking HGPIN are clear cell cribriform hyperplasia (CCCH), central zone histology, and basal cell hyperplasia. CCCH consists of numerous cribriform glands with clear cytoplasm that may grow either in a nodular pattern or as a more infiltrative lesion (Fig. 45.63) (169). Distinctive features of CCCH are the lesion’s pale cytoplasm, benign-appearing nuclei with (at most) small nucleoli, and a strikingly prominent basal cell layer around many of the cribriform glands. Glands within the central zone at the base of the prostate mimic PIN because they may show Roman bridge and cribriform patterns and are lined by tall, pseudostratified epithelium (Fig. 45.64) (183). Characteristic features include eosinophilic cytoplasm, an occasionally prominent basal cell layer, nuclear streaming parallel to the glandular bridges (in contrast to the more rigid bridges seen in PIN), and most importantly a lack of cytologic atypia. Otherwise, typical basal cell hyperplasia may show prominent nucleoli along with mitotic activity (162,163), which may be mistaken for HGPIN (Table 45.5).
Abundant evidence demonstrates that HGPIN is a precursor lesion to some carcinomas of the prostate, especially those in the peripheral zone. Comparing prostates with carcinoma to those without carcinoma, there is an increase in the size and number of HGPIN foci, in addition to an increased incidence of higher grade PIN (184). The finding of zones of HGPIN from which there appears to be budding off glands of carcinoma is further histologic evidence that HGPIN is a precursor to some prostate carcinomas (185). About 20% of HGPIN lesions harbor a TMPRSS2-ERG fusion gene, which is a common molecular abnormality detectable in about 50% of prostate cancers (186,187).
It is recommended that men do not need a routine repeat needle biopsy within the first year following the diagnosis of HGPIN on a single core as it is not associated with an increased risk of cancer on rebiopsy relative to men with a benign diagnosis on the initial biopsy (131). However, because of the lack of large studies on its long-term risk of cancer and the potential medicolegal consequences of not following up on an HGPIN diagnosis, a reasonable approach is to perform repeat biopsy 3 years following a diagnosis of HGPIN on a single core on needle biopsy (188). In men with two or more cores containing HGPIN, there is a higher risk of cancer on rebiopsy, and these men should undergo repeat biopsy within 6 months (189,190).
The significance of HGPIN on TUR is not clear, with conflicting data as to the risk for subsequent discovery of cancer (191–193). In an elderly patient with HGPIN on TUR, often, no further workup is instituted. In a younger man, a more aggressive workup to rule out a clinically significant tumor is warranted. If HGPIN is found on TUR and the specimen has not been put through entirely, the remainder should be processed to look for infiltrating carcinoma.
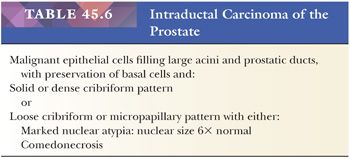
Rarely, intraductal carcinoma of the prostate (IDC-P) may be identified on biopsy material in the absence of infiltrating carcinoma. IDC-P has either architectural or cytologic atypia that clearly exceeds that seen in HGPIN (Table 45.6). IDC-P on prostate biopsies is frequently associated with Gleason score 8 to 10 cancer and poor prognostic parameters at radical prostatectomy. These findings support prior studies that IDC-P typically represents an advanced stage of tumor progression with intraductal spread of tumor, although in rare cases, it may be present unassociated with infiltrating carcinoma. We recommend that patients with IDC-P only on biopsy be treated with definitive therapy (194–199). In cases that are borderline between IDC-P and HGPIN, repeat biopsy is warranted.
CARCINOMA: CLINICAL STAGES T2 (PALPABLE) AND T1C (NONPALPABLE) CANCER DETECTED ON NEEDLE BIOPSY
METHODS OF PROCESSING RADICAL PROSTATECTOMY SPECIMENS
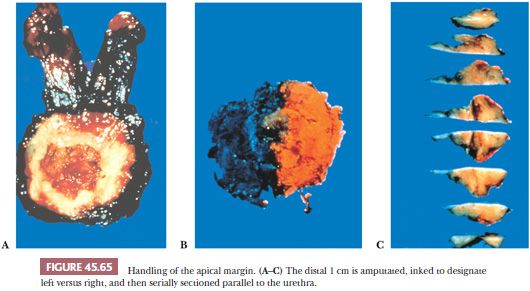
In institutions that do not totally embed radical prostatectomy specimens, these authors recommend two methods to sample the prostate, depending on whether gross tumor is visible (200–202). In both methods, routine sections include (a) amputation of the distal (apical) 1 cm of the prostate and serial section of this specimen parallel to the urethra (Fig. 45.65); (b) either a thin shave of the proximal (base) margins or amputation of the base analogous to that described for the apex; (c) base of seminal vesicles at the juncture with the prostate; and (d) right and left vas deferens margins. If there is grossly visible tumor, sections are submitted in which gross tumor is identified (Fig. 45.66). If no visible tumor is seen, the posterior sections are submitted along with one midanterior section from each side. No additional sections are required if this midanterior section shows no or only minimal tumor. If these sections demonstrate sizable tumor, then one should go back to the wet tissue and submit the anterior sections on the ipsilateral side because it indicates a large transition zone tumor component where anterior positive margins or anterior extraprostatic extension could be present.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


