1 Introduction
Regulatory authorities require that pharmaceutical products be manufactured according to the principles of good manufacturing practice (GMP) (also referred to as current good manufacturing practice, cGMP). Such authorities include the European Union (EU), the UK Medicines and Health Care Products Regulatory Agency (MHRA) and the US Food and Drug Administration (FDA). Products manufactured in the UK for the US market must satisfy the FDA. GMP guidelines were first given statutory authority in the USA and published in the UK in 1971 (see Immel, 2000 and Sharp, 2009 for a more detailed history).
Compliance must not, however, be seen as a regulatory burden. Failure in GMP can have massive consequences for the well-being of the patient and the finances of the manufacturer. For the manufacturer it can lead to litigation, losses associated with recall of product or loss of licence with attendant bad publicity. Furthermore, an incident in 2004 of failure in GMP for vaccine manufacture at a UK company led to a major shortage of fluvaccine in the USA.
Some products, such as injections, must be sterile (see Chapter 22), while others, such as oral drugs, need not be sterile but must be free from pathogens that can be contracted via the oral route (British Pharmacopoeia Commission, 2010, Appendix XVI D). More space in the literature is dedicated to quality of sterile products than that of non-sterile products, but this reflects the additional quality assurance requirements compared to those for non-sterile products (Sharp, 2000; Butson & Hawitt, 2008).
The manufacture of sterile products is carried out in both industry and hospitals. In the latter, batches tend to be much smaller, sometimes only one item, and the products are stored for a much shorter time, usually less than 24 hours (Beaney, 2005).
This chapter summarizes measures for the control during manufacture of one important feature of product quality: the level of microbial contamination. The chapter is designed to complement and be read in conjunction with Chapters 7, 17, 19, 21 and 22. It is not intended as a manual, but as an explanation of the principles involved, and more detailed information can be found in Section 8 (References and further reading).
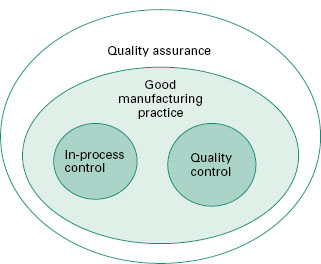
Several terms used in industrial and hospital production must be defined to enable the reader to follow this chapter. These definitions are given in sections 2.1-2.6. More detailed definitions are to be found in The Rules and Guidance for Pharmaceutical Manufacturers and Distributors (2007). The inter-relationship between quality assurance (QA), GMP, quality control (QC) and in-process control is shown in Figure 23.1.
Figure 23.1 The inter-relationship between quality assurance, good manufacturing practice, quality control and in-process control.
The UK Orange Guide (The Rules and Guidance for Pharmaceutical Manufacturers and Distributors, 2007) emphasizes the fundamental point:
Quality assurance is particularly important, and this type of manufacture must strictly follow carefully established and validated methods of preparation and procedure. Sole reliance for sterility or other quality aspects must not be placed on any terminal sterilization process or finished product test.
The difficulty in demonstrating quality is that the tests carried out are designed to show the absence of quality. For example, the test for sterility (Chapter 21) involves taking samples and testing for microorganisms. If 20 samples are tested, 3.4% of the batch needs to be contaminated to have a 50% chance of detecting that contamination. That level of contamination represents gross failure of GMP and problems would normally be detected by environmental monitoring. Indeed, it is not unknown for a batch to pass a sterility test but be rejected due to problems detected during environmental monitoring (section 5.2). Therefore it is important that a product be manufactured in a suitable environment by a procedure that minimizes the possibility of contamination occurring. At the end of this process the tests can be performed as an additional measure of quality.
2.1 Quality
There are many definitions of quality (see Sharp, 2000). For the purpose of pharmaceutical products the term quality is usually taken to mean fitness for purpose. Not only must the product have the desired therapeutic properties, it must also be safe for administration by the route intended. Sharp (2000, 2001) discussed several meanings of quality but summarizes it as follows:
in a nutshell, it is fit to be given to a patient in the confidence that it will have the desired effects and not damage him or her, in any way, through faults in manufacture.
2.2 Manufacture
Manufacture is the complete cycle of production of a medical product. This cycle includes the acquisition of all raw materials, their processing into a final product, and subsequent packaging and distribution.
2.3 Quality assurance (QA)
Quality assurance is a wide ranging concept covering all matters which individually or collectively influence the quality of a product. It is the total sum of the procedures needed to ensure the fitness of a pharmaceutical product for its intended use. QA incorporates GMP plus other factors.
2.4 Good m anufacturing practice (GMP)
GMP is that part of QA which is aimed at ensuring the product is consistently manufactured to a quality appropriate for its intended use and to meet the requirements of the regulatory authorities. GMP requires that: (1) the manufacturing process is fully defined before it begins; and (2) the necessary facilities are provided. In practice, this means that:
- Personnel must be adequately trained..
- Suitable premises and equipment must be employed.
- Correct materials must be used.
- Approved procedures must be adopted.
- Suitable storage and transport facilities must be available.
- Appropriate records must be made.
The reasons for GMP are (Sharp, 2001):
- the poor chance of the patient detecting that anything is wrong
- the weakness of product testing because:
- we can only test samples
- we cannot test for everything
- we can only test samples
- the dangers to patients of even only a small number of defective or wrongly labelled items in a batch (and it is very difficult to detect a small number of defectives).
It is about getting things right all along the line.
2.5 Quality control (QC)
QC is that part of GMP concerned with sampling, specifications and testing, as well as the organization, documentation and release procedures which ensure that the necessary and relevant tests are carried out, and that materials are not released for use, nor products released for sale or supply, until their quality has been judged satisfactory. For sterile products QC includes testing for sterility and pyrogens (see Chapters 21 and 22). The Rules and Guidance for Pharmaceutical Manufacturers and Distributors (2007) states that QC is not confined to laboratory operations, but must be involved in all decisions which may affect the quality of the product. The independence of QC from production is considered fundamental to the satisfactory operation of QC.
2.6 In-process control
This comprises any test on a product, the environment or the equipment that is used during the manufacturing process. An example of this is testing that an autoclave is functioning correctly (Gardner & Peel, 1998).
2.7 Validation
A documented programme that provides a high degree of assurance that a specific process, method or system will consistently produce a result meeting predetermined acceptance criteria.
3 Control of microbial contamination during manufacture: general a spects
A pharmaceutical product may become contaminated by a number of means and at several points during manufacture. There are several ways in which this risk can be minimized. Any such measures require an understanding of the risks involved (Chapter 17).
3.1 Risk assessment
GMP is informed by past mistakes and case studies have been valuable (Friedman, 2004). However a proactive approach is required. Nowadays a manufacturer is expected to demonstrate to the regulatory authorities that an extensive risk assessment has been carried out. Risk analysis must comply with ICH 9Q (EMEA, 2006) and is underpinned by a sound understanding of the process and of the microbial ecology of the environment and ingredients.
Several methods are employed (see EMEA, 2006; Kirupakar, 2007), including hazard analysis critical control points (HACCP), failure mode and effects analysis (FMEA), fault tree analysis (FTA), risk ranking and filtering (RRF) and hazard operability analysis (HAZOP). Only HACCP and FMEA are discussed here.
3.1.1 Hazard analysis critical control points (HACCP)
HACCP has been widely used in the food industry and is becoming more commonly used in the pharmaceutical industry (McCullogh, 2007; Sharp, 2000; WHO, 2003; Whyte, 2010). The original HACCP had seven steps:
1 Conduct a hazard analysis and identify preventive measures for each step of the process
2 Determine the critical control points.
3 Establish critical limits.
4 Establish a system to monitor the critical control points.
5 Establish the corrective action to be taken when monitoring indicates that the critical control points are not in a state of control.
6 Establish a system to verify that the HACCP procedure is working effectively.
7 Establish a record-keeping system.
However, HACCP has been modified so that it can be applied quantitatively not only to microbiology but also to pyrogens and particles (Tidswell, 2004; Whyte, 2010).
3.1.2 Failure mode and effects analysis (FMEA)
FMEA was first used in the engineering industry (Stamatis, 2003). It involves breaking the process down into many discrete steps. For each step scales are set for severity, occurrence and detection. The scores are multiplied and compared to an informed score at which risk becomes unacceptable.
3.2 Environmental cleanliness and hygiene
Microorganisms may be transferred to a product from working surfaces, fixtures and equipment. Pooled stagnant water is a frequent source of contamination. Thus it is essential that all working areas are kept clean, dry and tidy. Any cracks where microorganisms may accumulate must be eliminated. All walls, floors and ceilings should be easy to clean. This entails impervious and washable surfaces, free from open joints or ledges. Coving should be used at junctions between walls and floors or ceilings. All services such as pipes, light fittings and ventilation points should be sited so that inaccessible recesses are avoided. A rigorous disinfection policy must be in place (Chapter 19; Pharmig 2006). All equipment must be easy to dismantle and clean and should be inspected for cleanliness before use.
Fall-out of dust-and droplet-borne microorganisms from the atmosphere is an obvious route for contamination. ‘Clean’ air (section 4.1.4) is therefore a prerequisite during manufacturing processes and the spread of dust during manufacture or packaging must be avoided. Microorganisms may thrive in certain liquid preparations and creams and ointments (Chapter 22). The manufacture of such products should, as far as possible, be in a closed system; this serves a dual purpose as it also prevents evaporative loss.
Personnel are another source of potential contamination. High standards of personal hygiene are essential. Operatives should be free from communicable disease and open lesions on exposed body surfaces. To ensure high standards of personal cleanliness, adequate hand-washing and hand-disinfecting facilities and protective garments, including headgear and gloves, must be provided. Staff should be trained in the principles of GMP and in the practice (and theory) of the tasks assigned. Staff employed in the manufacture of sterile products should also receive training in basic microbiology.
3.3 Quality of starting materials
Raw materials account for a high proportion of the microorganisms introduced during the manufacture of pharmaceuticals, and the selection of materials of good microbiological quality aids in the control of contamination levels in both products and the environment. It is, however, common to have to accept raw materials which have some non-pathogenic microorganisms present and this must be considered during risk assessment. Whatever the means of prevention of growth or survival by chemical or in-process treatment, it should be regarded as critical and controlled accordingly (Sharp 2000).
Untreated raw materials that are derived from a natural source usually support an extensive and varied microflora. Products from animal sources such as gelatin, desiccated thyroid, pancreas and cochineal may be contaminated with animal-borne pathogens. For this reason some statutory bodies such as the British Pharmacopoeia require freedom of such materials from Escherichia coli and Salmonella spp. at a stated level before they can be used in the preparation of pharmaceutical products. The microflora of materials of plant origin such as gum acacia and tragacanth, agar, powdered rhubarb and starches may arise from those indigenous to plants and may include bacteria such as Erwinia spp., Pseudomonas spp., Lactobacillus spp., Bacillus spp., streptococci, moulds such as Cladosporium spp., Alternaria spp. and Fusarium spp. and non-mycelated yeasts, or those introduced during cultivation. For example, the use of untreated sewage as a fertilizer may result in animal-borne pathogens such as Salmonella spp. being present. Some refining processes modify the microflora of raw materials; for example, drying may concentrate the level of spore-forming bacteria and some solubilizing processes may introduce waterborne bacteria such as Escherichia coli. Synthetic raw materials are usually free from all but incidental microbial contamination.
The storage condition of raw materials, particularly hygroscopic substances, is important, and as a minimum water activity (Aw) of 0.70 is required for osmophilic yeasts, 0.80 for most spoilage moulds and 0.91 for most spoilage bacteria, precautions should be taken to ensure that dry materials are held below these levels (Chapter 17). Some packaging used for raw materials, such as unlined paper sacks, may absorb moisture and may itself be subject to microbial deterioration and so contaminate the contents; for this reason polythene-lined sacks are preferable. Some liquid or semisolid raw materials contain preservatives, but others such as syrups depend upon osmotic pressure to prevent the growth of osmophiles, which are often present. With this type of material it is important that they are held at a constant temperature, as any variation may result in evaporation of some of the water content followed by condensation and dilution of the surface layers to give an Aw value which may permit the growth of osmophiles and spoil the syrup.
The use of natural products with a high non-pathogenic microbial count is possible if a sterilization stage is included either before or during the manufacturing process. Such sterilization procedures (see also Chapter 21) may include heat treatment, filtration, irradiation, recrystallization from a bactericidal solvent such as an alcohol, or for dry products, where compatible, ethylene oxide gas. If the raw material is only a minor constituent and the final product is adequately preserved either by low Aw, chemically or by virtue of its pH, sugar or alcohol content, an in-process sterilization stage may not be necessary. If, however, the product is intended for parenteral or ophthalmic use a sterilization stage is essential.
The handling of contaminated raw materials as described previously may increase the airborne contamination level, and if there is a central dispensing area precautions may be necessary to prevent airborne cross-contamination, as well as that from contaminated measuring and weighing equipment. This presents a risk for all materials but in particular those stored in the liquid state where contamination may result in the bulk being spoiled.
3.4 Water
Many grades of water are used in pharmaceutical manufacturing (Table 23.1). Water for manufacturing may be potable mains water, water purified by ion exchange, reverse osmosis or distillation, or water for injection purposes (EMEA, 2002).
Table 23.1 Types of water for sterile manufacture
| Type | Properties | Use |
| Mains (potable) | Not sterile. Contains ions, chlorine | Initial washing if rinsed with purified water |
| Purified water | Potable water purified by distillation, ion exchange, reverse osmosis | Not sterile |
| Washing containers | ||
| Water for injections BP | Distilled water, free from pyrogens (some countries allow reverse osmosis) | |
| Water for injections in bulk | Final rinse | |
| Solutions to be sterilized | ||
| Sterile water for injections | Autoclaved in suitable container | Sterile solutions |
Most types of water are derived from municipal supplies. Such water is treated, sometimes by filtration, and always by chemicals, usually chlorine, to render it free from coliforms. This water is, however, not sterile. Its microbial and chemical content varies from region to region and the microbial count can increase on storage.
Water used for parenteral products, known as Water for Injections or Water For Injection (WFI), must be virtually apyrogenic (Chapter 22). The British Pharmacopoeia (British Pharmacopoeia Commission, 2010) and the US Pharmacopeia (2009a) specify an endotoxin level of no more than 0.25 IU/ml for WFI. In Europe such water is usually produced in a still specially designed to prevent pyrogens from being mechanically carried over into the distillate. In other countries reverse osmosis may also be used (US Pharmacopoeia, 2009a), but in Europe reverse osmosis is not approved (EMEA, 2002). WFI can be used immediately for the preparation of injections, provided it is sterilized within 4 hours of water collection. Alternatively, the water can be kept for longer periods at a temperature above 65°C (typically 80°C) to prevent bacterial growth with consequent pyrogen production. Ultraviolet radiation may be useful for treating WFI in order to reduce the bacterial count, but this must not be regarded as a sterilization process (Chapter 21). A more detailed account of water for pharmaceutical use may be found in EMEA (2002) and US Pharmacopeia (2009b).
3.5 Process design
The manufacturing process must be fully defined and capable of providing, with the facilities available, a product that is microbiologically acceptable and conforms to specifications. The process must be fully validated before starting to ensure that it is suitable for routine production operations. Processes and procedures must also be subject to frequent reappraisal and should be re-evaluated when any significant changes are made in the equipment or materials used.
3.6 Quality control and documentation
The lower the microbiological count of the starting materials, the more readily the quality of the product can be controlled. Microbiological standards should be set for all raw materials as well as microbial limits for in-process samples and the final product. Microbiological quality assurance also covers the validation of cleaning and dis-infectant solutions and the monitoring of the production environment by microbial counts. This monitoring should be carried out while normal production operations are in progress. In addition, sterile manufacture requires extra safeguards. Operators must be adequately trained and their aseptic technique monitored both by observation and microbiological testing. Air filter and sterilizer efficiency must also be evaluated (Chapter 21), whilst sterility testing (Chapter 21) and, where necessary testing for pyrogens (Chapter 22), are the final tests on the finished product.
Documentation is a vital part of quality assurance. Details of starting materials, packaging materials, and intermediate, bulk and finished products should be recorded so that the history of each batch may be traced. Distribution records must be kept. This information is of paramount importance in the event that a defective batch has to be recalled.
3.7 Packaging, storage and transport
Packaging serves a number of functions; it keeps the contents in, it should keep contaminants out and is labelled to permit identification of its contents. The product is contained within primary packaging. In industry these packages are then placed inside secondary packaging for storage and transport. This secondary packaging may take the form of cartons, boxes, trays or shrink wrapping.
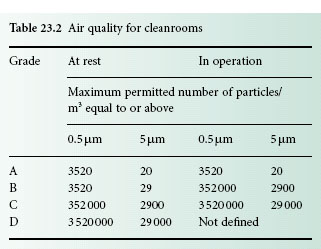
Consideration must be given to both the fabric of the packaging and its cleaning, and to the actual process of packaging. Where terminal sterilization is carried out, the packaging must be suitable for the process. Packaging of aseptically processed products into a sterile container (section 3.7) must be carried out in a grade A environment (Table 23.2).
< div class='tao-gold-member'>