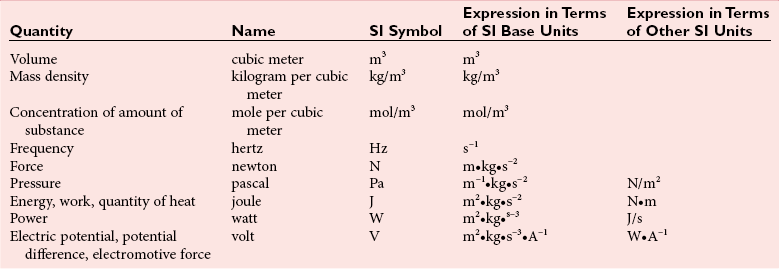
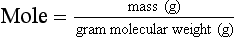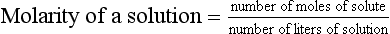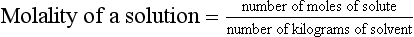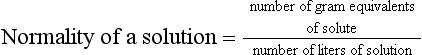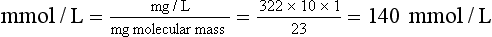Chapter 9 To reliably perform qualitative and quantitative analyses on body fluids and tissue, the clinical laboratorian must understand the basic principles and techniques of analytical chemistry. Valcárcel has generically defined analytical chemistry as “a metrological science that develops, optimizes, and applies measuring processes intended to derive quality analytical information in order to solve the measuring problems posed.”25 Factors that affect the analytical process and operation of the clinical laboratory include knowledge of (1) the concept of solute and solvent; (2) units of measurement; (3) chemicals; (4) reference materials; (5) basic techniques, such as volumetric sampling and dispensing, centrifugation, measurement of radioactivity, gravimetry, thermometry, buffer solution, and processing of solutions; and (6) safety. Many analyses in the clinical laboratory are concerned with determination of the presence or measurement of the concentration of substances in solutions, the solutions most often being blood, serum, urine, spinal fluid, or other body fluids (see Chapter 7). Base, derived, and supplemental units are the three classes of SI units.20 The eight fundamental base units are listed in Table 9-1. A derived unit is derived mathematically from two or more base units (Table 9-2). A supplemental unit is a unit that conforms to the SI but has not been classified as either base or derived. At present, only the radian (for plane angles) and the steradian (for solid angles) are classified this way. TABLE 9-1 TABLE 9-2 Examples of SI-Derived Units Important in Clinical Medicine, Expressed in Terms of Base Units The minute, hour, and day have had such long-standing use in everyday life that it is unlikely that new SI units derived from the second could supplant them. Some other non-SI units are still accepted, although they are rarely used by most individuals in their daily lives but have been very important in some specialized fields. Details of the SI system are found in an expanded version of this chapter.2 In practical application of units, certain values are too large or too small to be expressed conveniently. Numeric values are brought to convenient size when the unit is appropriately modified by official prefixes (Table 9-3). TABLE 9-3 *The Eleventh Conférence Générale des Poids et Mésures (CGPM) (1960, Resolution 12) adopted a first series of prefixes and symbols of prefixes to form the names and symbols of the decimal multiples and submultiples of SI units. Prefixes for 10−15 and 10−18 were added by the 12th CGPM (1964, Resolution 8) and those for 1015 and 1018 by the 15th CGPM (1975, Resolution 10), and those for 1021, 1024, and 10−24 were proposed by the Comité International des Poids et Mésures (CIPM) (1990) for approval by the 19th CGPM (1991). †Outside the United States, the spelling “deca” is used extensively. From The International System of Units (SI). Washington, DC: National Institute of Standards and Technology, 1991. To describe test results properly, it is important that all necessary information be included in the test description. Systems developed for expressing results produced by the clinical laboratory include the Laboratory Logical Observation Identifiers, Names, and Codes (Lab LOINC),15–17 and the Nomenclature, Properties, and Units (NPU) developed by the International Federation of Clinical Chemistry/International Union of Pure and Applied Chemistry (IFCC/IUPAC). The Lab LOINC system is a universal coding system for reporting laboratory and other clinical observations to facilitate electronic transmission of laboratory data within and between institutions (http://www.loinc.org). It has several thousand observations in its database. For each observation, there is a code, a long formal name, a short 30-character name, and synonyms. A mapping program termed “Regenstrief LOINC Mapping Assistant” (RELMA) is available to map local test codes to LOINC codes and to facilitate searching of the Lab LOINC database. Both Lab LOINC and RELMA are available at no cost from http://loinc.org/relma (accessed March 16, 2011). The Lab LOINC and NPU coding systems are used in context with existing standards, such as the Systematized Nomenclature of Medicine, Clinical Terms (SNOMED CT). Other such coding systems are the ASTM E1238 (American Society for Testing and Materials), HL7 version 2.2 (Health Level Seven; http://www.hl7.org; accessed March 16, 2011), and CEN ENV 1613—a standard developed by the European Committee for Standardization of the Comité Européen de Normalisation (CEN) Technical Committee 251 (http://www.cen.eu/ accessed March 16, 2011). SNOMED CT is a comprehensive clinical terminology, originally created by the College of American Pathologists (CAP) and, as of April 2007, owned, maintained, and distributed by the International Health Terminology Standards Development Organisation (IHTSDO) in Denmark (http://www.ihtsdo.org; accessed March 16, 2011). IHTSDO is a not-for-profit association that develops and promotes use of SNOMED CT to support safe and effective health information exchange. In practice, the CAP continues to support SNOMED CT operations under contract to the IHTSDO and provides SNOMED-related products and services as a licensee of the terminology. Preparation of many reagents and solutions used in the clinical laboratory requires “pure” water. Single-distilled water fails to meet the specifications for Clinical Laboratory Reagent Water (CLRW) established by the Clinical Laboratory and Standards Institute (CLSI).10 Because the terms deionized water and distilled water describe preparation techniques, they should be replaced by reagent grade water, followed by the designation of CLRW, which better defines the specifications of the water and is independent of the method of preparation (Table 9-4). TABLE 9-4 Clinical Laboratory and Standards Institute (CLSI) Specifications for Reagent Water *Microbiological content. The microbiological content of viable organisms, as determined by total colony count after incubation at 36 ± 1 °C for 14 hours, followed by 48 hours at 25 ± 1 °C, and reported as colony-forming units per mL (cfu/mL). †Specific resistance or resistivity. The electrical resistance in ohms measured between opposite faces of a 1-cm cube of an aqueous solution at a specified temperature. For these specifications, the resistivity will be corrected for 25 °C and reported in MΩ/cm. The higher the quantity of ionizable materials, the lower will be the resistivity and the higher the conductivity. ‡Particulate matter. When water is passed through a membrane filter with a mean pore size of 0.2 µm, it is considered to be free of particulate matter; when water is passed through a bed of activated carbon, it is considered to contain minimum organic material. From CLSI. Preparation and testing of reagent water in the clinical laboratory, 4th edition. CLSI Document C3-A4. Wayne, Pa: CLSI, 2006. At a minimum, water should be tested for microbiological content, pH, resistivity, and soluble silica,10 and the maximum interval in the testing cycle for purity of reagent water should be 1 week. It should be noted that measurements taken at the time of production may differ from those taken at the time and place of use. For example, if the water is piped a long distance, consideration must be given to deterioration en route to the site of use. To meet the specifications for high-performance liquid chromatography (HPLC), in some instances it may be necessary to add a final 0.1-µm membrane filter. The water can be tested by HPLC using a gradient program and monitoring with an ultraviolet (UV) detector. No peaks exceeding the analytical noise of the system should be found. Chemicals that meet specifications of the American Chemical Society (ACS) are described as reagent or analytical reagent grade. These specifications have also become the de facto standards for chemicals used in many high-purity applications. These are available in two forms: (1) lot-analyzed reagents, in which each individual lot is analyzed and the actual amount of impurity reported, and (2) maximum impurities reagents, for which maximum impurities are listed. The Committee on Analytical Reagents of the ACS periodically publishes “Reagent Chemicals” listing specifications (http://pubs.acs.org/reagents/index.html; accessed March 16, 2011). These reagent grade chemicals are of very high purity and are recommended for quantitative or qualitative analyses. A reference material is a material or substance with one or more physical or chemical properties that is sufficiently well established to be used for (1) calibrating instruments, (2) validating methods, (3) assigning values to materials, and (4) evaluating the comparability of results. Reference materials are of prime importance in establishing metrologic transferability (http://www.bipm.org; accessed March 16, 2011),1,26 a term defined as “the property of a measurement result whereby the result can be related to a reference through a documented unbroken chain of calibrations, each contributing to the measurement uncertainty.”13 Primary, secondary, standard, and certified are types of reference materials. Standard reference materials (SRMs) for clinical and molecular laboratories are available from the National Institute of Standards and Technology (NIST; http://ts.nist.gov; accessed March 16, 2011). Cholesterol, the first SRM developed by the NIST, was issued in 1967. It should be noted that not all SRMs have the properties and the degree of purity specified for a primary standard, but each has been well characterized for certain chemical or physical properties and is issued with a certificate that gives results of the characterization. These may then be used to characterize other materials. Examples of SRMs that are available from the NIST for use in clinical and molecular diagnostics laboratories include (1) pure crystalline standards (Table 9-5), (2) human-based standards (Table 9-6), (3) animal blood standards (Table 9-7), (4) standards containing drugs of abuse in urine and human hair (Table 9-8), and (5) SRMs used for DNA profiling/crime scene investigations (Table 9-9). TABLE 9-5 Standard Reference Materials (SRMs)—Pure Crystalline Standards Available from the National Institute of Standards and Technology (www.nist.gov; accessed March 16 2011). TABLE 9-6 Standard Reference Materials (SRMs)—Human Serum Based PCBs, Polychlorinated biphenyls. Available from the National Institute of Standards and Technology (www.nist.gov; accessed March 16, 2011). TABLE 9-7 Standard Reference Materials (SRMs)—Miscellaneous Available from the National Institute of Standards and Technology (www.nist.gov; accessed March 16, 2011). TABLE 9-8 Standard Reference Materials (SRMs) for Drugs of Abuse in Urine and Human Hair Available from the National Institute of Standards and Technology (www.nist.gov; accessed March 16, 2011). TABLE 9-9 Standard Reference Materials (SRMs) for Use in DA Profiling/Crime Scene Investigations PCR, Polymerase chain reaction; RFLP, restriction fragment length polymorphism. Available from the National Institute of Standards and Technology (www.nist.gov accessed March 16, 2011). Certified reference materials (CRMs) are available for clinical and molecular laboratories from the Institute for Reference Materials and Measurements (IRMM) in Geel, Belgium (http://www.irmm.jrc.be; accessed March 16, 2011). The IRMM is one of the seven institutes of the Joint Research Centre (JRC), a Directorate-General of the European Commission (EC). Other acronyms used to label IRMM reference materials include ERM (European Reference Materials), BCR (Community Bureau of Reference of the Commission of the European Communities), and the IFCC (International Federation of Clinical Chemistry). Examples of available IRMM standards are listed in Tables 9-10 and 9-11. Reference materials also are available from the World Health Organization (WHO; http://www.who.int/biologicals; accessed March 16, 2011). TABLE 9-10 Reference Materials Available from the Institute for Reference Materials and Measurements (www.irmm.jrc.be; accessed March 16, 2011) TABLE 9-11 Standards Certified for DNA Sequence Available from the Institute for Reference Materials and Measurements (www.irmm.jrc.be; accessed March 16, 2011)* *Availability: Each polypropylene vial contains approximately 1 ng plasmid DNA in a volume of 50 µL of a tris/EDTA solution. Basic practices used in clinical and molecular diagnostic laboratories include (1) optical, (2) chromatographic, (3) electrochemical, (4) electrophoretic, (5) mass spectrometric, (6) enzymatic, and (7) immunoassay techniques. These techniques are discussed in detail in Chapters 10 through 16. Here we discuss the basic techniques of volumetric sampling and dispensing, centrifugation, measurement of radioactivity, gravimetry, thermometry, controlling hydrogen ion concentration, and processing solutions. Transfer Pipettes: Transfer pipettes include both volumetric and Ostwald-Folin pipettes (Figure 9-1). They consist of a cylindrical bulb joined at both ends to narrower glass tubing. A calibration mark is etched around the upper suction tube, and the lower delivery tube is drawn out to a gradual taper. The bore of the delivery orifice should be sufficiently narrow to allow rapid outflow of liquid and incomplete drainage cannot cause measurement errors beyond tolerances specified. A volumetric transfer pipette (Figure 9-1, A) is calibrated to deliver accurately a fixed volume of a dilute aqueous solution. The reliability of the calibration of the volumetric pipette decreases with decreased size, and therefore special micropipettes have been developed. Ostwald–Folin pipettes (Figure 9-1, B) are similar to volumetric pipettes but have the bulb closer to the delivery tip and are used for accurate measurement of viscous fluids, such as blood or serum. In contrast to a volumetric pipette, an Ostwald-Folin pipette has an etched ring near the mouthpiece, indicating that it is a blow-out pipette. With the use of a pipetting bulb, the liquid is blown out of the pipette only after the blood or serum has drained to the last drop in the delivery tip. When filled with opaque fluids, such as blood, the top of the meniscus must be read. Controlled slow drainage is required with all viscous solutions so that no residual film is left on the walls of the pipette. Measuring Pipettes: The second principal type of pipette is the graduated or measuring pipette (Figure 9-1, C). This is a piece of glass tubing that is drawn out to a tip and graduated uniformly along its length. Two types are available. The Mohr pipette is calibrated between two marks on the stem, whereas the serologic pipette has graduated marks down to the tip. The serologic pipette (Figure 9-1, D) must be blown out to deliver the entire volume of the pipette and has an etched ring (or pair of rings) near the bulb end of the pipette signifying that it is a blow-out pipette. Mohr pipettes require controlled delivery of the solution between the calibration marks. Serologic pipettes have a larger orifice than do Mohr pipettes and thus drain faster. In practice, measuring pipettes are used principally for measurement of reagents and generally are not considered sufficiently accurate for measuring samples and calibrators.
Principles of Basic Techniques and Laboratory Safety
Concept of Solute and Solvent
Units of Measurement
International System of Units
Quantity
Name
Symbol
Length
meter
m
Mass
kilogram
kg
Time
second
S
Electrical current
ampere
A
Thermodynamic temperature
kelvin
K
Amount of substance
mole
mol
Luminous intensity
candela
cd
Catalytic amount
katal
kat
Factor
Prefix
Symbol
1024
yotta
Y
1021
zetta
Z
1018
exa
E
1015
peta
P
1012
tera
T
109
giga
G
106
mega
M
103
kilo
k
102
hecto
h
101
deka†
da
10−1
deci
d
10−2
centi
c
10−3
milli
m
10−6
micro
µ
10−9
nano
n
10−12
pico
p
10−15
femto
f
10−18
atto
a
10−21
zepto
z
10−24
yocto
y
Standardized Reporting of Test Results
Lab LOINC System
Applications
Chemicals
Reagent Grade Water
CLRW
Microbiological content,* colony-forming units per mL, cfu/mL (maximum)
10
pH
Not applicable
Resistivity,† MΩ per centimeter (MΩcm), 25 °C
≥10 (in line)
Silicate, mg SiO2/L (maximum)
0.05
Particulate matter‡
Water passed through 0.2-µm filter
Organics
Water passed through activated carbon
Testing for Water Purity
Reagent Grade or Analytical Reagent Grade (AR) Chemicals
Reference Materials
Standard Reference Materials (SRMs)
SRM Number
Analyte
998
Angiotensin I (human)
916a
Bilirubin
915b
Calcium Carbonate
911c
Cholesterol
921
Cortisol (hydroxycortisone)
914a
Creatinine
917b
D-Glucose (dextrose)
920
D-Mannitol
937
Iron Metal (clinical)
928
Lead Nitrate
924a
Lithium Carbonate
929a
Magnesium Gluconate Dihydrate
918b
Potassium Chloride
919b
Sodium Chloride
1595
Tripalmitin
912a
Urea
913a
Uric Acid
925
VMA (4-Hydroxy-3-Methoxy-DL-Mandelic Acid)
8327
Peptide Reference Materials
(For Molecular Mass and Purity Measurements)
SRM Number
Description
909b
Human Serum (Contains 12 Analytes)
1951b
Lipids in Frozen Human Serum
956b
Electrolytes in Frozen Human Serum
965a
Glucose in Frozen Human Serum
967
Creatinine in Frozen Human Serum
970
Ascorbic Acid in Frozen Human Serum
1952a
Cholesterol in Frozen Human Serum
968c
Fat-Soluble Vitamins in Human Serum
1589a
PCBs, Pesticides, and Dioxins/Furans in Human Serum
1599
Anticonvulsant Drug Level Assay (Valproic Acid and Carbamazepine)
900
Antiepilepsy Drug Level Assay
1955
Homocysteine and Folate in Human Serum
SRM Number
Description
955c
Lead in Caprine (Goat) Blood
966
Toxic Elements in Bovine Blood
1598
Inorganic Constituents in Bovine Serum
927d
Bovine Serum Albumin
2921
Cardiac Troponin Complex
2389
Amino Acids in HCl
1400
Bone Ash
1486
Bone Ash
SRM
Description
1508 a
Cocaine Metabolite in Urine
RM 8444
Cotinine in Urine
1507b
Marijuana Metabolite in Urine
2381
Morphine and Codeine in Urine
2382
Morphine Glucuronide in Urine
1511
Multi Drugs of Abuse in Urine
2379
Drugs of Abuse in Human Hair I
2379
Drugs of Abuse in Human Hair I
SRM
Description
2372
Human DNA Quantitation Standard
2390
DNA Profiling Standard—RFLP
2391b
PCR-Based DNA Profiling Standard
2392
Human Mitochondrial DNA Sequencing (3 Components)
2392-1
Human Mitochondrial DNA Sequencing (1 Component)
2394
Heteroplasmic Mitochondrial DNA Mutation Detection Standard
2395
Human Y-Chromosome DNA Profiling Standard
2396
Oxidative DNA Damage Mass Spectrometry Standard
2399
Fragile X Human DNA Triplet Repeat Standard
Certified Reference Materials (CRMs)
Number
Description
BCR-304
Lyophilized Human Serum
BCR-573; 574; and 575
Creatinine in Human Serum
IRMM-468 and 469
Thyroxine (T4) and Triiodothyronine (T3), Two Levels Each
ERM-DA451/IFCC
Cortisol Reference Panel of Fresh Frozen Human Serum
ERM-DA192 and 193
Cortisol in Human Serum
BCR-348R and BCR-DA347
Progesterone in Human Serum
BCR-576; 577; and 578
17-β-Estradiol in Human Serum
ERM-CE-194; 195; and 196
Pb and Cd in Lyophilized Bovine Blood
BCR-634; 635; and 636
Pb and Cd in Lyophilized Human Blood
BCR-637; 638; and 639
Al, Se, and Zn in Human Serum
BCR-393
Lyophilized APO A1 from Human Serum
BCR-457
Human Thyroglobin (Tg)
BCR-486
Purified Alpha Fetoprotein (AFP)
BCR-613
Prostate Specific Antigen (PSA) in the Reconstituted Material
BCR-405
Glycated Hemoglobin (HbA1c) in Human Hemolysate
ERM-DA470k
Human Serum Proteins
ERM-DA472/IFCC
C-Reactive Protein (CRP)
BCR-522
Hemiglobincyanide (HCN) in Bovine Blood Lysate
IRMM/IFCC-466 and 467
Hemoglobin Isolated from Whole Blood
BCR-410
Prostatic Acid Phosphatase from Human Prostate
BCR-647
Human Adenosine Deaminase (ADA1) from Human Erythrocytes
BCR-693
Human Pancreatic Lipase from Pancreatic Juice
BCR-6974
Human Pancreatic Lipase (Recombinant)
ERM-AD452/IFCC
γ-Glutamyltransferase from Pig Kidney
ERM-AD453/IFCC
Human Lactate Dehydrogenase Isoenzyme 1
ERM-AD454/IFCC
Alanine Aminotransferase from Pig Heart
ERM-AD455/IFCC
Creatine Kinase (CK-MB) from Human Heart
IRMM/IFCC-456
Human Pancreatic α-Amylase
ERM-AD457/IFCC
Aspartate Transaminase (AST)
Number
Plasmid DNA
IRMM/IFCC-490
Sequence of 609 bp DNA Fragment from Human Prothrombin Gene (G20210 Wildtype Sequence)
IRMM/IFCC-491
Sequence of 609 bp DNA Fragment from Human Prothrombin Gene (Point Mutation G20210A)
IRMM/IFCC-492
Sequence of 609 bp DNA Fragment from Human Prothrombin Gene (G20210 Wildtype and Point Mutation G20210A Sequences)
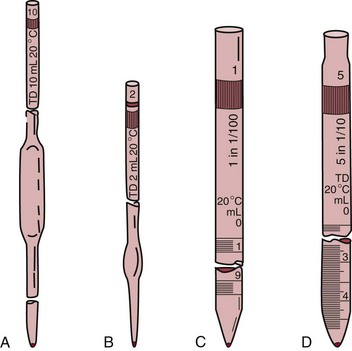
Basic Techniques and Procedures
Volumetric Sampling and Dispensing
Pipettes
Transfer and Measuring Pipettes
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree