http://evolve.elsevier.com/McCuistion/pharmacology/
Physiology of Pregnancy
Because pregnancy elicits many changes in the normal physiology of the body, the normal and expected pharmacokinetics and pharmacodynamics of drugs are also changed during pregnancy. Changes in drug action during pregnancy include the (1) effect of circulating steroid hormones on the liver’s metabolism of drugs; (2) reduced gastrointestinal (GI) motility and increased gastric pH; (3) increased glomerular filtration rate and increased renal perfusion, resulting in more rapid renal excretion of drugs; (4) expanded maternal circulating blood volume, resulting in dilution of drugs; and (5) alteration in the clearance of drugs in later pregnancy, resulting in a decrease in serum and tissue concentrations of drugs. Because of alterations in the normal physiology of the body, drugs should not be ordered in lower doses with longer intervals between doses because of the possibility of subtherapeutic serum concentrations.
Other factors, such as late pregnancy and labor, can alter the half-lives of some drugs. Antibiotics and barbiturates are examples of drugs that have shorter half-lives during pregnancy. In contrast to later pregnancy, labor can actually increase the half-life of some drugs (e.g., analgesics, hypnotics, antibiotics); it is believed that drug clearance decreases as a result of transient reduced blood flow associated with uterine contractions when the patient is in a supine position. There is also concern about the effects of certain disease states on medication usage during pregnancy. Disorders such as diabetes mellitus and gestational hypertension may result in decreased renal perfusion and subsequent drug accumulation.
The placenta plays an important role in drug use and metabolism. It was once thought that the placenta played a barrier role, but it is now known that the placenta has an important function as the organ of exchange for numerous substances, including drugs. It allows some substances to transfer quickly or slowly between mother and fetus, depending on variables such as (1) maternal and fetal blood flow; (2) molecular weight of the substance (low–molecular-weight substances cross more readily than do high–molecular-weight substances; most drugs have a low molecular weight, so they readily cross the placenta); (3) degree of ionization of the drug molecule (the more ionized the molecule, the less readily it crosses the placenta); (4) degree of protein binding (highly bound drugs do not cross readily); (5) metabolic activity of the placenta (the metabolic activity can biotransform molecules into active metabolites that can affect the fetus); and (6) maternal dose.
Guidelines for drug administration during pregnancy must include determination that the benefits of prescribing a drug outweigh potential short- or long-term risks to the maternal-fetal system. Careful selection and monitoring for the minimum effective dose for the shortest interval in the therapeutic range are required. Consideration must be given to alterations related to the physiologic changes of pregnancy.
Liver metabolism of drugs is much slower in the fetus as a result of immaturity of the liver, which can cause more evident or longer drug effects on the fetus than on the mother. The degree of fetal exposure to a drug and its breakdown products are more important to fetal outcome than the rate at which the drug is transported to the fetus.
The mechanisms by which drugs cross the placenta are analogous to the way in which drugs infiltrate breast tissue. Lactation results in increased blood flow to the breasts, and drugs accumulate in adipose breast tissue through simple diffusion. Long-term effects on infants from drugs in breast milk are unknown, but drugs that accumulate in breast milk are known, and the breastfeeding patient should be alerted to the potential accumulation.
Despite prenatal education, public service announcements, and information conveyed through the media, use of legal and illicit drugs by pregnant patients continues. Additionally, health care providers may prescribe drugs for maternal disorders that indirectly affect the fetus. It is estimated that half the drugs taken by pregnant patients are over-the-counter (OTC) drugs. The drugs most commonly ingested during pregnancy are iron supplements, vitamins, antiemetics, antacids, stool softeners, nasal decongestants, mild analgesics, and antibiotics. Although many drugs required by pregnant patients can be used safely, pregnant patients should be discouraged from using OTC drugs until they consult with their health care provider.
Drugs conclusively determined to be safe for the embryo are limited in number. Clinical trials can be resources for reliable drug information; however, it is unethical to test for the safety and efficacy of drugs in pregnant patients. Animal studies are required during drug testing, but the information obtained from such studies is difficult to extrapolate to humans. The U.S. Food and Drug Administration (FDA) recently changed its pregnancy category system to assist with safe prescribing for pregnant patients who require drugs. See http://www.fda.gov/Drugs/DevelopmentApprovalProcess/DevelopmentResources/Labeling/ucm093307.htm for more information.
There are many known teratogens, substances that cause developmental abnormalities. Timing, dose, and duration of exposure are crucial in determining the teratogenicity of a given drug. In humans, the teratogenic period begins 2 weeks after conception, before which the embryo is not susceptible to teratogenesis. After this 2-week point in fetal development, however, exposure to teratogens may result in either death of the embryo or minor cellular damage without congenital birth defects. Gestational week 2 through week 12 (first trimester) is the period of organogenesis, in which major structures and organs develop.
Table 49.1 shows adverse effects of selected illicit substances commonly used during pregnancy.
Therapeutic Drugs and Use of Herbs in Pregnancy
The most common indications for use of drugs during pregnancy are to supplement nutrition with iron, vitamins, and minerals and to treat nausea and vomiting, gastric acidity, and mild discomforts; however, caution must be exercised (Complementary and Alternative Therapies 49.1).
Iron
During pregnancy, approximately twice the normal amount of iron is needed to meet fetal and maternal daily requirements: 27 mg/day during pregnancy compared with 18 mg/day for nonpregnant women 19 to 30 years of age. Supplementation with iron is not generally necessary until the second trimester, when the fetus begins to store iron; the goal is to prevent maternal iron-deficiency anemia, not to supply the fetus. The fetus is adequately supplied through the placenta even though the mother is deficient. The greatest iron demand occurs in the third trimester: 22.4 mg/day, compared with 6.4 mg/day in the first trimester and 18.8 mg/day in the second trimester.
Although a normal diet generally provides the 18-mg recommended daily allowance (RDA) of iron for nonpregnant patients, pregnant patients at risk for anemia are usually instructed to supplement with 60 to 120 mg of elemental iron per day. The elemental iron content of the most common iron salts includes ferrous sulfate 20% (300 mg of ferrous sulfate is equivalent to 60 mg elemental iron), exsiccated ferrous sulfate 30%, ferrous gluconate 12%, and ferrous fumarate 33%. The estimated net iron cost of pregnancy is approximately 600 to 800 mg. This iron cost is due to the iron use by the fetus, placenta, and increased red blood cell (RBC) volume. Patients are advised to continue supplements for 6 weeks postpartum.
Pregnant patients generally have decreased hematocrit early in the third trimester. Those with levels below 30% will have their supplemental iron dosages increased, and a complete blood count (CBC) with platelet and ferritin will be measured. In those with true iron-deficiency anemia, response to iron supplementation is usually noted in 5 to 7 days with a modest reticulocytosis and an increase in hemoglobin in 3 weeks. No teratogenic effects have been reported with physiologic doses. In contrast, increasing evidence has associated prenatal iron supplementation with glucose impairment and hypertension in midpregnancy. Many OTC and prescription iron products (Table 49.2) are available in varying dosages, which differ in the amount of elemental iron contained in the form of iron salts.
TABLE 49.1
General Adverse Effects of Substances Commonly Abused During Pregnancy
| Substance | Maternal Effects | Fetal and Neonatal Effects∗ |
| Alcohol | 1 oz twice a week increased risk for spontaneous abortion. | Fetal alcohol syndrome (FAS): mild to moderate mental retardation, altered facial features, growth retardation, low birthweight, small head circumference, hypotonia, and poor motor coordination. Full FAS seen only in some children; others display only fetal alcohol effect (FAE). Pregnancy category: There is no known safe amount of alcohol use during pregnancy†. |
| Caffeine | 2 cups increase epinephrine concentrations after 30 min and decrease intervillous blood flow with potential for spontaneous abortion (dependent on amount and gestational period). | Excess consumption (>6-8 cups/d) likely toxic to embryo. Pregnancy category: C† |
| Cocaine | Clearance via urine is 72 h. Incidence of spontaneous abortion is increased in the first trimester. Continued use or sporadic use is related to premature delivery and abruptio placentae secondary to placental vasoconstriction and hyperextension. | In a newborn, renal clearance is 4-5 d because of liver immaturity and lack of cholinesterase. IUGR, decreased head circumference, and intrauterine cerebral infarction are also risks. Increased irritability, hyperreflexia, and tremulousness may occur along with deficient organization and interactive abilities. By month 4, the infant is still hypertonic, tremulous, and has impaired motor development. Studies suggest that subtle cognitive and behavioral impairments occur from the neonatal stage to adolescence. Pregnancy category: C† |
| Heroin | Heroin can cause first-trimester spontaneous abortion, premature delivery, and inadequate maternal caloric and protein intake. | Neonatal meconium aspiration syndrome; decreased weight and length through postnatal month 9, smaller head circumference at birth and during childhood development, impaired interactive abilities (hard to engage and console); inconsistent behavioral responses; increased tremulousness and irritability Pregnancy category: UK† |
| Cannabis | Studies show that women who use Cannabis during pregnancy are more likely to experience placental complications and to give birth to babies with lower birthweights. | Adverse fetal outcomes related to Cannabis use in pregnancy remain unclear. Incidence of meconium passage during labor is higher. Pregnancy category: X†. |
| Tobacco/nicotine | Degenerative placental lesions with areas of poor oxygen exchange; higher incidence of abruptio placentae and placenta previa; vaginal bleeding during pregnancy; possible PROM and possible amnionitis; less likely to choose to breastfeed | Short stature, smaller head and arm circumferences; no increase in mortality rate or congenital anomalies but some evidence of increased oral clefts; respiratory infections beyond the perinatal period were increased. Pregnancy category: C/D† |
| Methadone | Detoxification should be avoided during first trimester due to risk of spontaneous abortion and in the third trimester due to fetal stress and preterm labor. | Smaller weight and length through postnatal month 9 (catch up on weight and length occurs by 1 y); smaller head circumference (no catch-up); withdrawal-induced fetal distress occurs if the mother detoxifies after 32 weeks gestation. Pregnancy category: C† |
| Barbiturates | CNS depression; lethargy; sleepiness; mood alterations; impaired judgment; decreased fine motor skills. When taken during labor, drug may reduce the force and frequency of contractions, prolonging labor and delaying delivery. Selective anticonvulsant activity without anesthesia effects may warrant use in pregnancy for seizure disorders. Contraindicated with active labor and imminent delivery because no antagonist drug is available. | Rapidly cross placenta; with excessive use. High doses cause CNS depression, respiratory depression, hyperactivity, and decreased sucking reflex. Pregnancy category: D† |
| Tranquilizers | Effects are dose-dependent. Toxic reactions include ataxia, syncope, vertigo, and drowsiness. Tranquilizers are used to control acute eclamptic seizures during labor. | Benzodiazepine use in third trimester or labor in high doses is associated with hyperbilirubinemia, hypotonia, hypothermia, and poor sucking reflex. Effects may be enhanced if mother receives systemic analgesia. Fetal effects are prolonged. Pregnancy category: D† |
Adverse Reactions
Common side effects of iron supplements include nausea, constipation, black tarry stools, GI irritation, epigastric pain, vomiting, discoloration of urine, and diarrhea.
Nursing Implications
Liquid forms can cause temporary tooth discoloration and therefore should be diluted and administered through a straw. Iron supplements are best absorbed when administered with water or juice on an empty stomach. Vitamin C increases the absorption of iron. If gastric irritation does occur, administer the iron with food. Iron supplementation may inhibit the absorption of several drugs, and appropriate separation of doses should be followed (e.g., iron supplementation should be administered 2 hours before or 4 hours after antacids). Additional examples of drugs that may require separation in dose include levodopa, levothyroxine, methyldopa, penicillins, quinolones, and tetracyclines. For the same reasons, do not administer iron with milk, cereal, tea, coffee, or eggs.
Folic Acid
Folic acid supplementation as part of preconception planning improves the outcome of pregnancy. During pregnancy, folic acid (vitamin B9, folate) is needed in increased amounts. Folic acid deficiency early in pregnancy can result in spontaneous abortion or birth defects, especially neural tube defects; a failure of the embryonic neural tube to close properly can lead to spina bifida or skull and brain malformations. Deficiency of folic acid may also contribute to premature birth, low birthweight, and premature separation of the placenta (abruptio placentae). In the United States, approximately 4000 pregnancies a year are affected by neural tube defects (NTDs). Controlled clinical trials have demonstrated that folic acid supplementation can reduce this incidence by as much as 50%.
Normally, the RDA for folic acid is 180 mcg, but the U.S. Preventive Services Task Force recommends that women who are planning pregnancy take a supplement containing 0.4 mg to 0.8 mg of folic acid 1 month before and for the first 2 to 3 months after conception. The American Congress of Obstetricians and Gynecologists (ACOG) recommends that all women of childbearing age ingest 400 mcg of folic acid daily for birth defect prevention (during pregnancy, the RDA rises to 600 mcg). The reasoning behind the ACOG recommendation is the high incidence of unplanned and unrecognized pregnancies.
The neural tube closes within the first 4 weeks of pregnancy (18 to 26 days after conception), therefore it is important that women consume the recommended amounts of folic acid every day. For patients who have had a pregnancy that was affected by an NTD, higher doses of folic acid are recommended: 4 mg starting 1 to 3 months before conception.
The recommended amount should be ingested from folate-rich foods, such as dark green leafy vegetables, asparagus, papaya, strawberries, and oranges; from folate-enriched foods, such as bread, rice, cornmeal, pasta, and cereal; and from supplementation because the amount of naturally occurring folic acid ingested in foods varies from day to day, and the folic acid from these sources is not well absorbed.
TABLE 49.2
| Generic | Route and Dosage | Uses and Considerations |
Iron salts (ferrous sulfate, ferrous gluconate, ferrous fumarate) Caution: Read the label carefully. The amount of elemental iron differs according to the formulation. | PO: 27-100 mg/d of elemental iron Dose dependent on prepregnancy iron stores. | Hematinic for iron-deficiency anemia and prophylaxis for iron deficiency in pregnancy. Replaces iron stores needed for RBC development. Absorption PO is 5%-30% in intestines, therefore GI side effects may occur. Toxic reactions include pallor, hematemesis, shock, cardiovascular collapse, and metabolic acidosis. Contraindicated in patients with hypersensitivity or peptic ulcer. Decreased absorption of zinc, tetracycline and penicillamine and increased absorption with ascorbic acid, such as orange juice; decreased absorption with antacids, eggs, milk, coffee, and tea. Take at bedtime to avoid GI upset. Use straw for elixir to prevent staining of teeth, swallow tab/cap whole with full glass of water or juice, preferably on an empty stomach. Sit upright 30 min after dose to decrease reflux. Increase fluids, activity, and dietary bulk. Keep out of reach of children. Peak reticulocytosis: 5-10 d; hemoglobin values increase in 2-4 wk Pregnancy category A∗; PB: UK; t½: UK; onset: 3-10 d; duration: 3-4 mo |
Adverse Reactions
Side effects of folic acid supplementation are uncommon but include allergic bronchospasm, rash, pruritus, erythema, and general malaise. Patients should be aware that folic acid supplementation may cause urine to turn more intensely yellow.
Multiple Vitamins
Prenatal vitamin preparations are routinely recommended for pregnant women. These preparations generally supply vitamins A, B-complex, B12, C, calcium, D, E, iron, and other minerals.
Inadequate nutrition cannot be rectified through supplements alone; vitamins are used most effectively by the body when taken with meals, and calories and protein are not supplied by supplements.
Large doses of vitamins and minerals above the recommended amounts do not improve health and may cause harm to the pregnant patient and fetus. Some vitamins and minerals can be teratogenic or toxic when taken in large amounts.
Drugs for Minor Discomforts of Pregnancy
Many complaints associated with pregnancy are related to the GI tract and include nausea and vomiting, heartburn, and constipation. The etiology of nausea and vomiting is unclear, although research suggests that it is probably related to an increase in human chorionic gonadotropin (hCG) levels during pregnancy. Increased progesterone during pregnancy, which relaxes smooth muscle, contributes to heartburn and constipation. The elevation in female sex hormones during pregnancy changes the motility of the GI tract, and the enlarging uterus displaces the bowel.
Nausea and Vomiting
Nausea and vomiting (“morning sickness”) during early pregnancy are major complaints for about 88% of pregnant patients, but hyperemesis gravidarum—severe nausea and vomiting that may require hospitalization for hydration and nutrition—occurs with a much lower incidence (1% to 3%). Nonpharmacologic measures to decrease nausea and vomiting include (1) eating crackers, dry toast, or other carbohydrates before rising; (2) avoiding high-fat or highly seasoned foods; (3) eating small, frequent meals; (4) drinking fluids between, rather than with, meals; (5) drinking apple juice or flat soda between meals; (6) eating a high-protein bedtime snack; (7) stopping smoking; and (8) taking an iron supplement at bedtime. These measures work well for most patients, but if vomiting is severe, fluid replacement and pharmacologic measures may be necessary.
The FDA has approved pyridoxine hydrochloride and doxylamine succinate for treatment of morning sickness. Table 49.3 lists drugs used for management of nausea and vomiting during pregnancy with their dosages, uses, and considerations.
Many patients use ginger to help treat the nausea and vomiting associated with pregnancy. Studies suggest that ginger can be safely used in moderation, but as with all drugs and herbal supplements, encourage the pregnant patient to discuss use of ginger with her health care provider. Ginger can increase the risk for bleeding, particularly if the patient has a history of bleeding disorder.
Iron supplementation during pregnancy may add to the problems of nausea and vomiting. Taking these supplements with food or at bedtime may help decrease gastric distress. Prenatal vitamins should be taken at the time of day the patient is least likely to experience emesis because a high incidence of nausea and vomiting is associated with prenatal vitamins. For patients with continued iron-induced gastric distress, many health care providers recommend taking two children’s chewable multivitamins with iron. Salting food to taste may help replace vomited chloride; foods rich in potassium and magnesium may also help replace lost nutrients.
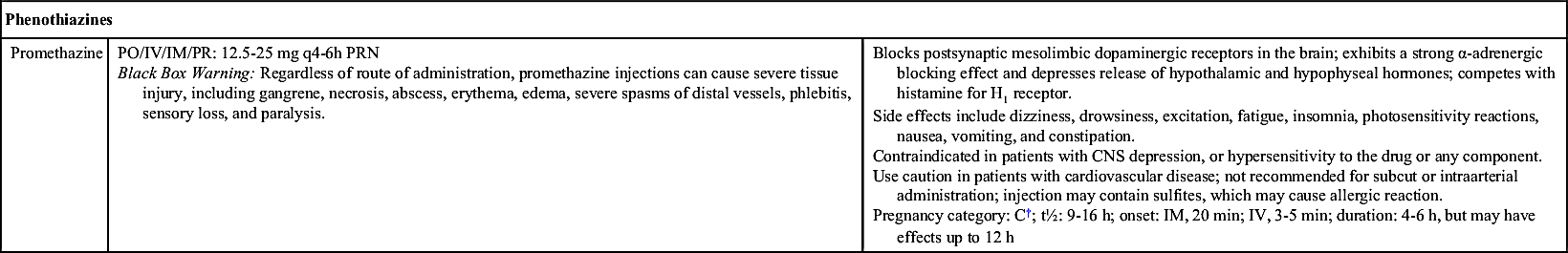
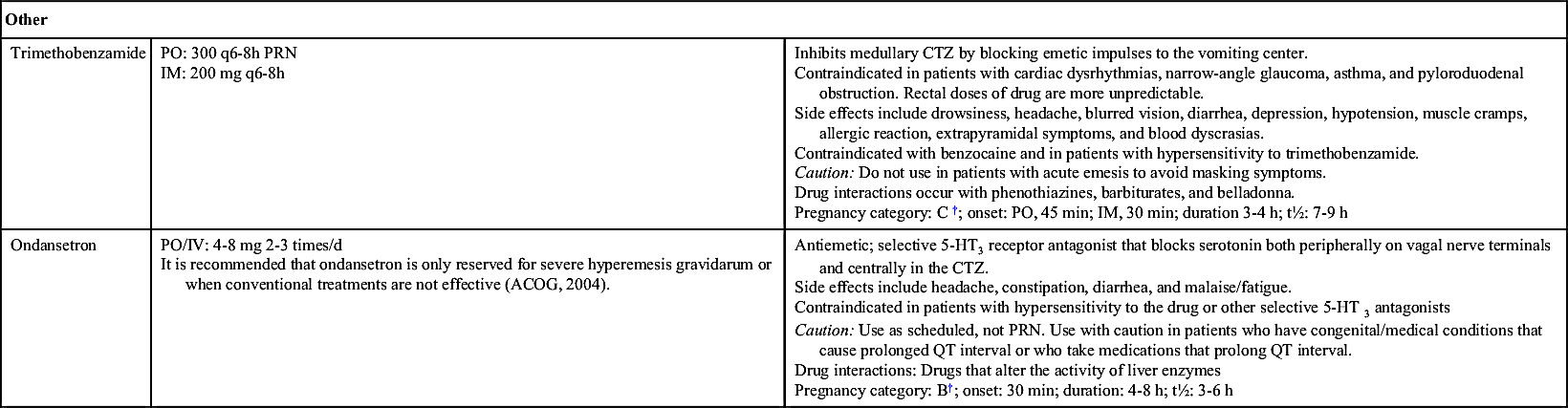
Patients whose symptoms persist and who experience weight loss and dehydration may require intravenous (IV) rehydration, including replacement of electrolytes and vitamins. Antiemetic therapy (probably with phenothiazines) may be used, and ondansetron may be administered only in severe cases of hyperemesis gravidarum.
Heartburn
Heartburn, or pyrosis, is a burning sensation in the epigastric and sternal regions that occurs with reflux of acidic stomach contents, which occurs in approximately 80% of pregnant patients. The normal increase in the hormone progesterone causes decreased motility of the GI tract during pregnancy. Progesterone also relaxes the cardiac sphincter—the sphincter that leads into the stomach from the esophagus, also called the lower esophageal sphincter—making reflux activity, or reverse peristalsis, more likely. During pregnancy, digestion and gastric emptying are slower than in the nonpregnant state. Heartburn is common when a pregnant patient sits or lies down soon after eating a normal meal, only to have her gravid uterus exert upward pressure on her stomach, causing increased reflux activity and the perception of hyperacidity. Heartburn is a disorder of the second and third trimesters of pregnancy.
Nonpharmacologic measures are preferred in the management of heartburn. These include (1) limiting the size of meals; (2) avoiding highly seasoned or greasy foods; (3) avoiding gas-forming foods (e.g., cabbage, onions); (4) eating slowly and chewing thoroughly; (5) avoiding citrus juices; (6) drinking adequate fluids, but not with meals; and (7) avoiding reclining immediately after eating.
Antacids should be considered first-line therapy if the patient does not respond to nonpharmacologic therapy. The antacids of choice for the pregnant patient include nonsystemic low-sodium products (those considered dietetically sodium free) that contain aluminum and magnesium (in the form of hydroxide) in combination. Discourage long-term use or large doses of magnesium antacids because fetal renal, respiratory, cardiovascular, and muscle problems may result. Sucralfate is likely safe during pregnancy because the drug is not systemically absorbed. Calcium carbonate antacid preparations may be avoided in pregnancy because of the rebound effect following acid neutralization. Chewable calcium carbonate tablets are frequently taken by pregnant patients for heartburn, but because they are calcium based, excessive use may contribute to constipation.
Most patients do not realize that remedies commonly used by nonpregnant patients (e.g., baking soda, sodium bicarbonate) can be harmful during pregnancy. Selection of the wrong antacid can result in diarrhea, constipation, or electrolyte imbalance. A combination of nonpharmacologic measures and minimal use of safe antacids should effectively meet the pregnant patient’s needs.
Liquid antacids are the preparations most commonly used in pregnancy because of their uniform dissolution, rapid action, and greater activity. Tablets are also acceptable, particularly for convenience, provided they are thoroughly chewed and the patient maintains adequate fluid intake.
Histamine2 (H2)-receptor antagonists can be used during pregnancy, but only if their use is recommended by a health care provider and initial treatment with antacids has failed. The teratogenicity of these medications is unknown. H2-receptor antagonists work by competitively and reversibly binding to the histamine receptors of the parietal cells, causing a reduction in gastric acid secretion. The onset of action is generally in 1 hour and can persist for 6 to 12 hours.
There is even less experience with the use of proton pump inhibitors. These medications work to suppress gastric acid secretion by inhibiting the proton pump on the surface of the parietal cells. With the release of OTC omeprazole, pregnant patients may wonder about its use for heartburn during pregnancy. Encourage patients to discuss the options with their health care provider. Currently, the use of omeprazole in pregnancy is limited to cases in which the benefits of therapy far outweigh the risks.
Table 49.4 presents medications for heartburn commonly used during pregnancy.
Constipation
Constipation is a frequent occurrence during pregnancy. Its cause may be related to hormonal changes—specifically progesterone, which decreases GI motility. As with heartburn, nonpharmacologic treatments for constipation should be tried first. These include (1) increased fluid intake, (2) increased dietary fiber intake, and (3) moderate physical exercise. If these methods do not work, treatment is indicated, and the safest agents are bulk-forming preparations that contain fiber because they are not systemically absorbed. Also, docusate sodium, a stool softener, would be appropriate as first-line treatment during pregnancy. Agents that should be reserved for occasional use include milk of magnesia, magnesium citrate, lactulose, sorbitol, bisacodyl, and senna.
Castor oil should be avoided during pregnancy because it can stimulate uterine contractions. Mineral oil should also be avoided because it can reduce the absorption of fat-soluble vitamins such as vitamin K. Low levels of vitamin K in the neonate can result in hemorrhage.
Pain
Through week 26 of pregnancy, headaches that result from hormonally induced body changes, sinus congestion, or eye strain are quite common. It is not unusual for the pregnant patient to experience backaches, joint pains, round ligament pain (resulting in mild abdominal aches and twinges), and pain from minor injuries. Nonpharmacologic pain-relief measures should be tried initially, including rest; a calming environment; relaxation exercises; alteration in routine; mental imagery; ice packs; warm, moist heat; postural changes; correct body mechanics; and changes in footwear.
TABLE 49.4
Over-the-Counter Antacids Commonly Used in Pregnancy
| Generic | Route and Dosage | Uses and Considerations |
| Aluminum hydroxide | PO: As directed∗ Recommended dose is 600 mg 5-6 times/d | Contains 320 mg of aluminum hydroxide gel per 300-mg tab or per 5 mL; ANC 8 contains saccharin and sorbitol. OTC preparation for heartburn secondary to reflux, it neutralizes gastric acidity. Side effects include constipation; adverse reactions include dehydration, hypophosphatemia (long-term use), GI obstruction. Effects are decreased with tetracycline, phenothiazine, benzodiazepines, isoniazid, and digoxin; follow dose with water. Pregnancy category: Not assigned†; PB: UK; onset: 15-30 min; peak: 0.5 h; duration: 1-3 h; t½: UK |
| Magnesium hydroxide and aluminum hydroxide with Simethicone | PO: Multiple formulations available OTC – take as directed∗ | Antiflatulant and neutralizes gastric acid. Liquid: Each 5 mL contains 400 mg aluminum hydroxide; 400 mg magnesium hydroxide; 40 mg simethicone, parabens, saccharin, and sorbitol; and 2 mg sodium. Tablets: Each contains 200 mg aluminum hydroxide, 200 mg magnesium hydroxide, and 20 mg simethicone. Tabs must be chewed thoroughly. Adverse effects include acid rebound. Aluminum-based antacids may cause constipation, whereas magnesium-based antacids have a laxative effect. Aluminum and magnesium–based combination antacids are given to balance the constipation and laxative effects. Do not administer magnesium-based antacids to patients with renal disease. Drug interactions: Concurrent administration with digoxin, indomethacin, or iron salts may decrease absorption of these drugs. Decreased pharmacologic effect with antacids and benzodiazepines, captopril, corticosteroids, fluoroquinolones, H2 antagonists, hydantoins, ketoconazole, penicillamine, phenothiazines, salicylates, and ticlopidine. Increased pharmacologic effect with levodopa, sulfonylureas, and valproic acid. Pregnancy category C†; PB: UK; t½: UK |
Acetaminophen
Acetaminophen, a para-aminophenol analgesic, is the most commonly ingested nonprescription drug during pregnancy. Acetaminophen may be used during all trimesters of pregnancy in therapeutic doses on a short-term basis for its analgesic and antipyretic effects. The drug is a weak prostaglandin inhibitor and does not have significant antiinflammatory effects. See Prototype Drug Chart 25.1 for the pharmacologic data for acetaminophen.
Pharmacokinetics
The rate of absorption of acetaminophen is dependent on the rate of gastric emptying. Acetaminophen is 10% to 25% protein bound and crosses the placenta during pregnancy; it is also found in low concentrations in breast milk. Acetaminophen is partially hepatically metabolized into inactive metabolites; however, a highly active metabolite (N-acetyl-p-benzoquinone) produced when the drug is taken in large doses can have potential liver and kidney toxicity. The half-life is 2 to 3 hours. There is no concrete evidence of fetal anomalies associated with the use of acetaminophen, and no adverse effects have been noted in breastfed infants of patients who used the drug while pregnant or breastfeeding.
Pharmacodynamics
The maximum daily dose of acetaminophen is 3000 mg per day, and the use of acetaminophen during pregnancy should not exceed 12 tablets per 24 hours of a 325-mg formulation (regular strength) or 8 tablets per 24 hours of a 500-mg (extra strength) formulation because of the potential for kidney and liver toxicity. The drug should be taken at 4- to 6-hour intervals. Onset of effects after oral ingestion is within 10 to 30 minutes, peak action occurs at 1 to 2 hours, and duration is from 3 to 5 hours.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree