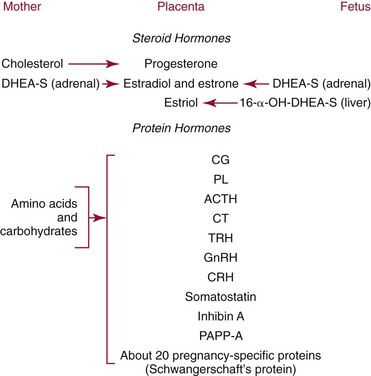
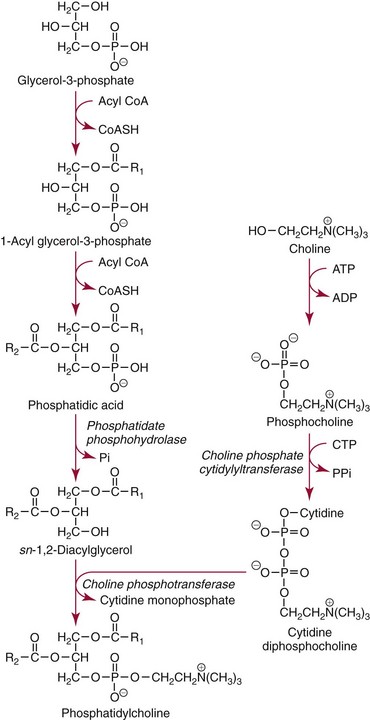
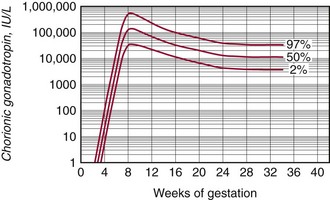
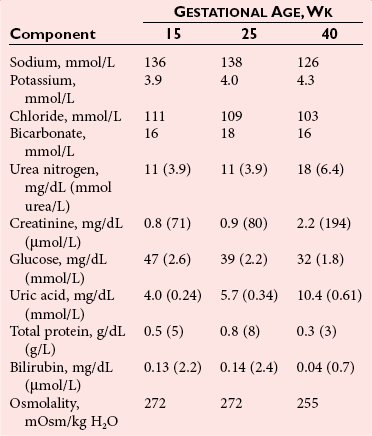
Chapter 57 Edward R. Ashwood, M.D., David G. Grenache, Ph.D., M.T.(A.S.C.P.), D.A.B.C.C., F.A.C.B. and Geralyn Lambert-Messerlian, Ph.D.* The clinical laboratory has an important role in managing pregnancy.25 In contrast to most clinical situations, when treating an expectant mother, a physician must simultaneously care for more than one patient. The health of the mother and that of her fetus are intertwined, each affecting the other; thus pregnancy management must consider both. This chapter reviews the biology of pregnancy and discusses laboratory tests used to detect, evaluate, and monitor both normal and abnormal pregnancies. Included are common tests, such as (1) chorionic gonadotropin (CG); (2) hematocrit; (3) blood type; (4) glucose tolerance testing; (5) prenatal testing and screening and diagnosis of fetal aneuplodies; and (6) esoteric tests, such as fetal lung maturity tests and amniotic fluid bilirubin examination. Ovulation occurs on approximately the 14th day of the regular menstrual cycle (see Chapter 56). If conception occurs, the ovum is fertilized, usually in the fallopian tube, and becomes a zygote, which is then carried down the tube into the uterus. The zygote divides, becoming a morula. After 50 to 60 cells are present, the morula develops a cavity, the primitive yolk sac, and thus becomes a blastocyst, which implants into the uterine wall about 5 days after fertilization. The cells on the exterior wall of the blastocyst, trophoblasts, synergistically invade the uterine endometrium and develop into chorionic villi, creating the placenta. Trophoblasts are subdivided into syncytiotrophoblasts and cytotrophoblasts, depending on location and cellular morphology. The placenta (1) keeps the maternal and fetal circulation systems separate, (2) nourishes the fetus, (3) eliminates fetal wastes, and (4) produces hormones vital to pregnancy. It is composed of large collections of fetal vessels called villi, which are surrounded by intervillous spaces in which maternal blood flows. For substances to move from the maternal circulation to the fetal circulation, they must cross through the trophoblasts and several membranes. The transfer of any substance depends largely on the (1) concentration gradient between the maternal and fetal circulatory systems, (2) presence or absence of circulating binding proteins, (3) lipid solubility of the substance, and (4) presence of facilitated transport, such as ion pumps or receptor-mediated endocytosis (Box 57-1). The placenta is an effective barrier to the movement of large proteins and hydrophobic compounds bound to plasma proteins. Maternal immunoglobulin (Ig)G crosses the placenta via receptor-mediated endocytosis. Because of its long half-life, maternally produced IgG protects a newborn for the first 6 months of life. Antibody assays with low limits of detection may be positive in infants up to age 18 months because of maternal antibodies. The placenta produces several protein and steroid hormones (Figure 57-1). The major protein hormones are CG and placental lactogen (PL). The steroids include progesterone, estradiol, estriol, and estrone. The placenta secretes most of its products into the maternal circulation; only small amounts reach the fetal circulation. Close proximity of the maternal blood vessels to the site of placental hormone production may explain some of this preferential accumulation of hormones in the maternal blood circulation. Generally, hormone production by the placenta increases in proportion to the increase in placental mass. Therefore concentrations of hormones derived from the placenta, such as PL, increase in maternal peripheral blood as the placenta increases in size. CG, which peaks at the end of the first trimester, is an exception. PL, also known as human placental lactogen (hPL) and human chorionic somatomammotropin (hCS), is a single polypeptide chain of 191 amino acids having two intramolecular disulfide bridges and a molecular mass of 22,279 Da. The structure of PL is exceptionally homologous (96%) with growth hormone (GH) and less so with prolactin (67%). Also, PL has potent growth and lactogenic properties. Five genes on chromosome 17 compose a gene family that codes for both GH and PL.279 PL production has been localized by immunofluorescence studies to the syncytiotrophoblast cells of the placenta. The increase in maternal serum PL concentration with advancing gestational age is directly correlated with the increasing mass of placental tissue and of functional syncytiotrophoblast tissue.333 Placental secretion near term is 1 to 2 g/d, the largest of any known human hormone. PL has many biological activities, including (1) lactogenic, (2) metabolic, (3) somatotropic, (4) luteotropic, (5) erythropoietic, and (6) aldosterone-stimulating effects. In addition, either directly or in synergism with prolactin, PL has a significant role in preparing the mammary glands for lactation. The many metabolic activities of PL closely resemble those of GH, including (1) inhibition of glucose uptake, (2) enhanced lipolysis leading to increased mobilization of free fatty acids, and (3) enhancement of nitrogen retention. Because glucose is the primary energy substrate for a fetus, it has been suggested that the glucose-sparing action of PL may provide a strategy to direct maternal metabolism toward greater use of fat for the mother’s requirements, thereby sparing maternal glucose for fetal use. Rare normal pregnancies have been reported in which complete absence of PL was noted. Although PL was used in the past to evaluate fetal well-being, currently no apparent clinical reason exists to measure PL.101 The placenta produces a wide variety of steroid hormones, including estrogen and progesterone with large amounts of estrogens produced at term. The chemistry of these steroids is described in Chapter 56. Maternal cholesterol is the main precursor for placental progesterone production. Biosynthesis of estrogens by the placenta differs from that of the ovaries because the placenta has no 17α-hydroxylase. Thus, each of the estrogens—estrone (E1), estradiol (E2), and estriol (E3)—must be synthesized from C-19 intermediates that already have a hydroxyl group at position 17. In nonpregnant women, the ovaries secrete 100 to 600 µg/d of estradiol, of which about 10% is metabolized to estriol. During late pregnancy, the placenta produces 50 to 150 mg/d of estriol and 15 to 20 mg/d of estradiol and estrone. Secretion of estrogens and progesterone throughout pregnancy ensures (1) appropriate development of the endometrium, (2) uterine growth, (3) adequate uterine blood supply, and (4) preparation of the uterus for labor. Although measurement of estriol in the third trimester was used in the past to assess fetal well-being, most obstetricians now consider this practice obsolete.100 Estriol measurements in the second trimester of pregnancy are useful in predicting fetal trisomy 21 and 18 (see later discussion on maternal serum screening for fetal defects). The volume of amniotic fluid increases progressively until 34 weeks’ gestation, when it decreases slightly through the 40th week and then more sharply declines until the 42nd week.312 The volume is 200 to 300 mL at 16 weeks, 400 to 1400 mL at 26 weeks, 300 to 2000 mL at 34 weeks, and 300 to 1400 mL at 40 weeks. The volume at any given moment is a function of several interrelated fluid fluxes. Direct measurements in primates and indirect measurements in humans have been used to derive a mathematical model of amniotic fluid volume.239 At term, total fluid fluxes into and out of the amniotic cavity are large (≈60 mL/h) and result in complete exchange of the amniotic fluid volume twice per day. Gross unidirectional fluid volume shifts occur episodically: into the amniotic cavity by fetal urination and out of the cavity by fetal swallowing. These unidirectional shifts begin at the end of the first trimester and increase linearly until approximately 30 weeks. Fetal swallowing and urination then exponentially increase, peaking at term at about 1000 mL/d. Bidirectional water exchanges—so-called intramembranous fluxes—occur across the following surfaces: (1) placenta (mother-fetus); (2) umbilical vessels, through the substance of the umbilical cord (fetus–amniotic fluid); (3) fetal skin (fetus–amniotic fluid); and (4) fetal membranes (amniotic fluid–mother). These exchanges increase in a linear fashion throughout pregnancy. At term, they are approximately 400 mL/d. The fetal tracheobronchial tree is filled with amniotic fluid. Although lung fluid transport contributes a small volume, fetal breathing of this fluid is the mechanism of surfactant transport from the fetal lungs into the amniotic fluid. Early in gestation, the composition of the amniotic fluid resembles a complex dialysate of the maternal serum. As a fetus grows, the amniotic fluid changes in several ways (Table 57-1). Most notably, sodium concentration and osmolality decrease and concentrations of urea, creatinine, and uric acid increase.39 The activities of many enzymes in amniotic fluid have been studied with respect to both gestational age and fetal status but have not been found to be clinically useful. The major lipids of interest are the phospholipids, whose type and concentrations reflect fetal lung maturity (discussed further later). Numerous steroid and protein hormones are also present in amniotic fluid.418 Concentrations of androgens and estrogens have been measured in futile attempts to predict fetal sex. The rare syndrome of congenital adrenal hyperplasia (CAH) has been diagnosed antenatally by measuring 17-hydroxyprogesterone and pregnanetriol in the amniotic fluid near term.190 Measurements of thyroid-stimulating hormone (TSH) and thyroxine in amniotic fluid may be useful in cases of fetal thyroid disease.348 No other diagnostic uses for amniotic fluid hormone measurements are in common use. Prostaglandins E1, E2, F1α, and F2α all are found in low concentrations in amniotic fluid and increase gradually during pregnancy. PGE2 and PGF2α concentrations are very high during active labor.109 Attempts to demonstrate an acute rise in PGE2 or PGF2α immediately before the onset of labor, at the initiation of parturition, have been unsuccessful. TABLE 57-1 Composition of Amniotic Fluid (Mean Values) From Benzie RJ, Doran TA, Harkins JL, Owen VM, Porter CJ. Composition of the amniotic fluid and maternal serum in pregnancy. Am J Obstet Gynecol 1974;119:798-810. During pregnancy, a woman undergoes dramatic physiologic and hormonal changes. The large quantities of estrogen, progesterone, PL, and corticosteroids produced during pregnancy affect various metabolic, physiologic, and endocrinologic systems. In addition, the woman experiences (1) an increase in resistance to angiotensin; (2) a predominance of lipid metabolism over glucose use; and (3) increased synthesis by the liver of thyroid- and steroid-binding proteins, fibrinogen, and other proteins characteristic of pregnancy. As a result of such changes, many of the laboratory reference intervals for nonpregnant patients are not appropriate for pregnant patients. Lockitch229 has developed reference intervals for more than 70 analytes in normal pregnancy. Her study group included 29 pregnant subjects tested from 16 weeks to term and also postpartum. Mean values for selected tests expressed as a percentage of control means are presented in Table 57-2. TABLE 57-2 HDL, High-density lipoprotein; LDL, low-density lipoprotein. Data from Lockitch G, ed. Handbook of diagnostic biochemistry and hematology in normal pregnancy. Boca Raton, Fla: CRC Press, 1993. The concentrations of several blood coagulation factors are increased during pregnancy. For example, plasma fibrinogen increases by approximately 65%, from 275 to 450 mg/dL; this increase contributes to the increase in sedimentation rate. Other clotting factors also increase, including factors VII, VIII, IX, and X. Prothrombin and factors V and XII do not change, whereas factors XI and XIII decrease slightly. Even though the platelet count remains unchanged in most women and prothrombin time and activated partial thromboplastin time shorten slightly (see Table 57-2), pregnancy increases the risk of thromboembolism to up to five times that of nonpregnant women. Increasing estrogen concentrations throughout pregnancy increase the secretion of prolactin up to tenfold. Conversely, high estrogen concentrations during pregnancy suppress the secretion of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) below the detection limit. Baseline concentrations of other pituitary hormones such as TSH remain nearly unchanged (see Table 57-2), but the GH response to provocative stimuli is blunted. Although normal pregnancy is a euthyroid state, many changes occur in thyroid function. High concentrations of TBG raise the concentration of total thyroxine (T4) and triiodothyronine (T3), but a slight decrease in free T4 concentration occurs during the second and third trimesters. A slight reciprocal increase in TSH was reported by Lockitch.229 Thyroglobulin is significantly increased, especially in the third trimester.151 Very few (<0.2%) pregnant individuals develop hyperthyroidism, and hypothyroidism is very rare.67 Postpartum thyroid dysfunction is common and is frequently unrecognized. The fetal thyroid-pituitary axis functions independently from the mother’s axis in most cases. However, if the mother has preexisting Graves disease (hyperthyroidism caused by autoantibodies that stimulate the thyroid), those antibodies can cross the placenta, causing hyperthyroidism in the fetus. If the mother has anti-TSH autoantibodies, the infant can develop transient hypothyroidism.229 In normal air-breathing lungs, a substance called pulmonary surfactant coats the alveolar epithelium and responds to alveolar volume changes by reducing surface tension in the alveolar wall during expiration. Surfactant is needed because surface tension is an inverse function of the radius of the airway. Thus small alveoli have a higher collapsing force than larger alveoli. Surfactant opposes the force and keeps the small alveoli from collapsing. Specialized alveolar cells called type II granular pneumocytes synthesize pulmonary surfactant and package it into laminated storage granules called lamellar bodies.186,351 These storage granules are 1 to 5 µm in diameter and contain phospholipids, cholesterol, and protein.276 Production starts as early as 20 weeks’ gestation,351 but adequate amounts do not accumulate until about 36 weeks. Exudation of pulmonary fluid (via the trachea) and fetal breathing movements transport lamellar bodies into the amniotic fluid. The internal structure of the particles is rearranged into a formation described as tubular myelin by those using electron microscopy.186 The newborn lung contains 100 times more surfactant per cm3 than the adult lung. Excessive surfactant is needed at birth as the newborn is transformed from breathing water to breathing air. Surfactant overcomes the surface tension produced in water-filled alveoli that are admitting air for the first time. Pulmonary surfactant is a complex mixture of lipids and proteins; less than 5% is composed of carbohydrates. Most of the lipid is phospholipid, and most of that is lecithin (phosphatidylcholine).65 Unlike lecithin from other tissues, pulmonary lecithin has two saturated fatty acids, usually palmitoyl groups. Other lipids present are phosphatidylglycerol (PG), phosphatidylinositol (PI), and phosphatidylethanolamine (see Chapter 27). Sphingomyelin is present in very small amounts (<2%). The protein fraction of lamellar bodies is approximately 4% and is composed of four surfactant-specific proteins: SP-A, SP-B, SP-C, and SP-D.177,297 Type II pneumocytes synthesize phosphatidylcholine in a manner quite different from that seen in other cells. Most other cells synthesize phosphatidylserine from cytidine diphosphate diacylglycerol and serine. The phosphatidylserine then is decarboxylated to yield phosphatidyl ethanolamine. The final step is the successive donation of three methyl groups via S-adenosylmethionine to form phosphatidylcholine. The pulmonary biosynthetic pathway is shown in Figure 57-2. The enzyme choline phosphotransferase forms PC directly from cytidine diphosphocholine and diacylglycerol. Phosphatidylinositol formation peaks at about 35 weeks. As PI decreases in concentration, PG begins to increase. For optimum healthcare during pregnancy, a woman should consult her physician before conception.8,253 Unfortunately, 49% of pregnancies in the United States are unintended—higher for unmarried women.179 Preconception evaluation should include a medical, reproductive, and family history; physical examination; and laboratory tests. The following laboratory tests are recommended as part of a preconception evaluation: (1) hematocrit, (2) blood type and Rh compatibility, (3) erythrocyte antibody screen, (4) Papanicolaou smear [or human papillomavirus (HPV) test], (5) urinalysis, (6) rubella titer, (7) Rapid Plasma Reagin (RPR) test, (8) gonococcal and chlamydia DNA test, (9) cystic fibrosis carrier status,162 (10) human immunodeficiency virus (HIV) antibody,7 and (11) hepatitis B surface antigen.14 Depending on demographic risks, genetic testing for disorders such as Tay-Sachs disease, thalassemia, and sickle cell disease may be offered.13 A careful diet history is warranted. Folic acid supplementation should be recommended to reduce the risk of neural tube defects.248 Women at high risk for diabetes mellitus should be screened for this disorder (see Chapter 46). Most individuals consult a physician a few days after a missed menses if they suspect they might be pregnant. Many laboratory tests are useful for managing normal and abnormal pregnancies. A urine pregnancy test result is positive (meaning that the test can detect a CG concentration of about 25 IU/L) in about half of pregnant females at the beginning of the missed menses—at about 2 weeks after conception.90 Screening for fetal neural tube defects and Down syndrome should be offered to all pregnant patients. Until 2002, screening was recommended at 16 to 18 weeks’ gestation,10 but Down syndrome screening can now be performed as early as 10 weeks.400 Depending on diabetes risk, glucose tolerance testing should be performed immediately or at 24 to 28 weeks (see Chapter 46 for details). Some physicians screen selected patients for preterm labor risk at 24 to 30 weeks using fetal fibronectin.18 Although PL and estriol measurements were used previously to predict fetal well-being, both tests are now obsolete for this purpose.100,101 Maternal observation and recording of fetal movements,9 ultrasound examination, and tests that monitor the fetal heart rate17 during random uterine contractions or fetal movement are the currently accepted methods for monitoring fetal well-being. Many different samples are used in clinical laboratory analysis before and during pregnancy. These include (1) paternal saliva, serum, and blood; (2) maternal saliva, serum, blood, and urine; (3) amniotic fluid obtained by amniocentesis101,347 or from pools of fluid in the vagina after rupture of the fetal membranes; (4) chorionic villi; (5) fetal blood obtained by percutaneous umbilical blood sampling; and (6) fetal tissue obtained by biopsy.105 The technique of amniocentesis is described in Chapter 7. Additional information is found in the section on tests for evaluating fetal lung maturity, later in this chapter. Qualitative tests for CG in blood or urine are used primarily to screen for pregnancy. Urine CG tests usually suffice to diagnose normal pregnancy when it has progressed beyond the first week after the first missed period. However, qualitative serum pregnancy tests may detect pregnancy earlier, and quantitative serum tests can help reveal problems in early pregnancy. False-positive or increased serum CG test results have been obtained from qualitative and quantitative assays when human antimouse antibodies (HAMA) or heterophile antibodies are present.91,157 If suspected, investigative experiments include testing a urine specimen for the presence of CG, serially diluting the serum to confirm an appropriate dose response, testing the serum using a different CG method, and retesting the serum after treatment with interfering antibody blocking agents. To establish accurate dates, obstetricians rely on (1) menstrual history, (2) physical examination, (3) fetal heart tones, (4) ultrasonography, and (5) detection and quantification of CG. In the first 8 weeks of pregnancy, the CG concentration in maternal serum rises geometrically (Figure 57-3). Detectable amounts (≈5 IU/L) are present in the serum 8 to 11 days after conception,234 which is in the third week of pregnancy as measured from the LMP.221 CG usually becomes detectable in the urine 1 to 3 days later, although this interval is highly variable.234 For women aged 13 to 40, serum CG concentrations of 5 IU/L or greater are consistent with pregnancy. Higher values are infrequently seen in older, nonpregnant women and are thought to be caused by CG secreted by the pituitary gland.350 Concentrations in approximately half of pregnant women reach 25 IU/L on the first day of their missed period. The peak concentration occurs at about 8 to 10 weeks and is about 100,000 IU/L. Subsequently, CG concentrations start to decline in serum and urine, and by the end of the second trimester, a 90% reduction from peak concentration has usually occurred. In the first trimester, maternal serum CG is about 96% to 98% intact, 1% to 3% β subunit, and up to 1% α subunit. During the second trimester, subunit synthesis becomes unbalanced and the serum distribution shifts to 92% to 98% intact, 1% to 7% α subunit, and up to 1% β subunit.280 Concentrations are approximately constant during the third trimester, with the predominant species being intact CG. The presence of twins approximately doubles CG concentrations.389 Conditions arising primarily in the mother include (1) ectopic pregnancy, (2) hyperemesis gravidarum, (3) preeclampsia, (4) HELLP syndrome (see later), (5) liver disease, (6) Graves disease, and (7) hemolytic disease of the newborn. The clinician must distinguish abnormal changes in laboratory tests from normal physiologic changes induced by pregnancy (see Table 57-2). When a fertilized egg implants in a location other than the body of the uterus, the condition is called an ectopic pregnancy. Most abnormal implantations occur in the fallopian tube; they can also occur in the abdomen, although this is rare. Tubal rupture and hemorrhage are common serious complications of ectopic pregnancy. About 25% of individuals with an ectopic pregnancy have three classic symptoms: lower abdominal pain, vaginal bleeding, and an adnexal mass.332 Of all individuals with these symptoms, about 15% have an ectopic pregnancy, and a smaller percentage have incomplete or complete spontaneous abortion. In 2006, the United States had 13.3 pregnancy-related deaths per 100,000 live births257—3% caused by ectopic pregnancy.181 A pregnant patient has about a 1 in 200 chance of dying from an ectopic pregnancy.155 Management of ectopic pregnancy is surgical (by laparoscopy) or medical (with intramuscular administration of methotrexate).15,21 Early detection and proper management of ectopic pregnancy are the most effective means of preventing maternal morbidity and mortality. Some have suggested that asymptomatic women at high risk for ectopic pregnancy should be screened with the use of ultrasound and laboratory tests.69 Ultrasound examination is used to evaluate women with symptoms. When ultrasound is nondiagnostic, quantitative measurements of serum CG are used to identify women with ectopic pregnancy or abnormal intrauterine pregnancy. These conditions frequently produce abnormal CG concentrations and slow rates of increase.58 An accurate gestational age is the best predictor of when an intrauterine pregnancy should be detected with transvaginal ultrasound. Failure to detect a gestational sac by sonography 24 days or longer after conception provides presumptive evidence of an ectopic pregnancy or fetal demise.193 In the absence of an accurate gestational age, a serum CG concentration is required to assist in interpreting a nondiagnostic ultrasound. An intrauterine pregnancy should be visible by ultrasonography at a CG concentration of 1500 to 2000 IU/L, but this concentration has a clinical sensitivity of only 42% and a clinical specificity of 81% for the detection of ectopic pregnancy when no intrauterine gestational sac is visualized.205 When CG concentrations are less than 1500 IU/L and ultrasonography is nondiagnostic, serial testing of CG may be very helpful. In normal intrauterine pregnancy, during the second through fifth weeks, the CG doubles every 1.5 days. After 5 weeks’ gestation, the doubling time gradually lengthens to 2 to 3 days.305 Before 7 weeks, calculation of the slope of log(CG) versus time in days is useful for identifying normal pregnancies.192 At least two CG determinations should be obtained from specimens collected 1 to 7 days apart. The log-linear slope, Δlog(CG)/Δtime [in log(IU/L)/d], is greater than 0.11 in the vast majority of normal pregnancies. In cases of ectopic pregnancy or spontaneous abortion, the slope is usually less than 0.11, and the CG concentrations often decrease. An increase in CG of at least 53% over 48 hours is 99% specific for excluding an ectopic pregnancy,33 but as many as 35% of ectopic gestations produce an increase greater than this.345 Serum progesterone concentration is often low in mothers with abnormal pregnancy.314 For example, a serum progesterone less than 6 ng/mL predicts an abnormal pregnancy outcome with 81% confidence for asymptomatic women within 8 weeks of their last menses,106 but average serum progesterone in nonviable pregnancies is 10 ng/mL. For women with clinical symptoms of abnormal pregnancy, measurement of both CG and progesterone is more predictive of abnormal pregnancy than a single CG measurement. In a large outcome study, 97% of patients with CG less than 3000 IU/L and progesterone less than 12.6 ng/mL had an abnormal pregnancy outcome, whereas those with CG greater than 3000 IU/L or progesterone greater than 12.6 ng/mL had a normal pregnancy.272 McCord and associates242 reported that in women at risk for ectopic pregnancy, a progesterone cutoff of 17.5 ng/mL detected 92% of ectopic cases (clinical sensitivity) but had a very poor clinical specificity of about 14%. Investigators concluded that patients with progesterone greater than 17.5 ng/mL needed no additional laboratory tests. Finally, in symptomatic patients, Stewart and colleagues362 reported a clinical sensitivity of 99% and a clinical specificity of 65% when a Δlog(CG)/Δtime greater than 0.14 is used to distinguish normal intrauterine pregnancy from ectopic pregnancy combined with inevitable abortion. A progesterone cutoff above 8 ng/mL had a clinical sensitivity of 81% and a clinical specificity of 88%. Preeclampsia is a pregnancy condition characterized by hypertension, proteinuria, and often edema, usually occurring late in the second trimester or early in the third trimester, and affecting 5 to 10% of pregnancies. It is a major cause of maternal and perinatal mortality. If the mother develops convulsions, the condition is called eclampsia. The cause has not been elucidated,359 but research suggests that increased circulating soluble fms-like tyrosine kinase 1 (sFlt-1) may have a role.222 The disorder manifests with placental ischemia and endothelial dysfunction that leads to intravascular deposition of fibrin with subsequent end-organ damage.320 Most maternal deaths are due to central nervous system complications, but ischemic liver damage may also occur. The only cure for preeclampsia is delivery of the placenta.359 The HELLP syndrome (hemolysis, elevated liver enzymes, and low platelet counts in association with preeclampsia) is a life-threatening obstetric complication that occurs in 0.1% of pregnancies. Its most prominent features are thrombocytopenia and disseminated intravascular coagulation (DIC; see Chapter 59). Most cases occur between the 27th and 36th weeks of pregnancy, but the syndrome also may occur postpartum. Women typically present with epigastric or right upper quadrant pain, malaise, nausea, vomiting, and headache.240,343 Jaundice occurs in 5% of patients. Lactate dehydrogenase (LD) concentrations may be very high, and alanine aminotransferase (ALT) and aspartate aminotransferase (AST) are usually 2 to 10 times their upper reference limits. Treatment is delivery. Postpartum management of the patient may require plasmapheresis or organ transplantation. Recurrence rates are 3 to 27%. Several liver disorders are unique to pregnancy.191,204 These include (1) hyperemesis gravidarum, (2) cholestasis of pregnancy, and (3) fatty liver of pregnancy. These disorders must be distinguished from the normal physiologic changes of pregnancy (see Table 57-2). Significant changes normally seen in pregnancy include a dilutional decrease in serum albumin and elevation of alkaline phosphatase (from the placenta). Notably, total bilirubin; 5′-nucleotidase, gamma-glutamyltranspeptidase (GGT), ALT, and AST are unchanged in mothers with a normal pregnancy. Changes in these analytes reflect hepatobiliary disease. Also discussed in this section are (1) non–pregnancy-related liver disease in pregnancy, (2) differential diagnosis, and (3) effect of pregnancy on preexisting liver disease. Hyperemesis gravidarum is characterized by nausea and vomiting and, in severe cases, dehydration and malnutrition. It typically occurs in the first trimester. When hyperemesis is severe enough to cause dehydration, abnormal liver enzyme values—usually less than four times the upper reference limit—are seen in approximately 50% of patients.191 Mild hyperbilirubinemia may occur. However, significant liver disease does not occur, and liver biopsy results are normal. Low birth weight babies are common, especially for women who develop malnutrition. Cholestasis of pregnancy usually occurs in the third trimester and is manifested clinically by diffuse pruritus and, in 10% of patients, jaundice. Typical features of cholestasis, including pale stools and dark urine, are present and last until delivery. Women who experience cholestasis while taking oral contraceptives usually develop cholestasis of pregnancy. Serum bilirubin rarely exceeds 5 mg/dL. Alkaline phosphatase is typically two to four times the upper reference limit. Aminotransferase enzyme concentrations are mildly elevated. Prothrombin time may be elevated because of vitamin K malabsorption. Although many clinicians order serum bile acids in this setting,130 this test is offered by only a few clinical laboratories and is rarely necessary for diagnosis. The condition itself is benign, but is associated with increased risk of premature birth and fetal death. It recurs with subsequent pregnancies.317 Fatty liver of pregnancy occurs in approximately 1 in 10,000 pregnancies and is characterized by accumulation of microvesicular fat in the hepatocytes.77 Many cases of this maternal disorder are caused by an inherited mitochondrial fatty acid oxidation disorder in the fetus, long-chain 3-hydroxyacyl-CoA dehydrogenase deficiency (LCHAD).379 Mothers carrying fetuses with this disorder are 50 times more likely to develop fatty liver of pregnancy.66 The disease typically occurs at week 37 and is manifested clinically by rapid onset of malaise, nausea, vomiting, and abdominal pain. Mild elevations in aminotransferase enzyme concentrations occur, with the AST elevation typically greater than the ALT elevation but with both less than six times the upper reference limit. Serum bilirubin is usually greater than 6 mg/dL. Life-threatening hypoglycemia may occur.320 Hyperuricemia, presumably from tissue destruction and renal failure, is characteristic. Liver histology shows acute fatty infiltration with little necrosis or inflammation. The fat is microvesicular and pericentral in the cell, similar to what is seen in Reye syndrome. If untreated, fulminant hepatic failure with hepatic encephalopathy ensues. Treatment is immediate termination of the pregnancy, at which time rapid recovery usually occurs. Infant and maternal mortality is approximately 50% and 20%.
Pregnancy and Its Disorders
Human Pregnancy
Conception, Embryo, and Fetus
Placenta
Function
Placental Hormones
Placental Lactogen
Placental Steroids
Amniotic Fluid
Volume and Dynamics
Composition
Maternal Adaptation
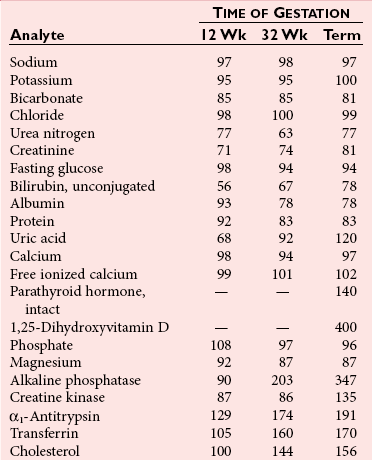

Hematologic Changes
Endocrine Changes
Functional Development of the Fetus
Lungs and Pulmonary Surfactant
Maternal and Fetal Health Assessment
Laboratory Testing
Clinical Specimens
Diagnosis and Dating of Pregnancy
Complications of Pregnancy
Abnormal Pregnancies
Ectopic Pregnancy and Threatened Abortion
Preeclampsia and Eclampsia
HELLP Syndrome
Liver Disease
Hyperemesis Gravidarum
Cholestasis of Pregnancy
Fatty Liver of Pregnancy
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Pregnancy and Its Disorders