135e | Less Common Hematologic Malignancies |
The most common lymphoid malignancies are discussed in Chap. 134, myeloid leukemias in Chaps. 132 and 133, myelodysplastic syndromes in Chap. 130, and myeloproliferative syndromes in Chap. 131. This chapter will focus on the more unusual forms of hematologic malignancy. The diseases discussed here are listed in Table 135e-1. Each of these entities accounts for less than 1% of hematologic neoplasms.
UNUSUAL LYMPHOID AND MYELOID MALIGNANCIES |
LYMPHOID MALIGNANCIES
Precursor B-cell and precursor T-cell neoplasms are discussed in Chap. 134. All the lymphoid tumors discussed here are mature B cell or T cell, natural killer (NK) cell neoplasms.
MATURE B-CELL NEOPLASMS
B-Cell Prolymphocytic Leukemia (B-PLL) This is a malignancy of medium-sized (about twice the size of a normal small lymphocyte), round lymphocytes with a prominent nucleolus and light blue cytoplasm on Wright’s stain. It dominantly affects the blood, bone marrow, and spleen and usually does not cause adenopathy. The median age of affected patients is 70 years, and men are more often affected than women (male-to-female ratio is 1.6). This entity is distinct from chronic lymphoid leukemia (CLL) and does not develop as a consequence of that disease.
Clinical presentation is generally from symptoms of splenomegaly or incidental detection of an elevated white blood cell (WBC) count. The clinical course can be rapid. The cells express surface IgM (with or without IgD) and typical B-cell markers (CD19, CD20, CD22). CD23 is absent, and about one-third of cases express CD5. The CD5 expression along with the presence of the t(11;14) translocation in 20% of cases leads to confusion in distinguishing B-PLL from the leukemic form of mantle cell lymphoma. No reliable criteria for the distinction have emerged. About half of patients have mutation or loss of p53, and deletions have been noted in 11q23 and 13q14. Nucleoside analogues like fludarabine and cladribine and combination chemotherapy (cyclophosphamide, doxorubicin, vincristine, and prednisone [CHOP]) have produced responses. CHOP plus rituximab may be more effective than CHOP alone, but the disease is sufficiently rare that large series have not been reported. Splenectomy can produce palliation of symptoms but appears to have little or no impact on the course of the disease.
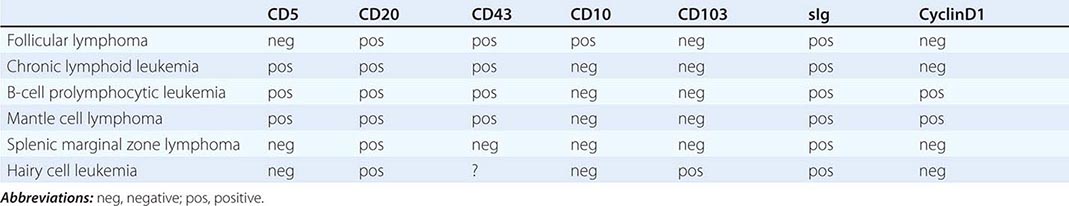
Splenic Marginal Zone Lymphoma (SMZL) This tumor of mainly small lymphocytes originates in the marginal zone of the spleen white pulp, grows to efface the germinal centers and mantle, and invades the red pulp. Splenic hilar nodes, bone marrow, and peripheral blood may be involved. The circulating tumor cells have short surface villi and are called villous lymphocytes. Table 135e-2 shows differences in tumor cells of a number of neoplasms of small lymphocytes that aid in the differential diagnosis. SMZL cells express surface immunoglobulin and CD20, but are negative for CD5, CD10, CD43, and CD103. Lack of CD5 distinguishes SMZL from CLL, and lack of CD103 separates SMZL from hairy cell leukemia.
IMMUNOPHENOTYPE OF TUMORS OF SMALL LYMPHOCYTES |
The median age of patients with SMZL is mid-fifties, and men and women are equally represented. Patients present with incidental or symptomatic splenomegaly or incidental detection of lymphocytosis in the peripheral blood with villous lymphocytes. Autoimmune anemia or thrombocytopenia may be present. The immunoglobulin produced by these cells contains somatic mutations that reflect transit through a germinal center, and ongoing mutations suggest that the mutation machinery has remained active. About 40% of patients have either deletions or translocations involving 7q21, the site of the FLNC gene (filamin Cγ, involved in cross-linking actin filaments in the cytoplasm). NOTCH2 mutations are seen in 25% of patients. Chromosome 8p deletions may also be noted. The genetic lesions typically found in extranodal marginal zone lymphomas [e.g., trisomy 3 and t(11;18)] are uncommon in SMZL.
The clinical course of disease is generally indolent with median survivals exceeding 10 years. Patients with elevated lactate dehydrogenase (LDH) levels, anemia, and hypoalbuminemia generally have a poorer prognosis. Long remissions can be seen after splenectomy. Rituximab is also active. A small fraction of patients undergo histologic progression to diffuse large B-cell lymphoma with a concomitant change to a more aggressive natural history. Experience with combination chemotherapy in SMZL is limited.
Hairy Cell Leukemia Hairy cell leukemia is a tumor of small lymphocytes with oval nuclei, abundant cytoplasm, and distinctive membrane projections (hairy cells). Patients have splenomegaly and diffuse bone marrow involvement. While some circulating cells are noted, the clinical picture is dominated by symptoms from the enlarged spleen and pancytopenia. The mechanism of the pancytopenia is not completely clear and may be mediated by both inhibitory cytokines and marrow replacement. The marrow has an increased level of reticulin fibers; indeed, hairy cell leukemia is a common cause of inability to aspirate bone marrow or so-called “dry tap” (Table 135e-3). Monocytopenia is profound and may explain a predisposition to atypical mycobacterial infection that is observed clinically. The tumor cells have strong expression of CD22, CD25, and CD103; soluble CD25 level in serum is an excellent tumor marker for disease activity. The cells also express tartrate-resistant acid phosphatase. The immunoglobulin genes are rearranged and mutated, indicating the influence of a germinal center. No specific cytogenetic abnormality has been found, but most cases contain the activating BRAF mutation V600E.
DIFFERENTIAL DIAGNOSIS OF “DRY TAP”–INABILITY TO ASPIRATE BONE MARROW |

The median age of affected patients is mid-fifties, and the male-to-female ratio is 5:1. Treatment options are numerous. Splenectomy is often associated with prolonged remission. Nucleosides including cladribine and deoxycoformycin are highly active but are also associated with further immunosuppression and can increase the risk of certain opportunistic infections. However, after brief courses of these agents, patients usually obtain very durable remissions during which immune function spontaneously recovers. Interferon α is also an effective therapy but is not as effective as nucleosides. Chemotherapy-refractory patients have responded to vemurafenib, a BRAF inhibitor.
Nodal Marginal Zone B-Cell Lymphoma This rare node-based disease bears an uncertain relationship with extranodal marginal zone lymphomas, which are often mucosa-associated and are called mucosa-associated lymphoid tissue (MALT) lymphomas, and SMZLs. Patients may have localized or generalized adenopathy. The neoplastic cell is a marginal zone B cell with monocytoid features and has been called monocytoid B-cell lymphoma in the past. Up to one-third of the patients may have extranodal involvement, and involvement of the lymph nodes can be secondary to the spread of a mucosal primary lesion. In authentic nodal primaries, the cytogenetic abnormalities associated with MALT lymphomas [trisomy 3 and t(11;18)] are very rare. The clinical course is indolent. Patients often respond to combination chemotherapy, although remissions have not been durable. Few patients have received CHOP plus rituximab, which is likely to be an effective approach to management.
Mediastinal (Thymic) Large B-Cell Lymphoma This entity was originally considered a subset of diffuse large B-cell lymphoma; however, additional study has identified it as a distinct entity with its own characteristic clinical, genetic, and immunophenotypic features. This is a disease that can be bulky in size but usually remains confined to the mediastinum. It can be locally aggressive, including progressing to produce a superior vena cava obstruction syndrome or pericardial effusion. About one-third of patients develop pleural effusions, and 5–10% can disseminate widely to kidney, adrenal, liver, skin, and even brain. The disease affects women more often than men (male-to-female ratio is 1:2–3), and the median age is 35–40 years.
The tumor is composed of sheets of large cells with abundant cytoplasm accompanied by variable, but often abundant, fibrosis. It is distinguished from nodular sclerosing Hodgkin’s disease by the paucity of normal lymphoid cells and the absence of lacunar variants of Reed-Sternberg cells. However, more than one-third of the genes that are expressed to a greater extent in primary mediastinal large B-cell lymphoma than in usual diffuse large B-cell lymphoma are also overexpressed in Hodgkin’s disease, suggesting a possible pathogenetic relationship between the two entities that affect the same anatomic site. Tumor cells may overexpress MAL. The genome of tumor cells is characterized by frequent chromosomal gains and losses. The tumor cells in mediastinal large B-cell lymphoma express CD20, but surface immunoglobulin and HLA class I and class II molecules may be absent or incompletely expressed. Expression of lower levels of class II HLA identifies a subset with poorer prognosis. The cells are CD5 and CD10 negative but may show light staining with anti-CD30. The cells are CD45 positive, unlike cells of classical Hodgkin’s disease.
Methotrexate, leucovorin, doxorubicin, cyclophosphamide, vincristine, prednisone, and bleomycin (MACOP-B) and rituximab plus CHOP are effective treatments, achieving 5-year survival of 75–87%. Dose-adjusted therapy with prednisone, etoposide, vincristine, cyclophosphamide, and doxorubicin (EPOCH) plus rituximab has produced 5-year survival of 97%. A role for mediastinal radiation therapy has not been definitively demonstrated, but it is frequently used, especially in patients whose mediastinal area remains positron emission tomography–avid after four to six cycles of chemotherapy.
Intravascular Large B-Cell Lymphoma This is an extremely rare form of diffuse large B-cell lymphoma characterized by the presence of lymphoma in the lumen of small vessels, particularly capillaries. It is also known as malignant angioendotheliomatosis or angiotropic large cell lymphoma. It is sufficiently rare that no consistent picture has emerged to define a clinical syndrome or its epidemiologic and genetic features. It is thought to remain inside vessels because of a defect in adhesion molecules and homing mechanisms, an idea supported by scant data suggesting absence of expression of β-1 integrin and ICAM-1. Patients commonly present with symptoms of small-vessel occlusion, skin lesions, or neurologic symptoms. The tumor cell clusters can promote thrombus formation. In general, the clinical course is aggressive and the disease is poorly responsive to therapy. Often a diagnosis is not made until very late in the course of the disease.
Primary Effusion Lymphoma This entity is another variant of diffuse large B-cell lymphoma that presents with pleural effusions, usually without apparent tumor mass lesions. It is most common in the setting of immune deficiency disease, especially AIDS, and is caused by human herpes virus 8 (HHV-8)/Kaposi’s sarcoma herpes virus (KSHV). It is also known as body cavity–based lymphoma. Some patients have been previously diagnosed with Kaposi’s sarcoma. It can also occur in the absence of immunodeficiency in elderly men of Mediterranean heritage, similar to Kaposi’s sarcoma but even less common.
The malignant effusions contain cells positive for HHV-8/KSHV, and many are also co-infected with Epstein-Barr virus. The cells are large with large nuclei and prominent nucleoli that can be confused with Reed-Sternberg cells. The cells express CD20 and CD79a (immunoglobulin-signaling molecule), although they often do not express immunoglobulin. Some cases aberrantly express T-cell markers such as CD3 or rearranged T-cell receptor genes. No characteristic genetic lesions have been reported, but gains in chromosome 12 and × material has been seen, similar to other HIV-associated lymphomas. The clinical course is generally characterized by rapid progression and death within 6 months.
Lymphomatoid Granulomatosis This is an angiocentric, angiodestructive lymphoproliferative disease comprised by neoplastic Epstein-Barr virus–infected monoclonal B cells accompanied and outnumbered by a polyclonal reactive T-cell infiltrate. The disease is graded based on histologic features such as cell number and atypia in the B cells. It is most often confused with extranodal NK–T cell lymphoma, nasal type, which can also be angiodestructive and is Epstein-Barr virus–related. The disease usually presents in adults (males > females) as a pulmonary infiltrate. Involvement is often entirely extranodal and can include kidney (32%), liver (29%), skin (25%), and brain (25%). The disease often but not always occurs in the setting of immune deficiency.
The disease can be remitting and relapsing in nature or can be rapidly progressive. The course is usually predicted by the histologic grade. The disease is highly responsive to combination chemotherapy and is curable in most cases. Some investigators have claimed that low-grade disease (grade I and II) can be treated with interferon α.
MATURE T-CELL AND NK CELL NEOPLASMS
T-Cell Prolymphocytic Leukemia This is an aggressive leukemia of medium-sized prolymphocytes involving the blood, marrow, nodes, liver, spleen, and skin. It accounts for 1–2% of all small lymphocytic leukemias. Most patients present with elevated WBC count (often >100,000/μL), hepatosplenomegaly, and adenopathy. Skin involvement occurs in 20%. The diagnosis is made from peripheral blood smear, which shows cells about 25% larger than those in small lymphocytes, with cytoplasmic blebs and nuclei that may be indented. The cells express T-cell markers like CD2, CD3, and CD7; two-thirds of patients have cells that are CD4+ and CD8–, and 25% have cells that are CD4+ and CD8+. T-cell receptor β chains are clonally rearranged. In 80% of patients, inversion of chromosome 14 occurs between q11 and q32. Ten percent have t(14;14) translocations that bring the T-cell receptor alpha/beta gene locus into juxtaposition with oncogenes TCL1 and TCL1b at 14q32.1. Chromosome 8 abnormalities are also common. Deletions in the ATM gene are also noted. Activating JAK3 mutations have also been reported.
The course of the disease is generally rapid, with median survival of about 12 months. Responses have been seen with the anti-CD52 antibody, nucleoside analogs, and CHOP chemotherapy. Small numbers of patients with T-cell prolymphocytic leukemia have also been treated with high-dose therapy and allogeneic bone marrow transplantation after remission has been achieved with conventional-dose therapy.
T-Cell Large Granular Lymphocytic Leukemia T-cell large granular lymphocytic leukemia (LGL leukemia) is characterized by increases in the number of LGLs in the peripheral blood (2000–20,000/μL) often accompanied by severe neutropenia, with or without concomitant anemia. Patients may have splenomegaly and frequently have evidence of systemic autoimmune disease, including rheumatoid arthritis, hypergammaglobulinemia, autoantibodies, and circulating immune complexes. Bone marrow involvement is mainly interstitial in pattern, with fewer than 50% lymphocytes on differential count. Usually the cells express CD3, T-cell receptors, and CD8; NK-like variants may be CD3–. The leukemic cells often express Fas and Fas ligand.
The course of the disease is generally indolent and dominated by the neutropenia. Paradoxically, immunosuppressive therapy with cyclosporine, methotrexate, or cyclophosphamide plus glucocorticoids can produce an increase in granulocyte counts. Nucleosides have been used anecdotally. Occasionally the disease can accelerate to a more aggressive clinical course.
Aggressive NK Cell Leukemia NK neoplasms are very rare, and they may follow a range of clinical courses from very indolent to highly aggressive. They are more common in Asians than whites, and the cells frequently harbor a clonal Epstein-Barr virus episome. The peripheral blood white count is usually not greatly elevated, but abnormal large lymphoid cells with granular cytoplasm are noted. The aggressive form is characterized by symptoms of fever and laboratory abnormalities of pancytopenia. Hepatosplenomegaly is common; node involvement is less common. Patients may have hemophagocytosis, coagulopathy, or multiorgan failure. Serum levels of Fas ligand are elevated.
The cells express CD2 and CD56 and do not have rearranged T-cell receptor genes. Deletions involving chromosome 6 are common. The disease can be rapidly progressive. Some forms of NK neoplasms are more indolent. They tend to be discovered incidentally with LGL lymphocytosis and do not manifest the fever and hepatosplenomegaly characteristic of the aggressive leukemia. The cells are also CD2 and CD56 positive, but they do not contain clonal forms of Epstein-Barr virus and are not accompanied by pancytopenia or autoimmune disease.
Extranodal NK/T-Cell Lymphoma, Nasal Type Like lymphomatoid granulomatosis, extranodal NK/T-cell lymphoma tends to be an angiocentric and angiodestructive lesion, but the malignant cells are not B cells. In most cases, they are CD56+ Epstein-Barr virus–infected cells; occasionally they are CD56– Epstein-Barr virus–infected cytotoxic T cells. They are most commonly found in the nasal cavity. Historically, this illness was called lethal midline granuloma, polymorphic reticulosis, and angiocentric immunoproliferative lesion. This form of lymphoma is prevalent in Asia, Mexico, and Central and South America; it affects males more commonly than females. When it spreads beyond the nasal cavity, it may affect soft tissue, the gastrointestinal tract, or the testis. In some cases, hemophagocytic syndrome may influence the clinical picture. Patients may have B symptoms. Many of the systemic manifestations of disease are related to the production of cytokines by the tumor cells and the cells responding to their signals. Deletions and inversions of chromosome 6 are common.
Many patients with extranodal NK/T-cell lymphoma, nasal type have excellent antitumor responses with combination chemotherapy regimens, particularly those with localized disease. Radiation therapy is often used after completion of chemotherapy. Four risk factors have been defined, including B symptoms, advanced stage, elevated LDH, and regional lymph node involvement. Patient survival is linked to the number of risk factors: 5-year survival is 81% for zero risk factors, 64% for one risk factor, 32% for two risk factors, and 7% for three or four risk factors. Combination regimens without anthracyclines have been touted as superior to CHOP, but data are sparse. High-dose therapy with stem cell transplantation has been used, but its role is unclear.
Enteropathy-Type T-Cell Lymphoma Enteropathy-type T-cell lymphoma is a rare complication of longstanding celiac disease. It most commonly occurs in the jejunum or the ileum. In adults, the lymphoma may be diagnosed at the same time as celiac disease, but the suspicion is that the celiac disease was a longstanding precursor to the development of lymphoma. The tumor usually presents as multiple ulcerating mucosal masses, but may also produce a dominant exophytic mass or multiple ulcerations. The tumor expresses CD3 and CD7 nearly always and may or may not express CD8. The normal-appearing lymphocytes in the adjacent mucosa often have a similar phenotype to the tumor. Most patients have the HLA genotype associated with celiac disease, HLA DQA1*0501 or DQB1*0201.
The prognosis of this form of lymphoma is typically (median survival is 7 months) poor, but some patients have a good response to CHOP chemotherapy. Patients who respond can develop bowel perforation from responding tumor. If the tumor responds to treatment, recurrence may develop elsewhere in the celiac disease–affected small bowel.
Hepatosplenic T-Cell Lymphoma Hepatosplenic T-cell lymphoma is a malignancy derived from T cells expressing the gamma/delta T-cell antigen receptor that affects mainly the liver and fills the sinusoids with medium-size lymphoid cells. When the spleen is involved, dominantly the red pulp is infiltrated. It is a disease of young people, especially young people with an underlying immunodeficiency or with an autoimmune disease that demands immunosuppressive therapy. The use of thiopurine and infliximab is particularly common in the history of patients with this disease. The cells are CD3+ and usually CD4– and CD8–. The cells may contain isochromosome 7q, often together with trisomy 8. The lymphoma has an aggressive natural history. Combination chemotherapy may induce remissions, but most patients relapse. Median survival is about 2 years. The tumor does not appear to respond to reversal of immunosuppressive therapy.
Subcutaneous Panniculitis-Like T-Cell Lymphoma Subcutaneous panniculitis-like T-cell lymphoma involves multiple subcutaneous collections of neoplastic T cells that are usually cytotoxic cells in phenotype (i.e., contain perforin and granzyme B and express CD3 and CD8). The rearranged T-cell receptor is usually alpha/beta-derived, but occasionally the gamma/delta receptors are involved, particularly in the setting of immunosuppression. The cells are negative for Epstein-Barr virus. Patients may have a hemophagocytic syndrome in addition to the skin infiltration; fever and hepatosplenomegaly may also be present. Nodes are generally not involved. Patients frequently respond to combination chemotherapy, including CHOP. When the disease is progressive, the hemophagocytic syndrome can be a component of a fulminant downhill course. Effective therapy can reverse the hemophagocytic syndrome.
Blastic NK Cell Lymphoma The neoplastic cells express NK cell markers, especially CD56, and are CD3 negative. They are large blastic-appearing cells and may produce a leukemia picture, but the dominant site of involvement is the skin. Morphologically, the cells are similar to the neoplastic cells in acute lymphoid and myeloid leukemia. No characteristic chromosomal abnormalities have been described. The clinical course is rapid, and the disease is largely unresponsive to typical lymphoma treatments.
Primary Cutaneous CD30+ T-Cell Lymphoma This tumor involves the skin and is composed of cells that appear similar to the cells of anaplastic T-cell lymphoma. Among cutaneous T-cell tumors, about 25% are CD30+ anaplastic lymphomas. If dissemination to lymph nodes occurs, it is difficult to distinguish between the cutaneous and systemic forms of the disease. The tumor cells are often CD4+, and the cells contain granules that are positive for granzyme B and perforin in 70% of cases. The typical t(2;5) of anaplastic T-cell lymphoma is absent; indeed, its presence should prompt a closer look for systemic involvement and a switch to a diagnosis of anaplastic T-cell lymphoma. This form of lymphoma has sporadically been noted as a rare complication of silicone on saline breast implants. Cutaneous CD30+ T-cell lymphoma often responds to therapy. Radiation therapy can be effective, and surgery can also produce long-term disease control. Five-year survival exceeds 90%.
Angioimmunoblastic T-Cell Lymphoma Angioimmunoblastic T-cell lymphoma is a systemic disease that accounts for about 15% of all T-cell lymphomas. Patients frequently have fever, advanced stage, diffuse adenopathy, hepatosplenomegaly, skin rash, polyclonal hypergammaglobulinemia, and a wide range of autoantibodies including cold agglutinins, rheumatoid factor, and circulating immune complexes. Patients may have edema, arthritis, pleural effusions, and ascites. The nodes contain a polymorphous infiltrate of neoplastic T cells and nonneoplastic inflammatory cells together with proliferation of high endothelial venules and follicular dendritic cells. The most common chromosomal abnormalities are trisomy 3, trisomy 5, and an extra × chromosome. Aggressive combination chemotherapy can induce regressions. The underlying immune defects make conventional lymphoma treatments more likely to produce infectious complications.
MYELOID MALIGNANCIES
The World Health Organization (WHO) system uses peripheral blood counts and smear analysis, bone marrow morphology, and cytogenetic and molecular genetic tests in order to classify myeloid malignancies into five major categories (Table 135e-4). In this chapter, we focus on chronic neutrophilic leukemia; atypical chronic myeloid leukemia, BCR-ABL1 negative; chronic myelomonocytic leukemia; juvenile myelomonocytic leukemia; chronic eosinophilic leukemia, not otherwise specified; mastocytosis; myeloproliferative neoplasm (MPN), unclassifiable (MPN-U); myelodysplastic syndrome (MDS)/MPN, unclassifiable (MDS/MPN-U); refractory anemia with ring sideroblasts associated with marked thrombocytosis (RARS-T); and myeloid and lymphoid neoplasms with eosinophilia and abnormalities of PDGFRA, PDGFRB, or FGFR1. This chapter also includes histiocytic and dendritic cell neoplasms, transient myeloproliferative disorders, and a broader discussion on primary eosinophilic disorders including hypereosinophilic syndrome (HES).
WORLD HEALTH ORGANIZATION CLASSIFICATION OF MYELOID MALIGNANCIES |
aAML-related precursor neoplasms include therapy-related MDS and myeloid sarcoma.
bEither monocytopenia or bicytopenia: hemoglobin level <10 g/dL, absolute neutrophil count <1.8 × 109/L, or platelet count <100 × 109/L. However, higher blood counts do not exclude the diagnosis in the presence of unequivocal histologic/cytogenetic evidence for MDS. cGenetic rearrangements involving platelet-derived growth factor receptor α/β (PDGFRA/PDGFRB) or fibroblast growth factor receptor 1 (FGFR1).
CHRONIC NEUTROPHILIC LEUKEMIA
Chronic neutrophilic leukemia (CNL) is characterized by mature neutrophilic leukocytosis with few or no circulating immature granulocytes. CNL is associated with activating mutations of the gene (CSF3R) encoding for the receptor for granulocyte colony-stimulating factor (G-CSF), also known as colony-stimulating factor 3 (CSF3). Patients with CNL might be asymptomatic at presentation but also display constitutional symptoms, splenomegaly, anemia, and thrombocytopenia. Median survival is approximately 2 years, and causes of death include leukemic transformation, progressive disease associated with severe cytopenias, and marked treatment-refractory leukocytosis. CNL is rare, with less than 200 reported cases. Median age at diagnosis is approximately 67 years, and the disease is equally prevalent in both genders.
Pathogenesis CSF3 is the main growth factor for granulocyte proliferation and differentiation. Accordingly, recombinant CSF3 is used for the treatment of severe neutropenia, including severe congenital neutropenia (SCN). Some patients with SCN acquire CSF3R mutations, and the frequency of such mutations is significantly higher (~80%) in patients who experience leukemic transformation. SCN-associated CSF3R mutations occur in the region of the gene coding for the cytoplasmic domain of CSF3R and result in truncation of the C-terminal-negative regulatory domain. A different class of CSF3R mutations is noted in ~90% of patients with CNL; these are mostly membrane proximal, with the most frequent being a C-to-T substitution at nucleotide 1853 (T618I). About 40% of the T618I-mutated cases also harbored SETBP1 mutations. CSF3R T618I induces a lethal myeloproliferative disorder in a mouse model and is associated with in vitro sensitivity to JAK inhibition.
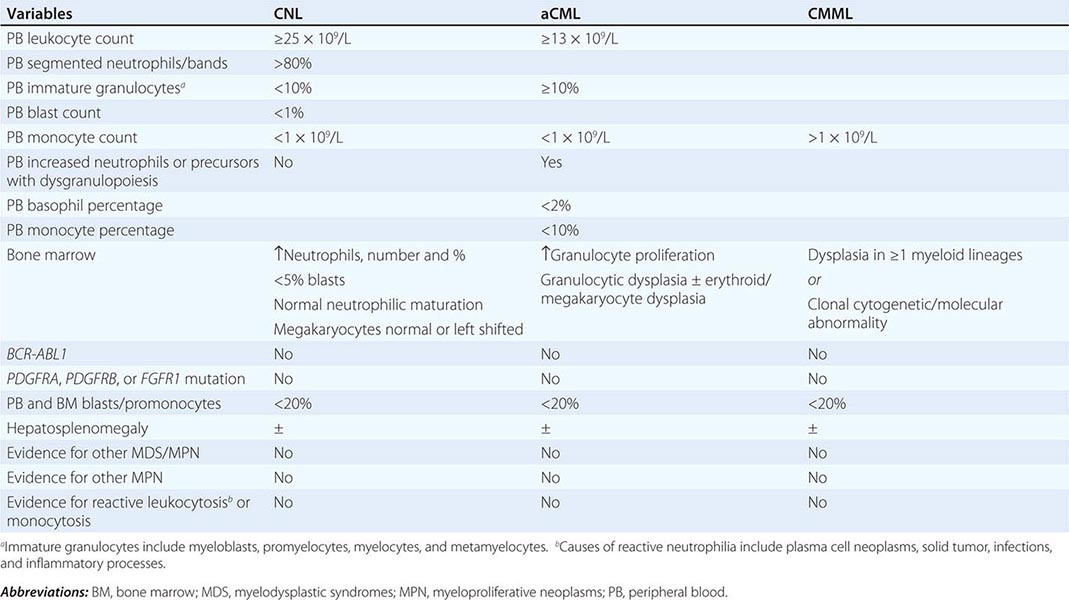
Diagnosis Diagnosis of CNL requires exclusion of the more common causes of neutrophilia including infections and inflammatory processes. In addition, one should be mindful of the association between some forms of metastatic cancer or plasma cell neoplasms with secondary neutrophilia. Neoplastic neutrophilia also occurs in other myeloid malignancies including atypical chronic myeloid leukemia and chronic myelomonocytic leukemia. Accordingly, the WHO diagnostic criteria for CNL are designed to exclude the possibilities of both secondary/reactive neutrophilia and leukocytosis associated with myeloid malignancies other than CNL (Table 135e-5): leukocytosis (≥25 × 109/L), >80% segmented/band neutrophils, <10% immature myeloid cells, <1% circulating blasts, and absence of dysgranulopoiesis or monocytosis. Bone marrow in CNL is hypercellular and displays increased number and percentage of neutrophils with a very high myeloid-to-erythroid ratio and minimal left shift, myeloid dysplasia, or reticulin fibrosis.
WORLD HEALTH ORGANIZATION DIAGNOSTIC CRITERIA FOR CHRONIC NEUTROPHILIC LEUKEMIA (CNL); ATYPICAL CHRONIC MYELOID LEUKEMIA, BCRABL1 NEGATIVE (ACML); AND CHRONIC MYELOMONOCYTIC LEUKEMIA (CMML) |
Treatment Current treatment in CNL is largely palliative and suboptimal in its efficacy. Several drugs alone or in combination have been tried, and none have shown remarkable efficacy. As such, allogeneic stem cell transplantation (ASCT) is reasonable to consider in the presence of symptomatic disease, especially in younger patients. Otherwise, cytoreductive therapy with hydroxyurea is probably as good as any treatment, and a more intensive combination chemotherapy may not have additional value. However, response to hydroxyurea therapy is often transient, and some have successfully used interferon α as an alternative drug. Response to treatment with ruxolitinib (a JAK1 and JAK2 inhibitor) has been reported but has not been confirmed.
ATYPICAL CHRONIC MYELOID LEUKEMIA
Atypical chronic myeloid leukemia, BCR-ABL1 negative (aCML) is formally classified under the MDS/MPN category of myeloid malignancies and is characterized by left shifted granulocytosis and dysgranulopoiesis. The differential diagnosis of aCML includes chronic myeloid leukemia (CML), which is distinguished by the presence of BCR-ABL1; CNL, which is distinguished by the absence of dysgranulopoiesis and presence of CSF3R mutations; and chronic myelomonocytic leukemia, which is distinguished by the presence of monocytosis (absolute monocyte count >1 × 109/L). The WHO diagnostic criteria for aCML are listed in Table 135e-5 and include granulocytosis (WBC ≥13 × 109/L), neutrophilia with dysgranulopoiesis, ≥10% immature granulocytes, <20% peripheral blood myeloblasts, <10% peripheral blood monocytes, <2% basophils, and absence of otherwise specific mutations such as BCR-ABL1. The bone marrow is hypercellular with granulocyte proliferation and dysplasia with or without erythroid or megakaryocytic dysplasia.
The molecular pathogenesis of aCML is incompletely understood; about one-fourth of the patients express SETBP1 mutations, which are, however, also found in several other myeloid malignancies, including CNL and chronic myelomonocytic leukemia. SETBP1 mutations in aCML were prognostically detrimental and mostly located between codons 858 and 871; similar mutations are seen with Schinzel-Giedion syndrome (a congenital disease with severe developmental delay and various physical stigmata including midface retraction, large forehead, and macroglossia).
In a series of 55 patients with WHO-defined aCML, median age at diagnosis was 62 years with female preponderance (57%); splenomegaly was reported in 54% of the patients, red cell transfusion requirement in 65%, abnormal karyotype in 20% (20q– and trisomy 8 being the most frequent), and leukemic transformation in 40%. Median survival was 25 months. Outcome was worse in patients with marked leukocytosis, transfusion requirement, and increased immature cells in the peripheral blood. Conventional chemotherapy is largely ineffective in the treatment of aCML. However, a favorable experience with ASCT was reported in nine patients; after a median follow-up of 55 months, the majority of the patients remained in complete remission.
CHRONIC MYELOMONOCYTIC LEUKEMIA
Chronic myelomonocytic leukemia (CMML) is classified under the WHO category of MDS/MPN and is defined by an absolute monocyte count (AMC) of >1 × 109/L in the peripheral blood. Median age at diagnosis ranges between 65 and 75 years, and there is a 2:1 male predominance. Clinical presentation is variable and depends on whether the disease presents with MDS-like or MPN-like phenotype; the former is associated with cytopenias and the latter with splenomegaly and features of myeloproliferation such as fatigue, night sweats, weight loss, and cachexia. About 20% of patients with CMML experience serositis involving the joints (arthritis), pericardium (pericarditis and pericardial effusion), pleura (pleural effusion), or peritoneum (ascites).
Pathogenesis Clonal cytogenetic abnormalities are seen in about one-third of patients with CMML and include trisomy 8 and abnormalities of chromosome 7. Almost all patients with CMML harbor somatic mutations involving epigenetic regulator genes (e.g., ASXL1, TET2), spliceosome pathway genes (e.g., SRSF2), DNA damage response genes (e.g., TP53), and tyrosine kinases/transcription factors (e.g., KRAS, NRAS, CBL, and RUNX1). However, none of these mutations are specific to CMML, and their precise pathogenetic contribution is unclear.
Diagnosis Reactive monocytosis is uncommon but has been reported in association with certain infections and inflammatory conditions. Clonal (i.e., neoplastic) monocytosis defines CMML but is also seen with juvenile myelomonocytic leukemia and acute myeloid leukemia with monocytic differentiation. The WHO diagnostic criteria for CMML are listed in Table 135e-5 and include persistent AMC >1 × 109/L, absence of BCR-ABL1, absence of the PDGFRA or PDGFRB mutations, <20% blasts and promonocytes in the peripheral blood and bone marrow, and dysplasia involving one or more myeloid lineages.
The bone marrow in CMML is hypercellular with granulocytic and monocytic proliferation. Dysplasia is often present and may involve one, two, or all myeloid lineages. On immunophenotyping, the abnormal cells often express myelomonocytic antigens such as CD13 and CD33, with variable expression of CD14, CD68, CD64, and CD163. Monocytic-derived cells are almost always positive for the cytochemical nonspecific esterases (e.g., butyrate esterase), whereas normal granulocytic precursors are positive for lysozyme and chloroacetate esterase. In CMML, it is common to have a hybrid cytochemical staining pattern with cells expressing both chloroacetate and butyrate esterases simultaneously (dual esterase staining).
Prognosis A meta-analysis showed median survival of 1.5 years in CMML. Numerous prognostic systems have attempted to better define and stratify the natural history of CMML. One of these, the Mayo prognostic model, assigns one point each to the following four independent prognostic variables: AMC >10 × 109/L, presence of circulating immature cells, hemoglobin <10 g/dL, and platelet count <100,000/mL. This model stratified patients into three risk groups: low (0 points), intermediate (1 point), and high (≥2 points), translating to median survival times of 32, 18, and 10 months, respectively.
A French study incorporated ASXL1 mutational status in 312 CMML patients. In a multivariable model, independent predictors of poor survival were WBC >15 × 109/L (3 points), ASXL1 mutations (2 points), age >65 years (2 points), platelet count <100,000/mL (2 points), and hemoglobin <10 g/dL in females and <11 g/dL in males (2 points). This model stratified patients into three groups: low (0–4 points), intermediate (5–7 points), and high risk (8–12 points), with median survival times of not reached, 38.5 months, and 14.4 months, respectively.
Treatment Current treatment consists of hydroxyurea and supportive care, including red cell transfusions and use of erythropoiesis-stimulating agents (ESAs). The value of hydroxyurea was reinforced by a randomized trial against oral etoposide. No other single or combination chemotherapy has been shown to be superior to hydroxyurea. ASCT is a viable treatment option for transplant-eligible patients with poor prognostic features. Given the MDS/MPN overlap phenotype and the presence of MDS-like genetic/methylation abnormalities in CMML, hypomethylating agents such as 5-azacitidine and decitabine have been used with limited efficacy.
JUVENILE MYELOMONOCYTIC LEUKEMIA
Juvenile myelomonocytic leukemia (JMML) is primarily a disease of early childhood and is included, along with CMML, in the MDS/MPN WHO category. Both CMML and JMML feature leukocytosis, monocytosis, and hepatosplenomegaly. Additional characteristic features in JMML include thrombocytopenia and elevated fetal hemoglobin. Myeloid progenitors in JMML display granulocyte-macrophage colony-stimulating factor (GM-CSF) hypersensitivity that has been attributed to dysregulated RAS/MAPK signaling. The latter is believed to result from mutually exclusive mutations involving RAS, PTPN11, and NF1. A third of patients with JMML that is not associated with Noonan’s syndrome carry PTPN11 mutations, whereas the incidence of NF1 in patients without neurofibromatosis type 1 and RAS mutations is approximately 15% each. Drug therapy is relatively ineffective in JMML, and the treatment of choice is ASCT, which results in a 5-year survival of approximately 50%.
MDS/MPN-U
The WHO classifies patients with morphologic and laboratory features that resemble both MDS and MPN as MDS/MPN overlap. This category includes CMML, aCML, and JMML, which have been described above. In addition, MDS/MPN includes a fourth category referred to as MDS/MPN, unclassifiable (MDS/MPN-U). Diagnosis of MDS/MPN-U requires the presence of both MDS and MPN features that are not adequate to classify patients as CMML, aCML, or JMML. MDS/MPN includes the provisional category of RARS-T.
RARS-T is classified in the MDS/MPN category because it shares dysplastic features with RARS and myeloproliferative features with essential thrombocythemia (ET). In one study, 111 patients with RARS-T were compared with 33 patients with RARS. The frequency of SF3B1 mutations in RARS-T (87%) was similar to that in RARS (85%). JAK2 V617F mutation was detected in 49% of RARS-T patients (including 48% of those mutated for SF3B1) but none of those with RARS. In RARS-T, SF3B1 mutations were more frequent in females (95%) than in males (77%), and mean ring sideroblast counts were higher in SF3B1-mutated patients. Median overall survival was 6.9 years in SF3B1-mutated patients versus 3.3 years in unmutated patients. Six-year survival was 67% in JAK2-mutated patients versus 32% in unmutated patients. Multivariable analysis identified younger age and JAK2 and SF3B1 mutations as favorable factors.
In one series, 85 patients with non-RARS-T MDS/MPN, median age was 70 years, and 72% were males. Splenomegaly at presentation was present in 33%, thrombocytosis in 13%, leukocytosis in 18%, JAK2 mutations in 30%, and abnormal karyotype in 51%; the most frequent cytogenetic abnormality was trisomy 8. Median survival was 12.4 months and favorably affected by thrombocytosis. Treatment with hypomethylating agents, immunomodulators, or ASCT did not appear to favorably affect survival.
MYELOPROLIFERATIVE NEOPLASM, UNCLASSIFIABLE (MPN-U)
The category of MPN-U includes MPN-like neoplasms that cannot be clearly classified as one of the other seven subcategories of MPN (Table 135e-4). Examples include patients presenting with unusual thrombosis or unexplained organomegaly with normal blood counts but found to carry MPN-characteristic mutations such as JAK2 and CALR or display bone marrow morphology that is consistent with MPN. It is possible that some cases of MPN-U represent earlier disease stages in polycythemia vera (PV) or ET that fail to meet the threshold hemoglobin levels (18.5 g/dL in men or 16.5 g/dL in women) or platelet counts (450 × 109/L) that are required by the WHO diagnostic criteria. Specific treatment interventions might not be necessary in asymptomatic patients with MPN-U, whereas patients with arterial thrombotic complications might require cytoreductive and aspirin therapy and those with venous thrombosis might require systemic anticoagulation.
TRANSIENT MYELOPROLIFERATIVE DISORDER (TMD)
TMD constitutes an often but not always transient phenomenon of abnormal megakaryoblast proliferation, which occurs in approximately 10% of infants with Down’s syndrome. TMD is usually recognized at birth and either undergoes spontaneous regression (75% of cases) or progresses into acute megakaryoblastic leukemia (AMKL) (25% of cases). Almost all patients with TMD and TMD-derived AMKL display somatic GATA1 mutations. TMD-associated GATA1 mutations constitute exon 2 insertions, deletions, or missense mutations, affecting the N-terminal transactivation domain of GATA-1, and result in loss of full-length (50-kDa) GATA-1 and its replacement with a shorter isoform (40-kDa) that retains friend of GATA-1 (FOG-1) binding. In contrast, inherited forms of exon 2 GATA1 mutations produce a phenotype with anemia, whereas exon 4 mutations that affect the N-terminal, FOG-1-interactive domain produce familial dyserythropoietic anemia with thrombocytopenia or X-linked macrothrombocytopenia.
EOSINOPHILIC DISORDERS
Eosinophilia refers to a peripheral blood absolute eosinophil count (AEC) that is above the upper normal limit of the reference range. The term hypereosinophilia is used when the AEC is >1500 × 109/L. Eosinophilia is operationally classified as secondary (nonneoplastic proliferation of eosinophils) and primary (proliferation of eosinophils that is either neoplastic or otherwise unexplained) (Table 135e-6). Secondary eosinophilia is by far the most frequent cause of eosinophilia and is often associated with infections, especially those related to tissue-invasive helminths; allergic/vasculitic diseases; drugs; and metastatic cancer. Primary eosinophilia is the focus of this chapter and is considered when a cause for secondary eosinophilia is not readily apparent.
DIAGNOSIS OF CHRONIC EOSINOPHILIC LEUKEMIA AND HYPEREOSINOPHILIC SYNDROME |
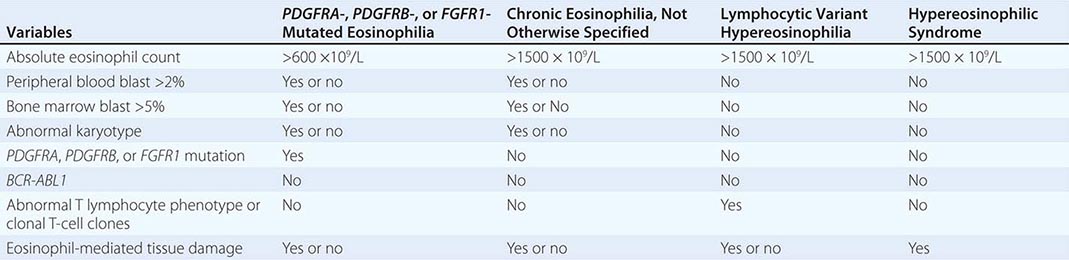
Primary Eosinophilia Primary eosinophilia is classified as clonal or idiopathic. Diagnosis of clonal eosinophilia requires morphologic, cytogenetic, or molecular evidence of a myeloid neoplasm. Idiopathic eosinophilia is considered when both secondary and clonal eosinophilias have been ruled out as a possibility. HES is a subcategory of idiopathic eosinophilia with persistent AEC of ≥1.5 × 109/L and associated with eosinophil-mediated organ damage (Table 135e-7). An HES-like disorder that is associated with clonal or phenotypically abnormal T cells is referred to as lymphocytic variant hypereosinophilia (Table 135e-7).
PRIMARY EOSINOPHILIA CLASSIFICATION |
Clonal Eosinophilia Examples of clonal eosinophilia include eosinophilia associated with acute myeloid leukemia (AML), MDS, CML, mastocytosis, and MDS/MPN overlap. Myeloid neoplasm-associated eosinophilia also includes the WHO MPN subcategory of chronic eosinophilic leukemia, not otherwise specified (CEL-NOS) and the WHO myeloid malignancy subcategory referred to as myeloid/lymphoid neoplasms with eosinophilia and mutations involving platelet-derived growth factor receptor (PDGFR) α/β or fibroblast growth factor receptor 1 (FGFR1).
The diagnostic workup for clonal eosinophilia that is not associated with morphologically overt myeloid malignancy should start with peripheral blood mutation screening for FIP1L1-PDGFRA and PDGFRB mutations using fluorescence in situ hybridization (FISH) or reverse transcription polymerase chain reaction. This is crucial because such eosinophilia is easily treated with imatinib. If mutation screening is negative, a bone marrow examination with cytogenetic studies is indicated. In this regard, one must first pay attention to the presence or absence of 5q33, 4q12, or 8p11.2 translocations, which, if present, would suggest PDGFRB-, PDGFRA-, or FGFR1-rearranged clonal eosinophilia, respectively. The presence of 5q33 or 4q12 translocations predicts favorable response to treatment with imatinib mesylate, whereas 8p11.2 translocations are associated with aggressive myeloid malignancies that are refractory to current drug therapy.
CEL-NOS is considered in the presence of cytogenetic/morphologic evidence of a myeloid malignancy that is otherwise not classifiable. Specifically, CEL-NOS is distinguished from HES by the presence of either a cytogenetic abnormality or greater than 2% peripheral blood blasts or greater than 5% bone marrow blasts (Table 135e-7). HES or idiopathic eosinophilia is considered in the absence of both morphologic and molecular evidence of clonal eosinophilia. However, before making a working diagnosis of HES, one has to exclude lymphocytic variant hypereosinophilia by excluding the presence of phenotypically abnormal T lymphocytes (by flow cytometry) and clonal T-cell gene rearrangements.
Chronic Eosinophilic Leukemia, Not Otherwise Specified (CEL-NOS) CEL-NOS is a subset of clonal eosinophilia that is neither molecularly defined nor classified as an alternative clinicopathologically assigned myeloid malignancy. We prefer to use the term strictly in patients with an HES phenotype who also display either a clonal cytogenetic/molecular abnormality or excess blasts in the bone marrow or peripheral blood. The WHO defines CEL-NOS in the presence of an AEC ≥1.5 × 109/L that is accompanied by either the presence of myeloblast excess (either >2% in the peripheral blood or 5–19% in the bone marrow) or evidence of myeloid clonality. Cytogenetic abnormalities in CEL, other than those that are associated with molecularly defined eosinophilic disorders, include trisomy 8 (the most frequent), t(10;11)(p14;q21), and t(7;12)(q11;p11). CEL-NOS does not respond to imatinib, and treatment strategies are often not different from those used in other similar MPNs and include ASCT for transplant-eligible patients with poor risk factors and participation in experimental treatment protocols otherwise.
PDGFR-Mutated Eosinophilia Both platelet-derived growth factor receptors α (PDGFRA located on chromosome 4q12) and β (PDGFRB located on chromosome 5q31-q32) are involved in MPN-relevant activating mutations. Clinical phenotype in both instances includes prominent blood eosinophilia and excellent response to imatinib therapy. In regard to PDGFRA mutations, the most popular is FIP1L1-PDGFRA, a karyotypically occult del(4)(q12) that was described in 2003 as an imatinib-sensitive activating mutation. Functional studies have demonstrated transforming properties in cell lines and the induction of MPN in mice. Cloning of the FIP1L1-PDGFRA fusion gene identified a novel molecular mechanism for generating this constitutively active fusion tyrosine kinase, wherein a ~800-kb interstitial deletion within 4q12 fuses the 5′ portion of FIP1L1 to the 3′ portion of PDGFRA. FIP1L1-PDGFRA occurs in a very small subset of patients who present with the phenotypic features of either systemic mastocytosis or HES, but the presence of the mutation reliably predicts complete hematologic and molecular response to imatinib therapy.
The association between eosinophilic myeloid malignancies and PDGFRB rearrangement was first characterized and published in 1994 when fusion of the tyrosine kinase–encoding region of PDGFRB to the ets-like gene, ETV6 [ETV6-PDGFRB, t(5;12)(q33;p13)] was demonstrated. The fusion protein was transforming to cell lines and resulted in constitutive activation of PDGFRB signaling. Since then, several other PDGFRB fusion transcripts with similar disease phenotypes have been described, cell line transformation and myeloproliferative disease (MPD) induction in mice has been demonstrated, and imatinib therapy was proven effective when used.
FGFR1-Mutated Eosinophilia The 8p11 myeloproliferative syndrome (EMS) (also known as human stem cell leukemic/lymphoma syndrome) constitutes a clinical phenotype with features of both lymphoma and eosinophilic MPN and characterized by a fusion mutation that involves the gene for fibroblast growth factor receptor 1 (FGFR1), which is located on chromosome 8p11. In EMS, both myeloid and lymphoid lineage cells exhibit the 8p11 translocation, thus demonstrating the stem cell origin of the disease. The disease features several 8p11-linked chromosome translocations, and some of the corresponding fusion FGFR1 mutants have been shown to transform cell lines and induce EMS- or CML-like disease in mice depending on the specific FGFR1 partner gene (ZNF198 or BCR, respectively). Consistent with this laboratory observation, some patients with BCR-FGFR1 mutation manifest a more indolent CML-like disease. The mechanism of FGFR1 activation in EMS is similar to that seen with PDGFRB-associated MPD; the tyrosine kinase domain of FGFR1 is juxtaposed to a dimerization domain from the partner gene. EMS is aggressive and requires combination chemotherapy followed by ASCT.
Hypereosinophilic Syndrome (HES) Blood eosinophilia that is neither secondary nor clonal is operationally labeled as being idiopathic. HES is a subcategory of idiopathic eosinophilia with persistent increase of the AEC to ≥1.5 × 109/L and presence of eosinophil-mediated organ damage, including cardiomyopathy, gastroenteritis, cutaneous lesions, sinusitis, pneumonitis, neuritis, and vasculitis. In addition, some patients manifest thromboembolic complications, hepatosplenomegaly, and either cytopenia or cytosis.
Bone marrow histologic and cytogenetic/molecular studies should be examined before a working diagnosis of HES is made. Additional blood studies that are currently recommended during the evaluation of HES include serum tryptase (an increased level suggests systemic mastocytosis and warrants molecular studies to detect FIP1L1-PDGFRA), T-cell immunophenotyping, and T-cell receptor antigen gene rearrangement analysis (a positive test suggests an underlying clonal or phenotypically abnormal T-cell disorder). In addition, initial evaluation in HES should include echocardiogram and measurement of serum troponin levels to screen for myocardial involvement by the disease.
Initial evaluation of the patient with eosinophilia should include tests that facilitate assessment of target organ damage, including complete blood count, chest x-ray, echocardiogram, and serum troponin level. An increased level of serum cardiac troponin has been shown to correlate with the presence of cardiomyopathy in HES. Typical echocardiographic findings in HES include ventricular apical thrombus, posterior mitral leaflet or tricuspid valve abnormality, endocardial thickening, dilated left ventricle, and pericardial effusion.
Glucocorticoids are the cornerstone of therapy in HES. Treatment with oral prednisone is usually started at 1 mg/kg per day and continued for 1–2 weeks before the dose is tapered slowly over the ensuing 2–3 months. If symptoms recur at a prednisone dose level of >10 mg/d, either hydroxyurea or interferon α is used as steroid-sparing agent. In patients who do not respond to usual therapy as outlined above, mepolizumab or alemtuzumab might be considered. Mepolizumab targets interleukin 5 (IL-5), a well-recognized survival factor for eosinophils. Alemtuzumab targets the CD52 antigen, which has been shown to be expressed by eosinophils but not by neutrophils.
MASTOCYTOSIS
Mast cell disease (MCD) is defined as tissue infiltration by morphologically and immunophenotypically abnormal mast cells. MCD is classified into two broad categories: cutaneous mastocytosis and systemic mastocytosis (SM). MCD in adults is usually systemic, and the clinical course can be either indolent or aggressive, depending on the respective absence or presence of impaired organ function. Symptoms and signs of MCD include urticaria pigmentosa, mast cell mediator release symptoms (e.g., headache, flushing, lightheadedness, syncope, anaphylaxis, pruritus, urticaria, angioedema, nausea, diarrhea, abdominal cramps), and organ damage (lytic bone lesions, osteoporosis, hepatosplenomegaly, cytopenia). Aggressive SM can be associated with another myeloid malignancy, including MPN, MDS, or MDS/MPN overlap (e.g., CMML), or present as overt mast cell leukemia. In general, life expectancy is near normal in indolent SM but significantly shortened in aggressive SM.
Diagnosis of SM is based on bone marrow examination that shows clusters of morphologically abnormal, spindle-shaped mast cells that are best evaluated by the use of immunohistochemical stains that are specific to mast cells (tryptase, CD117). In addition, mast cell immunophenotyping reveals aberrant CD25 expression by neoplastic mast cells. Other laboratory findings in SM include increased levels of serum tryptase, histamine and urine histamine metabolites, and prostaglandins. SM is associated with KIT mutations, usually KIT D816V, in the majority of patients. Accordingly, mutation screening for KIT D816V is diagnostically useful. However, the ability to detect KIT D816V depends on assay sensitivity and mast cell content of the test sample.
Both indolent and aggressive SM patients might experience mast cell mediator release symptoms, which are usually managed by both H1 and H2 histamine receptor blockers as well as cromolyn sodium. In addition, patients with propensity to vasodilatory shock should wear a medical alert bracelet and carry an Epi-Pen self-injector for self-administration of subcutaneous epinephrine. Urticaria pigmentosa shows variable response to both topical and systemic glucocorticoid therapy. Cytoreductive therapy is not recommended for indolent SM. In aggressive SM, either interferon α or cladribine is considered first-line therapy and benefits the majority of patients. In contrast, imatinib is ineffective in the treatment of PDGFR-unmutated SM.
DENDRITIC AND HISTIOCYTIC NEOPLASMS
Dendritic cell (DC) and histiocyte/macrophage neoplasms are extremely rare. DCs are antigen-presenting cells, whereas histiocyte/macrophages are antigen-processing cells. Bone marrow myeloid stem cells (CD34+) give rise to monocyte (CD14+, CD68+, CD11c+, CD1a–) and DC (CD14–, CD11c+/–, CD1a+/c) precursors. Monocyte precursors, in turn, give rise to macrophages (CD14+, CD68+, CD11c+, CD163+, lysozyme+) and interstitial DCs (CD68+, CD1a–). DC precursors give rise to Langerhans cell DCs (Birbeck granules, CD1a+, S100+, langerin+) and plasmacytoid DCs (CD68+, CD123+). Follicular DCs (CD21+, CD23+, CD35+) originate from mesenchymal stem cells. Dendritic and histiocytic neoplasms are operationally classified into macrophage/histiocyte-related and DC-related neoplasms. The former includes histiocytic sarcoma/malignant histiocytosis and the latter Langerhans cell histiocytosis, Langerhans cell sarcoma, interdigitating DC sarcoma, and follicular DC sarcoma.
Histiocytic Sarcoma/Malignant Histiocytosis Histiocytic sarcoma represents malignant proliferation of mature tissue histiocytes and is often localized. Median age at diagnosis is estimated at 46 years with slight male predilection. Some patients might have history of lymphoma, MDS, or germ cell tumors at time of disease presentation. The three typical disease sites are lymph nodes, skin, and the gastrointestinal system. Patients may or may not have systemic symptoms including fever and weight loss, and other symptoms include hepatosplenomegaly, lytic bone lesions, and pancytopenia. Immunophenotype includes presence of histiocytic markers (CD68, lysozyme, CD11c, CD14) and absence of myeloid or lymphoid markers. Prognosis is poor, and treatment is often ineffective. The term malignant histiocytosis refers to a disseminated disease and systemic symptoms. Lymphoma-like treatment induces complete remissions in some patients, and median survival is estimated at 2 years.
Langerhans Cell Histiocytosis Langerhans cells (LCs) are specialized DCs that reside in mucocutaneous tissue and upon activation become specialized for antigen presentation to T cells. LC histiocytosis (LCH; also known as histiocytosis X) represents neoplastic proliferation of LCs (S-100+, CD1a+, and Birbeck granules on electron microscopy). LCH incidence is estimated at 5 per million, and the disease typically affects children with a male predilection. Presentation can be either unifocal (eosinophilic granuloma) or multifocal. The former usually affects bones and less frequently lymph nodes, skin, and lung, whereas the latter is more disseminated. Unifocal disease often affects older children and adults, whereas multisystem disease affects infants. LCH of the lung in adults is characterized by bilateral nodules. Prognosis depends on organs involved. Only 10% of patients progress from unifocal to multiorgan disease. LCH of the lung might improve upon cessation of smoking.
Langerhans Cell Sarcoma Langerhans cell sarcoma (LCS) also represents neoplastic proliferation of LCs with overtly malignant morphology. The disease can present de novo or progress from antecedent LCH. There is a female predilection, and median age at diagnosis is estimated at 41 years. Immunophenotype is similar to that seen in LCH, and liver, spleen, lung, and bone are the usual sites of disease. Prognosis is poor, and treatment is generally ineffective.
Interdigitating Dendritic Cell Sarcoma Interdigitating DC sarcoma (IDCS), also known as reticulum cell sarcoma, represents neoplastic proliferation of interdigitating DCs. The disease is extremely rare and affects elderly adults with no sex predilection. Typical presentation is asymptomatic solitary lymphadenopathy. Immunophenotype includes S-100+ and negative for vimentin and CD1a. Prognosis ranges from benign local disease to widespread lethal disease.
Follicular Dendritic Cell Neoplasm Follicular DCs (FDCs) reside in B-cell follicles and present antigen to B cells. FDC neoplasms (FDCNs) are usually localized and often affect adults. FDCN might be associated with Castleman’s disease in 10–20% of cases, and increased incidence in schizophrenia has been reported. Cervical lymph nodes are the most frequent site of involvement in FDCN, and other sites include maxillary, mediastinal, and retroperitoneal lymph nodes; oral cavity; gastrointestinal system; skin; and breast. Sites of metastasis include lung and liver. Immunophenotype includes CD21, CD35, and CD23. Clinical course is typically indolent, and treatment includes surgical excision followed by regional radiotherapy and sometimes systemic chemotherapy.
Hemophagocytic Syndromes Hemophagocytic syndrome (HPS) represents nonneoplastic proliferation and activation of macrophages that induce cytokine-mediated bone marrow suppression and features of intense phagocytosis in bone marrow and liver. HPS may result from genetic or acquired disorders of macrophages. The former entail genetically determined inability to regulate macrophage proliferation and activation. Acquired HPS is often precipitated by viral infections, most notably Epstein-Barr virus. HPS might also accompany certain malignancies such as T-cell lymphoma. Clinical course is often fulminant and fatal.
136 | Plasma Cell Disorders |
The plasma cell disorders are monoclonal neoplasms related to each other by virtue of their development from common progenitors in the B-lymphocyte lineage. Multiple myeloma, Waldenström’s macroglobulinemia, primary amyloidosis (Chap. 137), and the heavy chain diseases comprise this group and may be designated by a variety of synonyms such as monoclonal gammopathies, paraproteinemias, plasma cell dyscrasias, and dysproteinemias. Mature B lymphocytes destined to produce IgG bear surface immunoglobulin molecules of both M and G heavy chain isotypes with both isotypes having identical idiotypes (variable regions). Under normal circumstances, maturation to antibody-secreting plasma cells and their proliferation is stimulated by exposure to the antigen for which the surface immunoglobulin is specific; however, in the plasma cell disorders, the control over this process is lost. The clinical manifestations of all the plasma cell disorders relate to the expansion of the neoplastic cells, to the secretion of cell products (immunoglobulin molecules or subunits, lymphokines), and to some extent to the host’s response to the tumor. Normal development of B lymphocytes is discussed in Chap. 372e and depicted in Fig. 134-2.
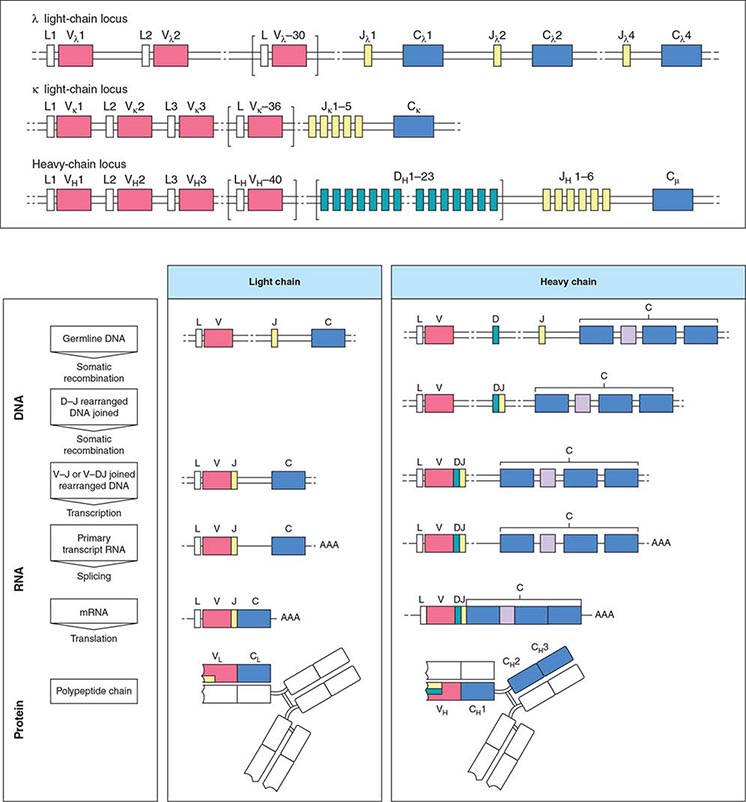
There are three categories of structural variation among immunoglobulin molecules that form antigenic determinants, and these are used to classify immunoglobulins. Isotypes are those determinants that distinguish among the main classes of antibodies of a given species and are the same in all normal individuals of that species. Therefore, isotypic determinants are, by definition, recognized by antibodies from a distinct species (heterologous sera) but not by antibodies from the same species (homologous sera). There are five heavy chain isotypes (M, G, A, D, E) and two light chain isotypes (κ, λ). Allotypes are distinct determinants that reflect regular small differences between individuals of the same species in the amino acid sequences of otherwise similar immunoglobulins. These differences are determined by allelic genes; by definition, they are detected by antibodies made in the same species. Idiotypes are the third category of antigenic determinants. They are unique to the molecules produced by a given clone of antibody-producing cells. Idiotypes are formed by the unique structure of the antigen-binding portion of the molecule.
Antibody molecules (Fig. 136-1) are composed of two heavy chains (~50,000 mol wt) and two light chains (~25,000 mol wt). Each chain has a constant portion (limited amino acid sequence variability) and a variable region (extensive sequence variability). The light and heavy chains are linked by disulfide bonds and are aligned so that their variable regions are adjacent to one another. This variable region forms the antigen recognition site of the antibody molecule; its unique structural features form idiotypes that are reliable markers for a particular clone of cells because each antibody is formed and secreted by a single clone. Because of the mechanics of the gene rearrangements necessary to specify the immunoglobulin variable regions (VDJ joining for the heavy chain, VJ joining for the light chain), a particular clone rearranges only one of the two chromosomes to produce an immunoglobulin molecule of only one light chain isotype and only one allotype (allelic exclusion) (Fig. 136-1). After exposure to antigen, the variable region may become associated with a new heavy chain isotype (class switch). Each clone of cells performs these sequential gene arrangements in a unique way. This results in each clone producing a unique immunoglobulin molecule. In most plasma cells, light chains are synthesized in slight excess, secreted as free light chains, and cleared by the kidney, but <10 mg of such light chains is excreted per day.
FIGURE 136-1 Immunoglobulin genetics and the relationship of gene segments to the antibody protein. The top portion of the figure is a schematic of the organization of the immunoglobulin genes, λ on chromosome 22, κ on chromosome 2, and the heavy chain locus on chromosome 14. The heavy chain locus is longer than 2 megabases, and some of the D region gene segments are only a few bases long, so the figure depicts the schematic relationship among the segments, not their actual size. The bottom portion of the figure outlines the steps in going from the noncontiguous germline gene segments to an intact antibody molecule. Two recombination events juxtapose the V-D-J (or V-J for light chains) segments. The rearranged gene is transcribed, and RNA splicing cuts out intervening sequences to produce an mRNA, which is then translated into an antibody light or heavy chain. The sites on the antibody that bind to antigen (the so called CDR3 regions) are encoded by D and J segments for heavy chains and the J segments for light chains. (From K Murphy: Janeway’s Immunobiology, 8th ed. Garland Science, 2011.)
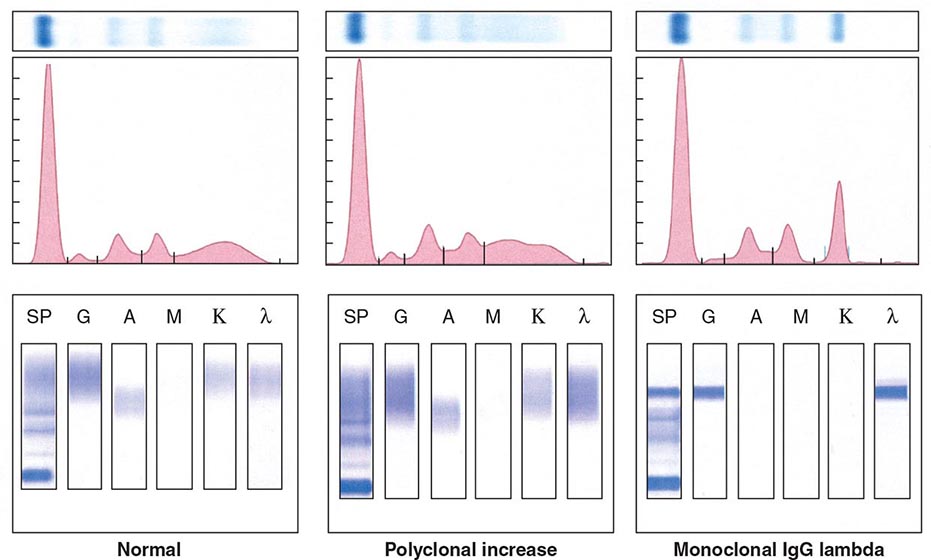
Electrophoretic analysis permits separation of components of the serum proteins (Fig. 136-2). The immunoglobulins move heterogeneously in an electric field and form a broad peak in the gamma region, which is usually increased in the sera of patients with plasma cell tumors. There is a sharp spike in this region called an M component (M for monoclonal). Less commonly, the M component may appear in the β2 or α2 globulin region. The monoclonal antibody must be present at a concentration of at least 5 g/L (0.5 g/dL) to be accurately quantitated by this method. This corresponds to ~109 cells producing the antibody. Confirmation of the type of immunoglobulin and that it is truly monoclonal is determined by immunoelectrophoresis that reveals a single heavy and/or light chain type. Hence immunoelectrophoresis and electrophoresis provide qualitative and quantitative assessment of the M component, respectively. Once the presence of an M component has been confirmed, the amount of M component in the serum is a reliable measure of the tumor burden, making M component an excellent tumor marker to manage therapy, yet it is not specific enough to be used to screen asymptomatic patients. In addition to the plasma cell disorders, M components may be detected in other lymphoid neoplasms such as chronic lymphocytic leukemia and lymphomas of B- or T-cell origin; nonlymphoid neoplasms such as chronic myeloid leukemia, breast cancer, and colon cancer; a variety of nonneoplastic conditions such as cirrhosis, sarcoidosis, parasitic diseases, Gaucher’s disease, and pyoderma gangrenosum; and a number of autoimmune conditions, including rheumatoid arthritis, myasthenia gravis, and cold agglutinin disease. Monoclonal proteins are also observed in immunosuppressed patients after organ transplant and, rarely, allogeneic transplant. At least two very rare skin diseases—lichen myxedematosus (also known as papular mucinosis) and necrobiotic xanthogranuloma—are associated with a monoclonal gammopathy. In papular mucinosis, highly cationic IgG is deposited in the dermis of patients. This organ specificity may reflect the specificity of the antibody for some antigenic component of the dermis. Necrobiotic xanthogranuloma is a histiocytic infiltration of the skin, usually of the face, that produces red or yellow nodules that can enlarge to plaques. Approximately 10% progress to myeloma. Five percent of patients with sensory motor neuropathy also have a monoclonal paraprotein.
FIGURE 136-2 Representative patterns of serum electrophoresis and immunofixation. The upper panels represent agarose gel, middle panels are the densitometric tracing of the gel, and lower panels are immunofixation patterns. Panel on the left illustrates the normal pattern of serum protein on electrophoresis. Because there are many different immunoglobulins in the serum, their differing mobilities in an electric field produce a broad peak. In conditions associated with increases in polyclonal immunoglobulin, the broad peak is more prominent (middle panel). In monoclonal gammopathies, the predominance of a product of a single cell produces a “church spire” sharp peak, usually in the γ globulin region (right panel). The immunofixation (lower panel) identifies the type of immunoglobulin. For example, normal and polyclonal increase in immunoglobulins produce no distinct bands; however, the right panel shows distinct bands in IgG and lambda protein lanes, confirming the presence of IgG lambda monoclonal protein. (Courtesy of Dr. Neal I. Lindeman; with permission.)
The nature of the M component is variable in plasma cell disorders. It may be an intact antibody molecule of any heavy chain subclass, or it may be an altered antibody or fragment. Isolated light or heavy chains may be produced. In some plasma cell tumors such as extramedullary or solitary bone plasmacytomas, less than one-third of patients will have an M component. In ~20% of myelomas, only light chains are produced and, in most cases, are secreted in the urine as Bence Jones proteins. The frequency of myelomas of a particular heavy chain class is roughly proportional to the serum concentration, and therefore, IgG myelomas are more common than IgA and IgD myelomas. In approximately 1% of patients with myeloma, biclonal or triclonal gammopathy is observed.
MULTIPLE MYELOMA
DEFINITION
Multiple myeloma represents a malignant proliferation of plasma cells derived from a single clone. The tumor, its products, and the host response to it result in a number of organ dysfunctions and symptoms, including bone pain or fracture, renal failure, susceptibility to infection, anemia, hypercalcemia, and occasionally clotting abnormalities, neurologic symptoms, and manifestations of hyperviscosity.
ETIOLOGY
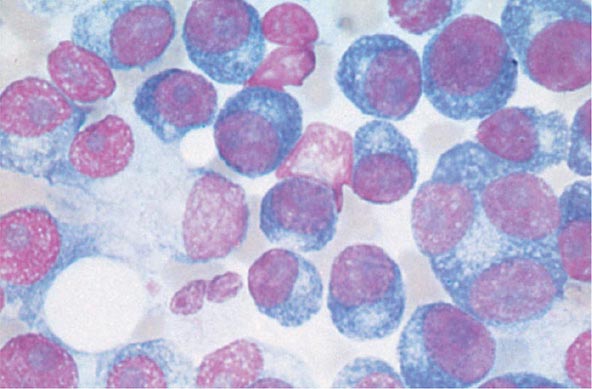
The cause of myeloma is not known. Myeloma occurred with increased frequency in those exposed to the radiation of nuclear warheads in World War II after a 20-year latency. Myeloma has been seen more commonly than expected among farmers, wood workers, leather workers, and those exposed to petroleum products. A variety of chromosomal alterations have been found in patients with myeloma: hyperdiploidy, 13q14 deletions, translocations t(11;14)(q13;q32), t(4;14)(p16;q32), and t(14;16), and 17p13 deletions. Evidence is strong that errors in switch recombination—the genetic mechanism to change antibody heavy chain isotype—participate in the transformation process. However, no common molecular pathogenetic pathway has yet emerged. Genome sequencing studies have failed to identify any recurrent mutation with frequency >20%; N-ras, K-ras, and B-raf mutations are most common and combined occur in over 40% of patients. There is also evidence of complex clusters of subclonal variants at diagnosis that acquire additional mutations over time, indicative of genomic evolution that may drive disease progression. The neoplastic event in myeloma may involve cells earlier in B-cell differentiation than the plasma cell. Interleukin (IL) 6 may play a role in driving myeloma cell proliferation. It remains difficult to distinguish benign from malignant plasma cells based on morphologic criteria in all but a few cases (Fig. 136-3).
FIGURE 136-3 Multiple myeloma (marrow). The cells bear characteristic morphologic features of plasma cells, round or oval cells with an eccentric nucleus composed of coarsely clumped chromatin, a densely basophilic cytoplasm, and a perinuclear clear zone containing the Golgi apparatus. Binucleate and multinucleate malignant plasma cells can be seen.
INCIDENCE AND PREVALENCE
An estimated 24,050 new cases of myeloma were diagnosed in 2014, and 11,090 people died from the disease in the United States. Myeloma increases in incidence with age. The median age at diagnosis is 70 years; it is uncommon under age 40. Males are more commonly affected than females, and blacks have nearly twice the incidence of whites. Myeloma accounts for 1.3% of all malignancies in whites and 2% in blacks, and 13% of all hematologic cancers in whites and 33% in blacks.
GLOBAL CONSIDERATIONS
![]() The incidence of myeloma is highest in African Americans and Pacific Islanders; intermediate in Europeans and North American whites; and lowest in people from developing countries including Asia. The higher incidence in more developed countries may result from the combination of a longer life expectancy and more frequent medical surveillance. Incidence of multiple myeloma in other ethnic groups including native Hawaiians, female Hispanics, American Indians from New Mexico, and Alaskan natives is higher relative to U.S. whites in the same geographic area. Chinese and Japanese populations have a lower incidence than whites. Immunoproliferative small-intestinal disease with alpha heavy chain disease is most prevalent in the Mediterranean area. Despite these differences in prevalence, the characteristics, response to therapy, and prognosis of myeloma are similar worldwide.
The incidence of myeloma is highest in African Americans and Pacific Islanders; intermediate in Europeans and North American whites; and lowest in people from developing countries including Asia. The higher incidence in more developed countries may result from the combination of a longer life expectancy and more frequent medical surveillance. Incidence of multiple myeloma in other ethnic groups including native Hawaiians, female Hispanics, American Indians from New Mexico, and Alaskan natives is higher relative to U.S. whites in the same geographic area. Chinese and Japanese populations have a lower incidence than whites. Immunoproliferative small-intestinal disease with alpha heavy chain disease is most prevalent in the Mediterranean area. Despite these differences in prevalence, the characteristics, response to therapy, and prognosis of myeloma are similar worldwide.
PATHOGENESIS AND CLINICAL MANIFESTATIONS
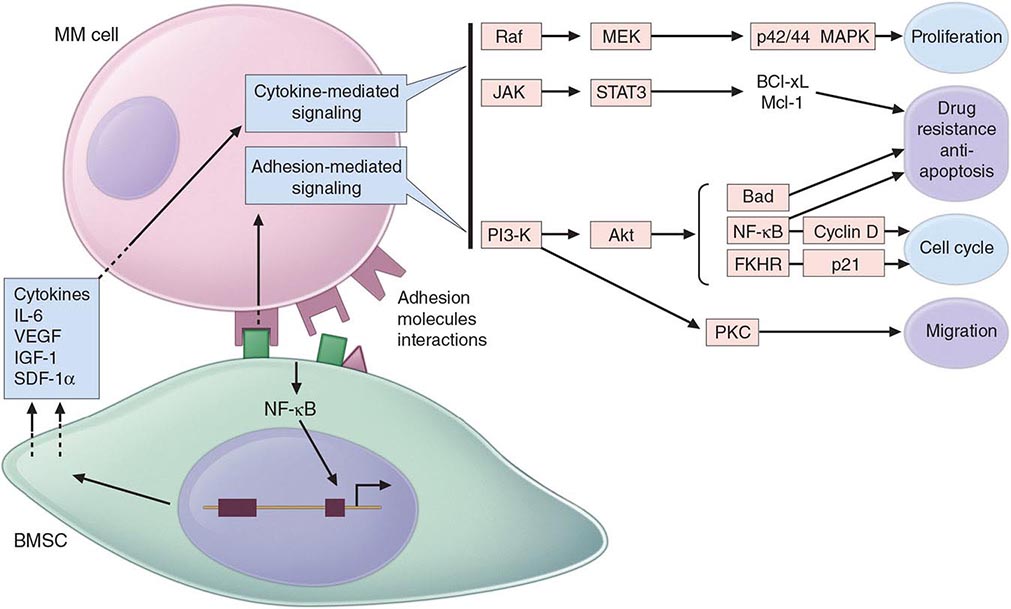
Multiple myeloma (MM) cells bind via cell-surface adhesion molecules to bone marrow stromal cells (BMSCs) and extracellular matrix (ECM), which triggers MM cell growth, survival, drug resistance, and migration in the bone marrow milieu (Fig. 136-4). These effects are due both to direct MM cell–BMSC binding and to induction of various cytokines, including IL-6, insulin-like growth factor type I (IGF-I), vascular endothelial growth factor (VEGF), and stromal cell–derived growth factor (SDF)-1α. Growth, drug resistance, and migration are mediated via Ras/Raf/mitogen-activated protein kinase, PI3K/Akt, and protein kinase C signaling cascades, respectively.
FIGURE 136-4 Pathogenesis of multiple myeloma. Multiple myeloma (MM) cells interact with bone marrow stromal cells (BMSCs) and extracellular matrix proteins via adhesion molecules, triggering adhesion-mediated signaling as well as cytokine production. This triggers cytokine-mediated signaling that provides growth, survival, and antiapoptotic effects as well as development of drug resistance.
Bone pain is the most common symptom in myeloma, affecting nearly 70% of patients. Unlike the pain of metastatic carcinoma, which often is worse at night, the pain of myeloma is precipitated by movement. Persistent localized pain in a patient with myeloma usually signifies a pathologic fracture. The bone lesions of myeloma are caused by the proliferation of tumor cells, activation of osteoclasts that destroy bone, and suppression of osteoblasts that form new bone. The increased osteoclast activity is mediated by osteoclast activating factors (OAFs) made by the myeloma cells (OAF activity can be mediated by several cytokines, including IL-1, lymphotoxin, VEGF, receptor activator of NF-κB [RANK] ligand, macrophage inhibitory factor [MIP]-1α, and tumor necrosis factor [TNF]). The bone lesions are lytic in nature and are rarely associated with osteoblastic new bone formation due to their suppression by dickhoff-1 (DKK-1) produced by myeloma cells. Therefore, radioisotopic bone scanning is less useful in diagnosis than is plain radiography. The bony lysis results in substantial mobilization of calcium from bone, and serious acute and chronic complications of hypercalcemia may dominate the clinical picture (see below). Localized bone lesions may expand to the point that mass lesions may be palpated, especially on the skull (Fig. 136-5), clavicles, and sternum; and the collapse of vertebrae may lead to spinal cord compression. The next most common clinical problem in patients with myeloma is susceptibility to bacterial infections. The most common infections are pneumonias and pyelonephritis, and the most frequent pathogens are Streptococcus pneumoniae, Staphylococcus aureus, and Klebsiella pneumoniae in the lungs and Escherichia coli and other gram-negative organisms in the urinary tract. In ~25% of patients, recurrent infections are the presenting features, and >75% of patients will have a serious infection at some time in their course. The susceptibility to infection has several contributing causes. First, patients with myeloma have diffuse hypogammaglobulinemia if the M component is excluded. The hypogammaglobulinemia is related to both decreased production and increased destruction of normal antibodies. Moreover, some patients generate a population of circulating regulatory cells in response to their myeloma that can suppress normal antibody synthesis. In the case of IgG myeloma, normal IgG antibodies are broken down more rapidly than normal because the catabolic rate for IgG antibodies varies directly with the serum concentration. The large M component results in fractional catabolic rates of 8–16% instead of the normal 2%. These patients have very poor antibody responses, especially to polysaccharide antigens such as those on bacterial cell walls. Most measures of T-cell function in myeloma are normal, but a subset of CD4+ cells may be decreased. Granulocyte lysozyme content is low, and granulocyte migration is not as rapid as normal in patients with myeloma, probably the result of a tumor product. There are also a variety of abnormalities in complement functions in myeloma patients. All these factors contribute to the immune deficiency of these patients. Some commonly used therapeutic agents, e.g., dexamethasone, suppress immune responses and increase susceptibility to bacterial and fungal infection, and bortezomib predisposes to herpesvirus reactivation.
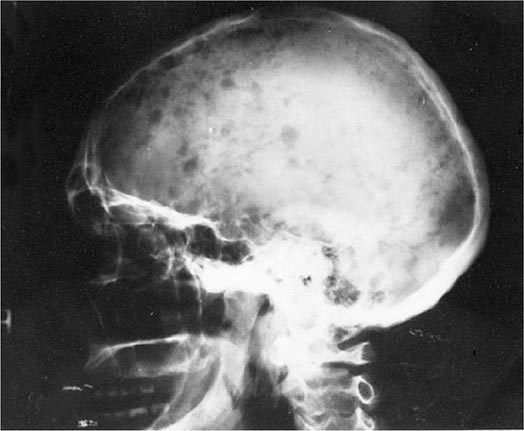
FIGURE 136-5 Bony lesions in multiple myeloma. The skull demonstrates the typical “punched out” lesions characteristic of multiple myeloma. The lesion represents a purely osteolytic lesion with little or no osteoblastic activity. (Courtesy of Dr. Geraldine Schechter; with permission.)
Renal failure occurs in nearly 25% of myeloma patients, and some renal pathology is noted in more than 50%. Many factors contribute to this. Hypercalcemia is the most common cause of renal failure. Glomerular deposits of amyloid, hyperuricemia, recurrent infections, frequent use of nonsteroidal anti-inflammatory agents for pain control, use of iodinated contrast dye for imaging, bisphosphonate use, and occasional infiltration of the kidney by myeloma cells all may contribute to renal dysfunction. However, tubular damage associated with the excretion of light chains is almost always present. Normally, light chains are filtered, reabsorbed in the tubules, and catabolized. With the increase in the amount of light chains presented to the tubule, the tubular cells become overloaded with these proteins, and tubular damage results either directly from light chain toxic effects or indirectly from the release of intracellular lysosomal enzymes. The earliest manifestation of this tubular damage is the adult Fanconi’s syndrome (a type 2 proximal renal tubular acidosis), with loss of glucose and amino acids, as well as defects in the ability of the kidney to acidify and concentrate the urine. The proteinuria is not accompanied by hypertension, and the protein is nearly all light chains. Generally, very little albumin is in the urine because glomerular function is usually normal. When the glomeruli are involved, nonselective proteinuria is also observed. Patients with myeloma also have a decreased anion gap [i.e., Na+ – (Cl– + HCO3–)] because the M component is cationic, resulting in retention of chloride. This is often accompanied by hyponatremia that is felt to be artificial (pseudohyponatremia) because each volume of serum has less water as a result of the increased protein. Renal dysfunction due to light chain deposition disease, light chain cast nephropathy, and amyloidosis is partially reversible with effective therapy. Myeloma patients are susceptible to developing acute renal failure if they become dehydrated.
Normocytic and normochromic anemia occurs in ~80% of myeloma patients. It is usually related to the replacement of normal marrow by expanding tumor cells, to the inhibition of hematopoiesis by factors made by the tumor, to reduced production of erythropoietin by the kidney, and to the effects of long-term therapy. In addition, mild hemolysis may contribute to the anemia. A larger than expected fraction of patients may have megaloblastic anemia due to either folate or vitamin B12 deficiency. Granulocytopenia and thrombocytopenia are rare except when therapy-induced. Clotting abnormalities may be seen due to the failure of antibody-coated platelets to function properly; the interaction of the M component with clotting factors I, II, V, VII, or VIII; antibody to clotting factors; or amyloid damage of endothelium. Deep venous thrombosis is also observed with use of thalidomide, lenalidomide, or pomalidomide in combination with dexamethasone. Raynaud’s phenomenon and impaired circulation may result if the M component forms cryoglobulins, and hyperviscosity syndromes may develop depending on the physical properties of the M component (most common with IgM, IgG3, and IgA paraproteins). Hyperviscosity is defined based on the relative viscosity of serum as compared with water. Normal relative serum viscosity is 1.8 (i.e., serum is normally almost twice as viscous as water). Symptoms of hyperviscosity occur at a level greater than 4 centipoise (cP), which is usually reached at paraprotein concentrations of ~40 g/L (4 g/dL) for IgM, 50 g/L (5 g/dL) for IgG3, and 70 g/L (7 g/dL) for IgA; however, depending on chemical and physical properties of the paraprotein molecule, it can occasionally be observed at lower levels.
Although neurologic symptoms occur in a minority of patients, they may have many causes. Hypercalcemia may produce lethargy, weakness, depression, and confusion. Hyperviscosity may lead to headache, fatigue, shortness of breath, exacerbation or precipitation of heart failure, visual disturbances, ataxia, vertigo, retinopathy, somnolence, and coma. Bony damage and collapse may lead to cord compression, radicular pain, and loss of bowel and bladder control. Infiltration of peripheral nerves by amyloid can be a cause of carpal tunnel syndrome and other sensorimotor mono- and polyneuropathies. Neuropathy associated with monoclonal gammopathy of undetermined significance (MGUS) and myeloma is more frequently sensory than motor neuropathy and is associated with IgM more than other isotypes. In >50% of patients with neuropathy, the IgM monoclonal protein is directed against myelin-associated globulin (MAG). Sensory neuropathy is also a side effect of thalidomide and bortezomib therapy.
Many of the clinical features of myeloma, e.g., cord compression, pathologic fractures, hyperviscosity, sepsis, and hypercalcemia, can present as medical emergencies. Despite the widespread distribution of plasma cells in the body, tumor expansion is dominantly within bone and bone marrow and, for reasons unknown, rarely causes enlargement of spleen, lymph nodes, or gut-associated lymphatic tissue.
DIAGNOSIS AND STAGING
The diagnosis of myeloma requires marrow plasmacytosis (>10%), a serum and/or urine M component, and end organ damage detailed in Table 136-1. Bone marrow plasma cells are CD138 and either monoclonal kappa or lambda light chain positive. The most important differential diagnosis in patients with myeloma involves their separation from individuals with MGUS or smoldering multiple myeloma (SMM). MGUS is vastly more common than myeloma, occurring in 1% of the population older than age 50 years and in up to 10% of individuals older than age 75 years. The diagnostic criteria for MGUS, SMM, and myeloma are described in Table 136-1. Although ~1% of patients per year with MGUS go on to develop myeloma, all myeloma is preceded by MGUS. Non-IgG subtype, abnormal kappa/lambda free light chain ratio, and serum M protein >15 g/L (1.5 g/dL) are associated with higher incidence of progression of MGUS to myeloma. Absence of all three features predicts a 5% chance of progression, whereas higher risk MGUS with the presence of all three features predicts a 60% chance of progression over 20 years. The features responsible for higher risk of progression from SMM to MM are bone marrow plasmacytosis >10%, abnormal kappa/lambda free light chain ratio, and serum M protein >30 g/L (3 g/dL). Patients with only one of these three features have a 25% chance of progression to MM in 5 years, whereas patients with high-risk SMM with all three features have a 76% chance of progression. There are two important variants of myeloma—solitary bone plasmacytoma and solitary extramedullary plasmacytoma. These lesions are associated with an M component in <30% of the cases, they may affect younger individuals, and both are associated with median survivals of ≥10 years. Solitary bone plasmacytoma is a single lytic bone lesion without marrow plasmacytosis. Extramedullary plasmacytomas usually involve the submucosal lymphoid tissue of the nasopharynx or paranasal sinuses without marrow plasmacytosis. Both tumors are highly responsive to local radiation therapy. If an M component is present, it should disappear after treatment. Solitary bone plasmacytomas may recur in other bony sites or evolve into myeloma. Extramedullary plasmacytomas rarely recur or progress.
DIAGNOSTIC CRITERIA FOR MULTIPLE MYELOMA, MYELOMA VARIANTS, AND MONOCLONAL GAMMOPATHY OF UNDETERMINED SIGNIFICANCE |