447 | Migraine and Other Primary Headache Disorders |
The general principles around headache as a cardinal symptom are covered elsewhere (Chap. 21); here we discuss disorders in which headache and associated features occur in the absence of any exogenous cause. The most common are migraine, tension-type headache, and the trigeminal autonomic cephalalgias, notably cluster headache; the complete list is summarized in Table 447-1.
PRIMARY HEADACHE DISORDERS, MODIFIED FROM INTERNATIONAL CLASSIFICATION OF HEADACHE DISORDERS-III-BETA (HEADACHE CLASSIFICATION COMMITTEE OF THE INTERNATIONAL HEADACHE SOCIETY, 2013) |

MIGRAINE
Migraine, the second most common cause of headache, and the most common headache-related, and indeed neurologic, cause of disability in the world, afflicts approximately 15% of women and 6% of men over a 1-year period. It is usually an episodic headache associated with certain features such as sensitivity to light, sound, or movement; nausea and vomiting often accompany the headache. A useful description of migraine is a recurring syndrome of headache associated with other symptoms of neurologic dysfunction in varying admixtures (Table 447-2). Migraine can often be recognized by its activators, referred to as triggers.
SYMPTOMS ACCOMPANYING SEVERE MIGRAINE ATTACKS IN 500 PATIENTS |
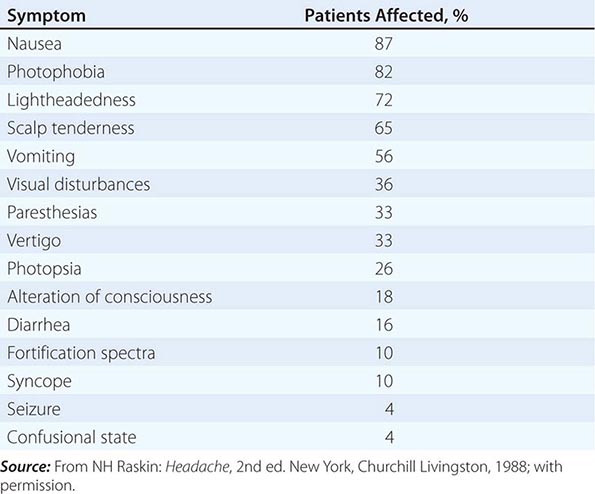
The brain of the migraineur is particularly sensitive to environmental and sensory stimuli; migraine-prone patients do not habituate easily to sensory stimuli. This sensitivity is amplified in females during the menstrual cycle. Headache can be initiated or amplified by various triggers, including glare, bright lights, sounds, or other afferent stimulation; hunger; let-down from stress; physical exertion; stormy weather or barometric pressure changes; hormonal fluctuations during menses; lack of or excess sleep; and alcohol or other chemical stimulation, such as with nitrates. Knowledge of a patient’s susceptibility to specific triggers can be useful in management strategies involving lifestyle adjustments.
Pathogenesis The sensory sensitivity that is characteristic of migraine is probably due to dysfunction of monoaminergic sensory control systems located in the brainstem and hypothalamus (Fig. 447-1).
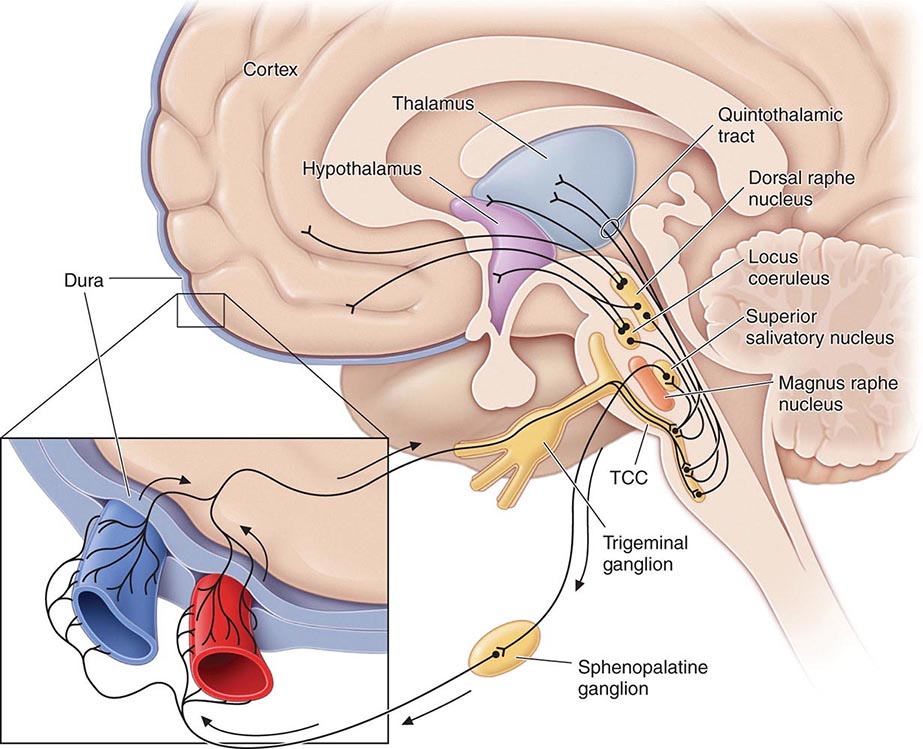
FIGURE 447-1 Brainstem pathways that modulate sensory input. The key pathway for pain in migraine is the trigeminovascular input from the meningeal vessels, which passes through the trigeminal ganglion and synapses on second-order neurons in the trigeminocervical complex (TCC). These neurons in turn project in the quintothalamic tract and, after decussating in the brainstem, synapse on neurons in the thalamus. Important modulation of the trigeminovascular nociceptive input comes from the dorsal raphe nucleus, locus coeruleus, and nucleus raphe magnus.
Activation of cells in the trigeminal nucleus results in the release of vasoactive neuropeptides, particularly calcitonin gene–related peptide (CGRP), at vascular terminations of the trigeminal nerve and within the trigeminal nucleus. CGRP receptor antagonists, gepants, have now been shown to be effective in the acute treatment of migraine, and monoclonal antibodies to CGRP have been shown effective in two early phase clinical trials. Centrally, the second-order trigeminal neurons cross the midline and project to ventrobasal and posterior nuclei of the thalamus for further processing. Additionally, there are projections to the periaqueductal gray and hypothalamus, from which reciprocal descending systems have established antinociceptive effects. Other brainstem regions likely to be involved in descending modulation of trigeminal pain include the nucleus locus coeruleus in the pons and the rostroventromedial medulla.
Pharmacologic and other data point to the involvement of the neurotransmitter 5-hydroxytryptamine (5-HT; also known as serotonin) in migraines. Approximately 60 years ago, methysergide was found to antagonize certain peripheral actions of 5-HT and was introduced as the first drug capable of preventing migraine attacks. The triptans were designed to stimulate selectively subpopulations of 5-HT receptors; at least 14 different 5-HT receptors exist in humans. The triptans are potent agonists of 5-HT1B and 5-HT1D receptors, and some are active at the 5-HT1F receptors; the latter’s exclusive agonists are called ditans. Triptans arrest nerve signaling in the nociceptive pathways of the trigeminovascular system, at least in the trigeminal nucleus caudalis and trigeminal sensory thalamus, in addition to cranial vasoconstriction, while ditans, now shown conclusively to be effective in acute migraine, act only at neural targets. An interesting range of neural targets is now being actively pursed for the acute and preventive management of migraine.
Data also support a role for dopamine in the pathophysiology of migraine. Most migraine symptoms can be induced by dopaminergic stimulation. Moreover, there is dopamine receptor hypersensitivity in migraineurs, as demonstrated by the induction of yawning, nausea, vomiting, hypotension, and other symptoms of a migraine attack by dopaminergic agonists at doses that do not affect nonmigraineurs. Dopamine receptor antagonists are effective therapeutic agents in migraine, especially when given parenterally or concurrently with other antimigraine agents. Moreover, hypothalamic activation, anterior to that seen in cluster headache, has now been shown in the premonitory phase of migraine using functional imaging, and this may hold a key to understanding some part of the role of dopamine in the disorder.
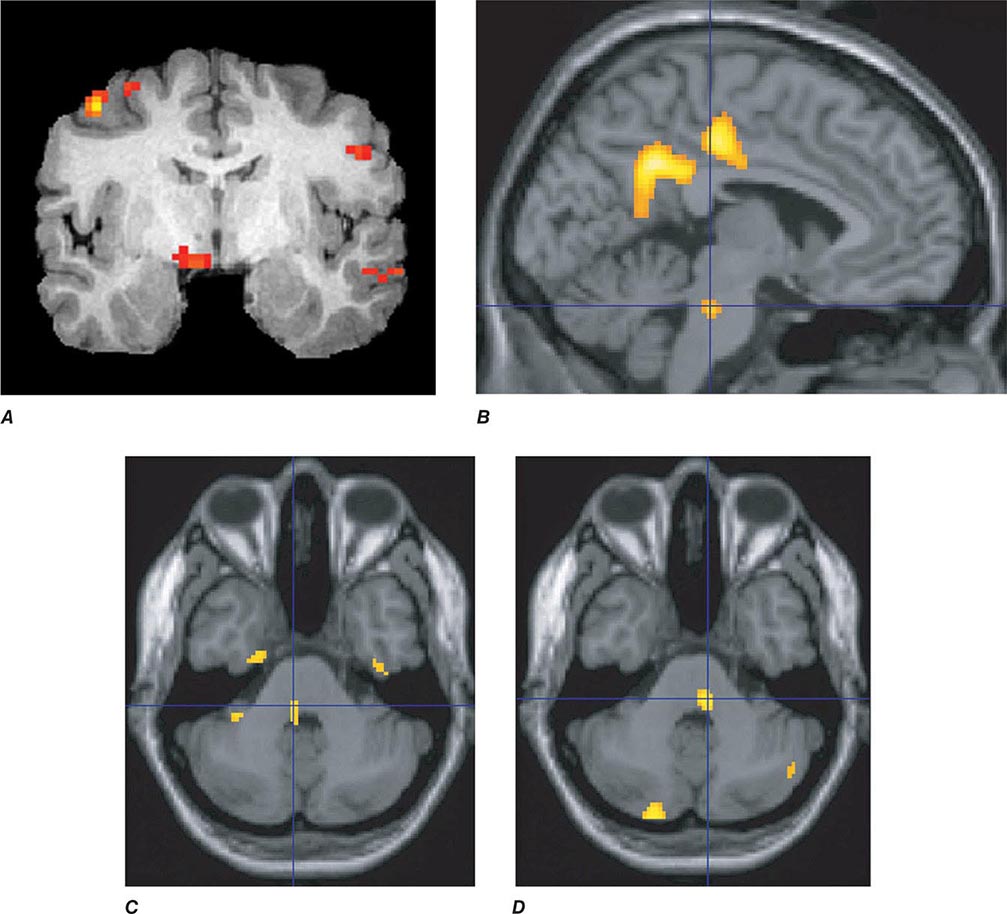
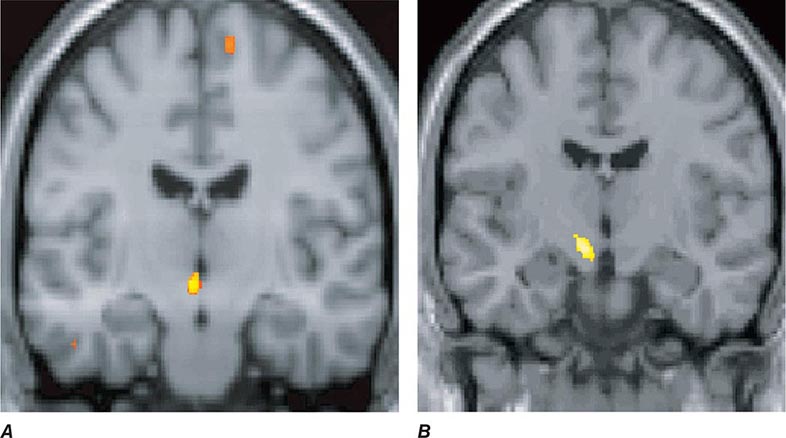
Migraine genes identified by studying families with familial hemiplegic migraine (FHM) reveal involvement of ion channels, suggesting that alterations in membrane excitability can predispose to migraine. Mutations involving the Cav2.1 (P/Q)–type voltage-gated calcium channel CACNA1A gene are now known to cause FHM 1; this mutation is responsible for about 50% of FHMs. Mutations in the Na+-K+ATPase ATP1A2 gene, designated FHM 2, are responsible for about 20% of FHMs. Mutations in the neuronal voltage-gated sodium channel SCN1A cause FHM 3. Functional neuroimaging has suggested that brainstem regions in migraine (Fig. 447-2) and the posterior hypothalamic gray matter region close to the human circadian pacemaker cells of the suprachiasmatic nucleus in cluster headache (Fig. 447-3) are good candidates for specific involvement in primary headache.
FIGURE 447-2 Positron emission tomography (PET) activation in migraine. Hypothalamic, dorsal midbrain, and dorsolateral pontine activation is seen in triggered attacks in the premonitory phase before pain, whereas in migraine attacks, dorsolateral pontine activation persists, as it does in chronic migraine (not shown). The dorsolateral pontine area, which includes the noradrenergic locus coeruleus, is fundamental to the expression of migraine. Moreover, lateralization of changes in this region of the brainstem correlates with lateralization of the head pain in hemicranial migraine; the scans shown in panels C and D are of patients with acute migraine headache on the right and left side, respectively. (Panel A from FH Maniyar et al: Brain 137:232, 2014; panel B from SK Afridi et al: Arch Neurol 2005;62:1270; Panels C and D from SK Afridi et al: Brain 128:932, 2005.)
FIGURE 447-3 A. Posterior hypothalamic gray matter activation by positron emission tomography in a patient with acute cluster headache. (From A May et al: Lancet 352:275, 1998.) B. High-resolution T1-weighted magnetic resonance image obtained using voxel-based morphometry demonstrates increased gray matter activity, lateralized to the side of pain in a patient with cluster headache. (From A May et al: Nat Med 5:836, 1999.)
Diagnosis and Clinical Features Diagnostic criteria for migraine headache are listed in Table 447-3. A high index of suspicion is required to diagnose migraine: the migraine aura, consisting of visual disturbances with flashing lights or zigzag lines moving across the visual field or of other neurologic symptoms, is reported in only 20–25% of patients. A headache diary can often be helpful in making the diagnosis; this is also helpful in assessing disability and the frequency of treatment for acute attacks. Patients with episodes of migraine that occur daily or near-daily are considered to have chronic migraine (see “Chronic Daily Headache” in Chap. 21). Migraine must be differentiated from tension-type headache (discussed below), the most common primary headache syndrome seen in the population. Migraine has several forms that have been defined (Table 447-1): migraine with and without aura and chronic migraine, the latter occurring 15 days or more a month, as the most important. Migraine at its most basic level is headache with associated features, and tension-type headache is headache that is featureless. Most patients with disabling headache probably have migraine.
SIMPLIFIED DIAGNOSTIC CRITERIA FOR MIGRAINE |
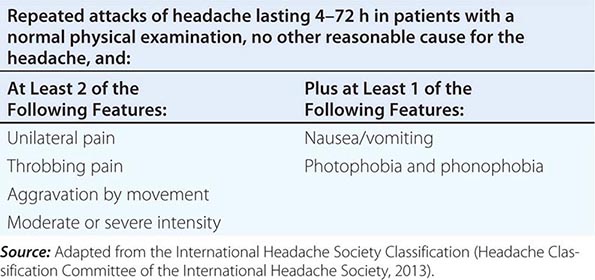
Patients with acephalgic migraine (typical aura without headache, 1.2.1.2 in Table 447-1) experience recurrent neurologic symptoms, often with nausea or vomiting, but with little or no headache. Vertigo can be prominent; it has been estimated that one-third of patients referred for vertigo or dizziness have a primary diagnosis of migraine. Migraine aura can have prominent brainstem symptoms, and the terms basilar artery and basilar-type migraine have now been replaced by migraine with brainstem aura (Table 447-1).
TENSION-TYPE HEADACHE
Clinical Features The term tension-type headache (TTH) is commonly used to describe a chronic head-pain syndrome characterized by bilateral tight, band-like discomfort. The pain typically builds slowly, fluctuates in severity, and may persist more or less continuously for many days. The headache may be episodic or chronic (present >15 days per month).
A useful clinical approach is to diagnose TTH in patients whose headaches are completely without accompanying features such as nausea, vomiting, photophobia, phonophobia, osmophobia, throbbing, and aggravation with movement. Such an approach neatly separates migraine, which has one or more of these features and is the main differential diagnosis, from TTH. The International Headache Society’s main definition of TTH allows an admixture of nausea, photophobia, or phonophobia in various combinations, although the appendix definition does not; this illustrates the difficulty in distinguishing these two clinical entities. In clinical practice, dichotomizing patients on the basis of the presence of associated features (migraine) and the absence of associated features (TTH) is highly recommended. Indeed patients whose headaches fit the TTH phenotype and who have migraine at other times, along with a family history of migraine, migrainous illnesses of childhood, or typical migraine triggers to their migraine attacks, may be biologically different from those who have TTH headache with none of the features. TTH may be infrequent (episodic) or occur on 15 days or more a month (chronic).
Pathophysiology The pathophysiology of TTH is incompletely understood. It seems likely that TTH is due to a primary disorder of central nervous system pain modulation alone, unlike migraine, which involves a more generalized disturbance of sensory modulation. Data suggest a genetic contribution to TTH, but this may not be a valid finding: given the current diagnostic criteria, the studies undoubtedly included many migraine patients. The name tension-type headache implies that pain is a product of nervous tension, but there is no clear evidence for tension as an etiology. Muscle contraction has been considered to be a feature that distinguishes TTH from migraine, but there appear to be no differences in contraction between the two headache types.
TRIGEMINAL AUTONOMIC CEPHALALGIAS, INCLUDING CLUSTER HEADACHE
The trigeminal autonomic cephalalgias (TACs) describe a grouping of primary headaches including cluster headache, paroxysmal hemicrania, SUNCT (short-lasting unilateral neuralgiform headache attacks with conjunctival injection and tearing)/SUNA (short-lasting unilateral neuralgiform headache attacks with cranial autonomic symptoms), and hemicrania continua (Table 447-1). TACs are characterized by relatively short-lasting attacks of head pain associated with cranial autonomic symptoms, such as lacrimation, conjunctival injection, or nasal congestion (Table 447-7). Pain is usually severe and may occur more than once a day. Because of the associated nasal congestion or rhinorrhea, patients are often misdiagnosed with “sinus headache” and treated with decongestants, which are ineffective.
CLINICAL FEATURES OF THE TRIGEMINAL AUTONOMIC CEPHALALGIAS |
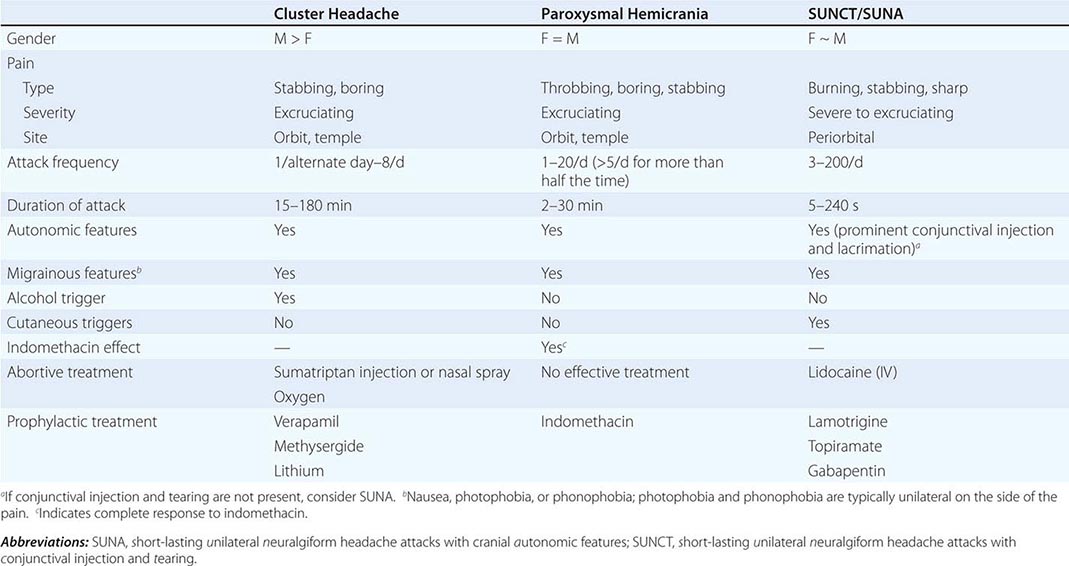
TACs must be differentiated from short-lasting headaches that do not have prominent cranial autonomic syndromes, notably trigeminal neuralgia, primary stabbing headache, and hypnic headache. The cycling pattern and length, frequency, and timing of attacks are useful in classifying patients. Patients with TACs should undergo pituitary imaging and pituitary function tests because there is an excess of TAC presentations in patients with pituitary tumor–related headache.
Cluster Headache Cluster headache is a relatively rare form of primary headache with a population frequency of approximately 0.1%. The pain is deep, usually retroorbital, often excruciating in intensity, nonfluctuating, and explosive in quality. A core feature of cluster headache is periodicity. At least one of the daily attacks of pain recurs at about the same hour each day for the duration of a cluster bout. The typical cluster headache patient has daily bouts of one to two attacks of relatively short-duration unilateral pain for 8 to 10 weeks a year; this is usually followed by a pain-free interval that averages a little less than 1 year. Cluster headache is characterized as chronic when there is less than 1 month of sustained remission without treatment. Patients are generally perfectly well between episodes. Onset is nocturnal in about 50% of patients, and men are affected three times more often than women. Patients with cluster headache tend to move about during attacks, pacing, rocking, or rubbing their head for relief; some may even become aggressive during attacks. This is in sharp contrast to patients with migraine, who prefer to remain motionless during attacks.
Cluster headache is associated with ipsilateral symptoms of cranial parasympathetic autonomic activation: conjunctival injection or lacrimation, rhinorrhea or nasal congestion, or cranial sympathetic dysfunction such as ptosis. The sympathetic deficit is peripheral and likely to be due to parasympathetic activation with injury to ascending sympathetic fibers surrounding a dilated carotid artery as it passes into the cranial cavity. When present, photophobia and phonophobia are far more likely to be unilateral and on the same side of the pain, rather than bilateral, as is seen in migraine. This phenomenon of unilateral photophobia/phonophobia is characteristic of TACs. Cluster headache is likely to be a disorder involving central pacemaker neurons in the posterior hypothalamic region (Fig. 447-3).
PAROXYSMAL HEMICRANIA
Paroxysmal hemicrania (PH) is characterized by frequent unilateral, severe, short-lasting episodes of headache. Like cluster headache, the pain tends to be retroorbital but may be experienced all over the head and is associated with autonomic phenomena such as lacrimation and nasal congestion. Patients with remissions are said to have episodic PH, whereas those with the nonremitting form are said to have chronic PH. The essential features of PH are unilateral, very severe pain; short-lasting attacks (2–45 min); very frequent attacks (usually more than five a day); marked autonomic features ipsilateral to the pain; rapid course (<72 h); and excellent response to indomethacin. In contrast to cluster headache, which predominantly affects males, the male-to-female ratio in PH is close to 1:1.
Indomethacin (25–75 mg tid), which can completely suppress attacks of PH, is the treatment of choice. Although therapy may be complicated by indomethacin-induced gastrointestinal side effects, currently there are no consistently effective alternatives. Topiramate is helpful in some cases. Piroxicam has been used, although it is not as effective as indomethacin. Verapamil, an effective treatment for cluster headache, does not appear to be useful for PH. In occasional patients, PH can coexist with trigeminal neuralgia (PH-tic syndrome); similar to cluster-tic syndrome, each component may require separate treatment.
Secondary PH has been reported with lesions in the region of the sella turcica, including arteriovenous malformation, cavernous sinus meningioma, pituitary pathology and epidermoid tumors. Secondary PH is more likely if the patient requires high doses (>200 mg/d) of indomethacin. In patients with apparent bilateral PH, raised cerebrospinal fluid (CSF) pressure should be suspected. It is important to note that indomethacin reduces CSF pressure. When a diagnosis of PH is considered, magnetic resonance imaging (MRI) is indicated to exclude a pituitary lesion.
SUNCT/SUNA
SUNCT (short-lasting unilateral neuralgiform headache attacks with conjunctival injection and tearing) is a rare primary headache syndrome characterized by severe, unilateral orbital or temporal pain that is stabbing or throbbing in quality. Diagnosis requires at least 20 attacks, lasting for 5–240 s; ipsilateral conjunctival injection and lacrimation should be present. In some patients, conjunctival injection or lacrimation is missing, and the diagnosis of SUNA (short-lasting unilateral neuralgiform headache attacks with cranial autonomic symptoms) can be made.
DIAGNOSIS The pain of SUNCT/SUNA is unilateral and may be located anywhere in the head. Three basic patterns can be seen: single stabs, which are usually short-lived; groups of stabs; or a longer attack comprising many stabs between which the pain does not completely resolve, thus giving a “saw-tooth” phenomenon with attacks lasting many minutes. Each pattern may be seen in the context of an underlying continuous head pain. Characteristics that lead to a suspected diagnosis of SUNCT are the cutaneous (or other) triggers of attacks, a lack of refractory period to triggering between attacks, and the lack of a response to indomethacin. Apart from trigeminal sensory disturbance, the neurologic examination is normal in primary SUNCT.
The diagnosis of SUNCT/SUNA is often confused with trigeminal neuralgia (TN) particularly in first-division TN (Chap. 455). Minimal or no cranial autonomic symptoms and a clear refractory period to triggering indicate a diagnosis of TN.
SECONDARY (SYMPTOMATIC) SUNCT SUNCT can be seen with posterior fossa or pituitary lesions. All patients with SUNCT/SUNA should be evaluated with pituitary function tests and a brain MRI with pituitary views.
Hemicrania Continua The essential features of hemicrania continua are moderate and continuous unilateral pain associated with fluctuations of severe pain; complete resolution of pain with indomethacin; and exacerbations that may be associated with autonomic features, including conjunctival injection, lacrimation, and photophobia on the affected side. The age of onset ranges from 11 to 58 years; women are affected twice as often as men. The cause is unknown.