PART 6: Nutrition and Weight Loss
95e | Nutrient Requirements and Dietary Assessment |
Nutrients are substances that are not synthesized in sufficient amounts in the body and therefore must be supplied by the diet. Nutrient requirements for groups of healthy persons have been determined experimentally. The absence of essential nutrients leads to growth impairment, organ dysfunction, and failure to maintain nitrogen balance or adequate status of other nutrients. For good health, we require energy-providing nutrients (protein, fat, and carbohydrate), vitamins, minerals, and water. Requirements for organic nutrients include 9 essential amino acids, several fatty acids, glucose, 4 fat-soluble vitamins, 10 water-soluble vitamins, dietary fiber, and choline. Several inorganic substances, including 4 minerals, 7 trace minerals, 3 electrolytes, and the ultratrace elements, must also be supplied by diet.
The amounts of the essential nutrients that are required by individuals differ by age and physiologic state. Conditionally essential nutrients are not required in the diet but must be supplied to individuals who do not synthesize them in adequate amounts, such as those with genetic defects, those with pathologic conditions such as infection or trauma with nutritional implications, and developmentally immature infants. For example, inositol, taurine, arginine, and glutamine may be needed by premature infants. Many other organic and inorganic compounds that are present in foods, such as pesticides and lead, also have health effects.
ESSENTIAL NUTRIENT REQUIREMENTS
Energy For weight to remain stable, energy intake must match energy output. The major components of energy output are resting energy expenditure (REE) and physical activity; minor components include the energy cost of metabolizing food (thermic effect of food, or specific dynamic action) and shivering thermogenesis (e.g., cold-induced thermogenesis). The average energy intake is ~2600 kcal/d for American men and ~1800 kcal/d for American women, though these estimates vary with body size and activity level. Formulas for roughly estimating REE are useful in assessing the energy needs of an individual whose weight is stable. Thus, for males, REE = 900 + 10m, and for females, REE = 700 + 7m, where is m mass in kilograms. The calculated REE is then adjusted for physical activity level by multiplying by 1.2 for sedentary, 1.4 for moderately active, or 1.8 for very active individuals. The final figure, the estimated energy requirement (EER), provides an approximation of total caloric needs in a state of energy balance for a person of a certain age, sex, weight, height, and physical activity level. For further discussion of energy balance in health and disease, see Chap. 97.
Protein Dietary protein consists of both essential and nonessential amino acids that are required for protein synthesis. The nine essential amino acids are histidine, isoleucine, leucine, lysine, methionine/cystine, phenylalanine/tyrosine, threonine, tryptophan, and valine. Certain amino acids, such as alanine, can also be used for energy and gluconeogenesis. When energy intake is inadequate, protein intake must be increased, because ingested amino acids are diverted into pathways of glucose synthesis and oxidation. In extreme energy deprivation, protein-calorie malnutrition may ensue (Chap. 97).
For adults, the recommended dietary allowance (RDA) for protein is ~0.6 g/kg desirable body mass per day, assuming that energy needs are met and that the protein is of relatively high biologic value. Current recommendations for a healthy diet call for at least 10–14% of calories from protein. Most American diets provide at least those amounts. Biologic value tends to be highest for animal proteins, followed by proteins from legumes (beans), cereals (rice, wheat, corn), and roots. Combinations of plant proteins that complement one another in biologic value or combinations of animal and plant proteins can increase biologic value and lower total protein requirements. In healthy people with adequate diets, the timing of protein intake over the course of the day has little effect.
Protein needs increase during growth, pregnancy, lactation, and rehabilitation after injury or malnutrition. Tolerance to dietary protein is decreased in renal insufficiency (with consequent uremia) and in liver failure. Normal protein intake can precipitate encephalopathy in patients with cirrhosis of the liver.
Fat and Carbohydrate Fats are a concentrated source of energy and constitute, on average, 34% of calories in U.S. diets. However, for optimal health, fat intake should total no more than 30% of calories. Saturated fat and trans fat should be limited to <10% of calories and polyunsaturated fats to <10% of calories, with monounsaturated fats accounting for the remainder of fat intake. At least 45–55% of total calories should be derived from carbohydrates. The brain requires ~100 g of glucose per day for fuel; other tissues use about 50 g/d. Some tissues (e.g., brain and red blood cells) rely on glucose supplied either exogenously or from muscle proteolysis. Over time, adaptations in carbohydrate needs are possible during hypocaloric states. Like fat (9 kcal/g), carbohydrate (4 kcal/g), and protein (4 kcal/g), alcohol (ethanol) provides energy (7 kcal/g). However, it is not a nutrient.
Water For adults, 1–1.5 mL of water per kilocalorie of energy expenditure is sufficient under usual conditions to allow for normal variations in physical activity, sweating, and solute load of the diet. Water losses include 50–100 mL/d in the feces; 500–1000 mL/d by evaporation or exhalation; and, depending on the renal solute load, ≥1000 mL/d in the urine. If external losses increase, intakes must increase accordingly to avoid underhydration. Fever increases water losses by ~200 mL/d per °C; diarrheal losses vary but may be as great as 5 L/d in severe diarrhea. Heavy sweating, vigorous exercise, and vomiting also increase water losses. When renal function is normal and solute intakes are adequate, the kidneys can adjust to increased water intake by excreting up to 18 L of excess water per day (Chap. 404). However, obligatory urine outputs can compromise hydration status when there is inadequate water intake or when losses increase in disease or kidney damage.
Infants have high requirements for water because of their large ratio of surface area to volume, their inability to communicate their thirst, and the limited capacity of the immature kidney to handle high renal solute loads. Increased water needs during pregnancy are ~30 mL/d. During lactation, milk production increases daily water requirements so that ~1000 mL of additional water is needed, or 1 mL for each milliliter of milk produced. Special attention must be paid to the water needs of the elderly, who have reduced total body water and blunted thirst sensation and are more likely to be taking medications such as diuretics.
Other Nutrients See Chap. 96e for detailed descriptions of vitamins and trace minerals.
DIETARY REFERENCE INTAKES AND RECOMMENDED DIETARY ALLOWANCES
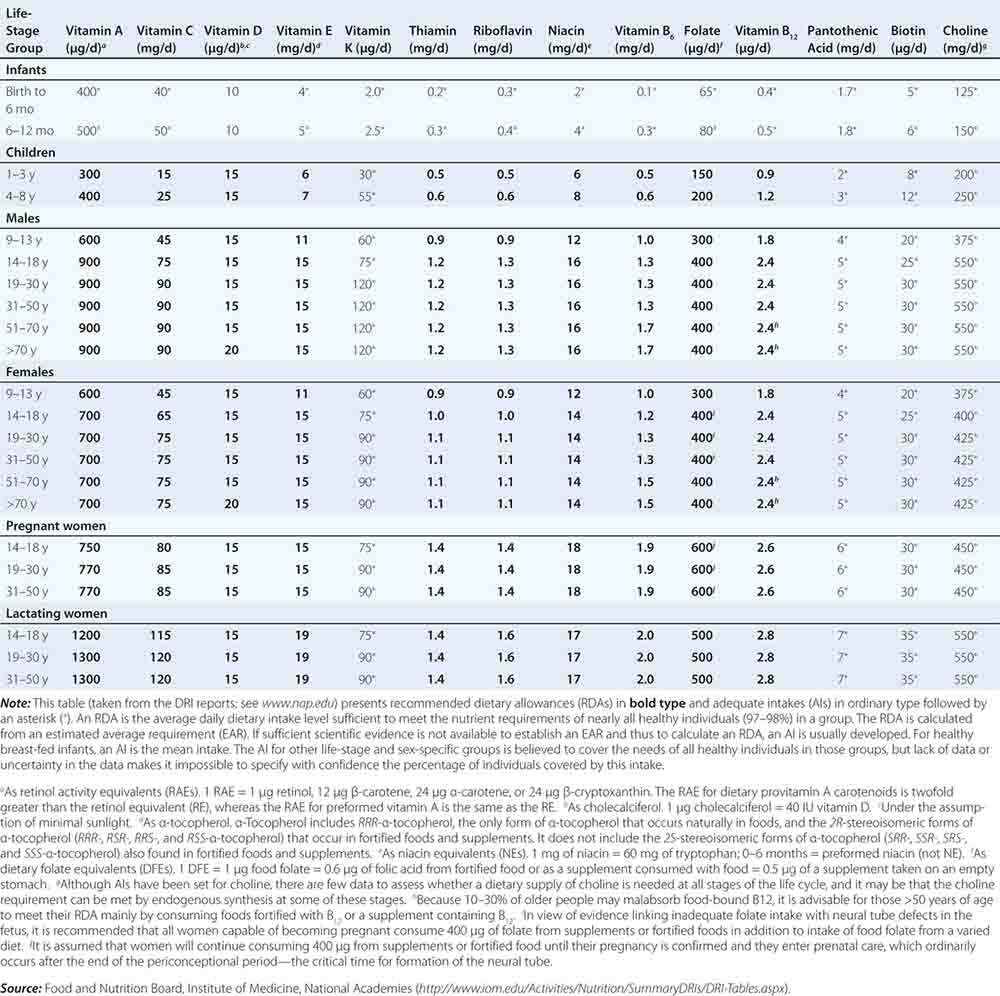
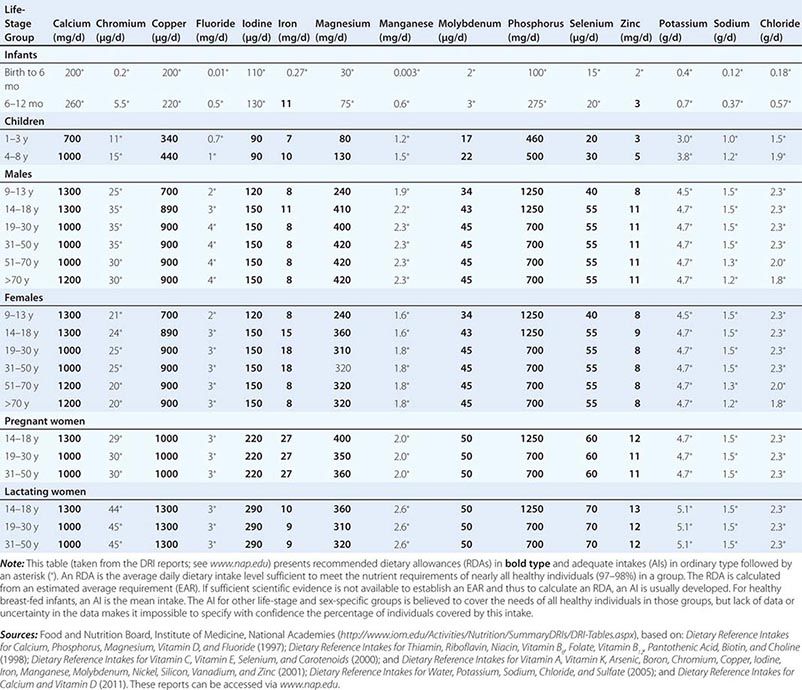
Fortunately, human life and well-being can be maintained within a fairly wide range for most nutrients. However, the capacity for adaptation is not infinite—too much, as well as too little, intake of a nutrient can have adverse effects or alter the health benefits conferred by another nutrient. Therefore, benchmark recommendations regarding nutrient intakes have been developed to guide clinical practice. These quantitative estimates of nutrient intakes are collectively referred to as the dietary reference intakes (DRIs). The DRIs have supplanted the RDAs—the single reference values used in the United States until the early 1990s. DRIs include the estimated average requirement (EAR) for nutrients as well as other reference values used for dietary planning for individuals: the RDA, the adequate intake (AI), and the tolerable upper level (UL). The DRIs also include acceptable macronutrient distribution ranges (AMDRs) for protein, fat, and carbohydrate. The current DRIs for vitamins and elements are provided in Tables 95e-1 and 95e-2, respectively. Table 95e-3 provides DRIs for water and macronutrients. EERs are discussed in Chap. 97 on energy balance in health and disease.
DIETARY REFERENCE INTAKES (DRIs): RECOMMENDED DIETARY ALLOWANCES AND ADEQUATE INTAKES FOR VITAMINS |
DIETARY REFERENCE INTAKES (DRIs): RECOMMENDED DIETARY ALLOWANCES AND ADEQUATE INTAKES FOR ELEMENTS |
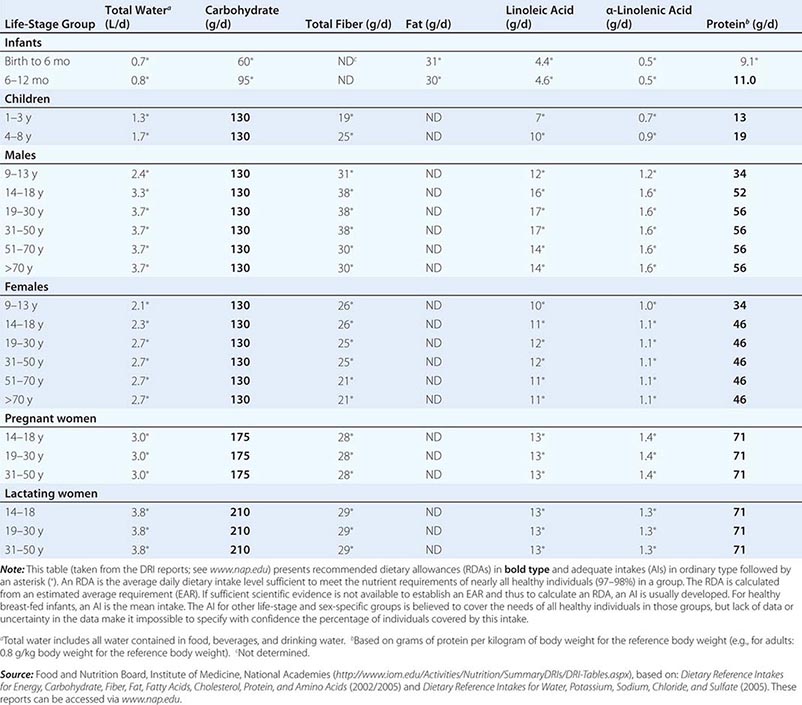
DIETARY REFERENCE INTAKES (DRIs): RECOMMENDED DIETARY ALLOWANCES AND ADEQUATE INTAKES FOR TOTAL WATER AND MACRONUTRIENTS |
Estimated Average Requirement When florid manifestations of the classic dietary-deficiency diseases such as rickets (deficiency of vitamin D and calcium), scurvy (deficiency of vitamin C), xerophthalmia (deficiency of vitamin A), and protein-calorie malnutrition were common, nutrient adequacy was inferred from the absence of their clinical signs. Later, biochemical and other changes were found to be evident long before the deficiency became clinically apparent. Consequently, criteria of adequacy are now based on biologic markers when they are available. Priority is given to sensitive biochemical, physiologic, or behavioral tests that reflect early changes in regulatory processes; maintenance of body stores of nutrients; or, if available, the amount of a nutrient that minimizes the risk of chronic degenerative disease. Current efforts focus on this last variable, but relevant markers often are not available.
The EAR is the amount of a nutrient estimated to be adequate for half of the healthy individuals of a specific age and sex. The types of evidence and criteria used to establish nutrient requirements vary by nutrient, age, and physiologic group. The EAR is not an effective estimate of nutrient adequacy in individuals because it is a median requirement for a group; 50% of individuals in a group fall below the requirement and 50% fall above it. Thus, a person with a usual intake at the EAR has a 50% risk of inadequate intake. For these reasons, other standards, described below, are more useful for clinical purposes.
Recommended Dietary Allowances The RDA is the average daily dietary intake level that meets the nutrient requirements of nearly all healthy persons of a specific sex, age, life stage, or physiologic condition (e.g., pregnancy or lactation). The RDA, which is the nutrient-intake goal for planning diets of individuals, is defined statistically as two standard deviations above the EAR to ensure that the needs of any given individual are met. The online tool at http://fnic.nal.usda.gov/interactiveDRI/ allows health professionals to calculate individualized daily nutrient recommendations for dietary planning based on the DRIs for persons of a given age, sex, and weight. The RDAs are used to formulate food guides such as the U.S. Department of Agriculture (USDA) MyPlate Food Guide for individuals (www.supertracker.usda.gov/default.aspx), to create food-exchange lists for therapeutic diet planning, and as a standard for describing the nutritional content of foods and nutrient-containing dietary supplements.
The risk of dietary inadequacy increases as intake falls below the RDA. However, the RDA is an overly generous criterion for evaluating nutrient adequacy. For example, by definition, the RDA exceeds the actual requirements of all but ~2–3% of the population. Therefore, many people whose intake falls below the RDA may still be getting enough of the nutrient. On food labels, the nutrient content in a food is stated by weight or as a percent of the daily value (DV), a variant of the RDA used on the nutrition facts panel that, for an adult, represents the highest RDA for an adult consuming 2000 kcal.
Adequate Intake It is not possible to set an RDA for some nutrients that do not have an established EAR. In this circumstance, the AI is based on observed or experimentally determined approximations of nutrient intakes in healthy people. In the DRIs, AIs rather than RDAs are proposed for nutrients consumed by infants (up to age 1 year) as well as for chromium, fluoride, manganese, sodium, potassium, pantothenic acid, biotin, choline, and water consumed by persons of all ages. Vitamin D and calcium recommendations were recently revised, and more precise estimates are now available.
Tolerable Upper Levels of Nutrient Intake Healthy individuals derive no established benefit from consuming nutrient levels above the RDA or AI. In fact, excessive nutrient intake can disturb body functions and cause acute, progressive, or permanent disabilities. The tolerable UL is the highest level of chronic nutrient intake (usually daily) that is unlikely to pose a risk of adverse health effects for most of the population. Data on the adverse effects of large amounts of many nutrients are unavailable or too limited to establish a UL. Therefore, the lack of a UL does not mean that the risk of adverse effects from high intake is nonexistent. Nutrients in commonly eaten foods rarely exceed the UL. However, highly fortified foods and dietary supplements provide more concentrated amounts of nutrients per serving and thus pose a potential risk of toxicity. Nutrient supplements are labeled with supplement facts that express the amount of nutrient in absolute units or as the percentage of the DV provided per recommended serving size. Total nutrient consumption, including that in foods, supplements, and over-the-counter medications (e.g., antacids), should not exceed RDA levels.
Acceptable Macronutrient Distribution Ranges The AMDRs are not experimentally determined but are rough ranges for energy-providing macronutrient intakes (protein, carbohydrate, and fat) that the Institute of Medicine’s Food and Nutrition Board considers to be healthful. These ranges are 10–35% of calories for protein, 20–35% of calories for fat, and 45–65% of calories for carbohydrate. Alcohol, which also provides energy, is not a nutrient; therefore, no recommendations are not provided.
FACTORS ALTERING NUTRIENT NEEDS
The DRIs are affected by age, sex, rate of growth, pregnancy, lactation, physical activity level, concomitant diseases, drugs, and dietary composition. If requirements for nutrient sufficiency are close to levels indicating excess of a nutrient, dietary planning is difficult.
Physiologic Factors Growth, strenuous physical activity, pregnancy, and lactation all increase needs for energy and several essential nutrients. Energy needs rise during pregnancy due to the demands of fetal growth and during lactation because of the increased energy required for milk production. Energy needs decrease with loss of lean body mass, the major determinant of REE. Because lean tissue, physical activity, and health often decline with age, energy needs of older persons, especially those over 70, tend to be lower than those of younger persons.
Dietary Composition Dietary composition affects the biologic availability and use of nutrients. For example, the absorption of iron may be impaired by large amounts of calcium or lead; likewise, non-heme iron uptake may be impaired by a lack of ascorbic acid and amino acids in the meal. Protein use by the body may be decreased when essential amino acids are not present in sufficient amounts—a rare scenario in U.S. diets. Animal foods, such as milk, eggs, and meat, have high biologic values, with most of the needed amino acids present in adequate amounts. Plant proteins in corn (maize), soy, rice, and wheat have lower biologic values and must be combined with other plant or animal proteins or fortified with the amino acids that are deficient to achieve optimal use by the body.
Route of Intake The RDAs apply only to oral intakes. When nutrients are administered parenterally, similar values can sometimes be used for amino acids, glucose (carbohydrate), fats, sodium, chloride, potassium, and most vitamins because their intestinal absorption rate is nearly 100%. However, the oral bioavailability of most mineral elements may be only half that obtained by parenteral administration. For some nutrients that are not readily stored in the body or that cannot be stored in large amounts, timing of administration may also be important. For example, amino acids cannot be used for protein synthesis if they are not supplied together; instead, they will be used for energy production, although in healthy individuals eating adequate diets, the distribution of protein intake over the course of the day has little effect on health.
Disease Dietary deficiency diseases include protein-calorie malnutrition, iron-deficiency anemia, goiter (due to iodine deficiency), rickets and osteomalacia (vitamin D deficiency), and xeropthalmia (vitamin A deficiency), megaloblastic anemia (vitamin B12 or folic acid deficiency), scurvy (vitamin C/ascorbic acid deficiency), beriberi (thiamin deficiency), and pellagra (niacin and tryptophan deficiency) (Chaps. 96e and 97). Each deficiency disease is characterized by imbalances at the cellular level between the supply of nutrients or energy and the body’s nutritional needs for growth, maintenance, and other functions. Imbalances and excesses in nutrient intakes are recognized as risk factors for certain chronic degenerative diseases, such as saturated fat and cholesterol in coronary artery disease; sodium in hypertension; obesity in hormone-dependent endometrial and breast cancers; and ethanol in alcoholism. Because the etiology and pathogenesis of these disorders are multifactorial, diet is only one of many risk factors. Osteoporosis, for example, is associated with calcium deficiency, sometimes secondary to vitamin D deficiency, as well as with risk factors related to environment (e.g., smoking, sedentary lifestyle), physiology (e.g., estrogen deficiency), genetic determinants (e.g., defects in collagen metabolism), and drug use (chronic steroid and aromatase inhibitors) (Chap. 425).
DIETARY ASSESSMENT
In clinical situations, nutritional assessment is an iterative process that involves: (1) screening for malnutrition, (2) assessing the diet and other data to establish either the absence or the presence of malnutrition and its possible causes, (3) planning and implementing the most appropriate nutritional therapy, and (4) reassessing intakes to make sure that they have been consumed. Some disease states affect the bioavailability, requirements, use, or excretion of specific nutrients. In these circumstances, specific measurements of various nutrients or their biomarkers may be required to ensure adequate replacement (Chap. 96e).
Most health care facilities have nutrition-screening processes in place for identifying possible malnutrition after hospital admission. Nutritional screening is required by the Joint Commission, which accredits and certifies health care organizations in the United States. However, there are no universally recognized or validated standards. The factors that are usually assessed include abnormal weight for height or body mass index (e.g., BMI <19 or >25); reported weight change (involuntary loss or gain of >5 kg in the past 6 months) (Chap. 56); diagnoses with known nutritional implications (e.g., metabolic disease, any disease affecting the gastrointestinal tract, alcoholism); present therapeutic dietary prescription; chronic poor appetite; presence of chewing and swallowing problems or major food intolerances; need for assistance with preparing or shopping for food, eating, or other aspects of self-care; and social isolation. The nutritional status of hospitalized patients should be reassessed periodically—at least once every week.
A more complete dietary assessment is indicated for patients who exhibit a high risk of or frank malnutrition on nutritional screening. The type of assessment varies with the clinical setting, the severity of the patient’s illness, and the stability of the patient’s condition.
Acute-Care Settings In acute-care settings, anorexia, various other diseases, test procedures, and medications can compromise dietary intake. Under such circumstances, the goal is to identify and avoid inadequate intake and to assure appropriate alimentation. Dietary assessment focuses on what patients are currently eating, whether or not they are able and willing to eat, and whether or not they experience any problems with eating. Dietary intake assessment is based on information from observed intakes; medical records; history; clinical examination; and anthropometric, biochemical, and functional status evaluations. The objective is to gather enough information to establish the likelihood of malnutrition due to poor dietary intake or other causes in order to assess whether nutritional therapy is indicated (Chap. 98e).
Simple observations may suffice to suggest inadequate oral intake. These include dietitians’ and nurses’ notes; observation of a patient’s frequent refusal to eat or the amount of food eaten on trays; the frequent performance of tests and procedures that are likely to cause meals to be skipped; adherence to nutritionally inadequate diet orders (e.g., clear liquids or full liquids) for more than a few days; the occurrence of fever, gastrointestinal distress, vomiting, diarrhea, or a comatose state; and the presence of diseases or use of treatments that involve any part of the alimentary tract. Acutely ill patients with diet-related diseases such as diabetes need assessment because an inappropriate diet may exacerbate these conditions and adversely affect other therapies. Abnormal biochemical values (serum albumin levels <35 g/L [<3.5 mg/dL]; serum cholesterol levels <3.9 mmol/L [<150 mg/dL]) are nonspecific but may indicate a need for further nutritional assessment.
Most therapeutic diets offered in hospitals are calculated to meet individual nutrient requirements and the RDA if they are eaten. Exceptions include clear liquids, some full-liquid diets, and test diets (such as those adhered to in preparation for gastrointestinal procedures), which are inadequate for several nutrients and should not be used, if possible, for more than 24 h. However, because as much as half of the food served to hospitalized patients is not eaten, it cannot be assumed that the intakes of hospitalized patients are adequate. Dietary assessment should compare how much and what kinds of food the patient has consumed with the diet that has been provided. Major deviations in intakes of energy, protein, fluids, or other nutrients of special concern for the patient’s illness should be noted and corrected.
Nutritional monitoring is especially important for patients who are very ill and who have extended lengths of hospital stay. Patients who are fed by enteral and parenteral routes also require special nutritional assessment and monitoring by physicians and/or dietitians with certification in nutritional support (Chap. 98e).
Ambulatory Settings The aim of dietary assessment in the outpatient setting is to determine whether or not the patient’s usual diet is a health risk in itself or if it contributes to existing chronic disease-related problems. Dietary assessment also provides the basis for planning a diet that fulfills therapeutic goals while ensuring patient adherence. The outpatient dietary assessment should review the adequacy of present and usual food intakes, including vitamin and mineral supplements, oral nutritional supplements, medical foods, other dietary supplements, medications, and alcohol, because all of these may affect the patient’s nutritional status. The assessment should focus on the dietary constituents that are most likely to be involved or compromised by a specific diagnosis as well as on any comorbidities that are present. More than one day’s intake should be reviewed to provide a better representation of the usual diet.
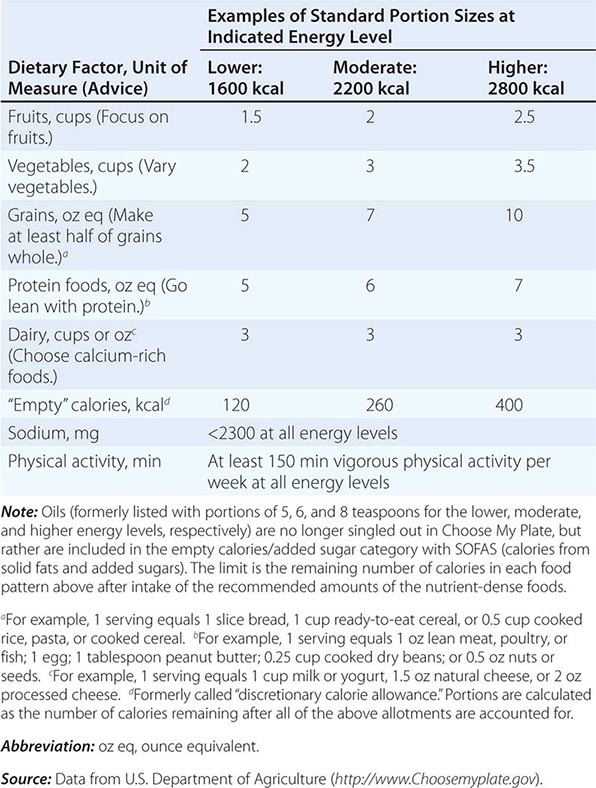
There are many ways to assess the adequacy of a patient’s habitual diet. These include use of a food guide, a food-exchange list, a diet history, or a food-frequency questionnaire. A commonly used food guide for healthy persons is the USDA’s Choose My Plate, which is useful as a rough guide for avoiding inadequate intakes of essential nutrients as well as likely excesses in the amounts of fat (especially saturated and trans fats), sodium, sugar, and alcohol consumed (Table 95e-4). The Choose My Plate graphic emphasizes a balance between calories and nutritional needs, encouraging increased intake of fruits and vegetables, whole grains, and low-fat milk in conjunction with reduced intake of sodium and high-calorie sugary drinks. The Web version of the guide provides a calculator that tailors the number of servings suggested for healthy patients of different weights, sexes, ages, and life-cycle stages to help them to meet their needs while avoiding excess (http://www.supertracker.usda.gov/default.aspx and www.ChooseMyPlate.gov). Patients who follow ethnic or unusual dietary patterns may need extra instruction on how foods should be categorized and on the appropriate portion sizes that constitute a serving. The process of reviewing the guide with patients helps them transition to healthier dietary patterns and identifies food groups eaten in excess of recommendations or in insufficient quantities. For persons on therapeutic diets, assessment against food-exchange lists may be useful. These include, for example, American Diabetes Association food-exchange lists for diabetes and the Academy of Nutrition and Dietetics food-exchange lists for renal disease.
CHOOSE MY PLATE: A GUIDE TO INDIVIDUALIZED DIETARY PLANNING |
NUTRITIONAL STATUS ASSESSMENT
Full nutritional status assessment is reserved for seriously ill patients and those at very high nutritional risk when the cause of malnutrition is still uncertain after the initial clinical evaluation and dietary assessment. It involves multiple dimensions, including documentation of dietary intake, anthropometric measurements, biochemical measurements of blood and urine, clinical examination, health history elicitation, and functional status evaluation. Therapeutic dietary prescriptions and menu plans for most diseases are available from most hospitals and from the Academy of Nutrition and Dietetics. For further discussion of nutritional assessment, see Chap. 97.
GLOBAL CONSIDERATIONS
![]() The DRIs (e.g., the EAR, the UL, and energy needs) are estimates of physiologic requirements based on experimental evidence. Assuming that appropriate adjustments are made for age, sex, body size, and physical activity level, these estimates should be applicable to individuals in most parts of the world. However, the AIs are based on customary and adequate intakes in U.S. and Canadian populations, which appear to be compatible with good health, rather than on a large body of direct experimental evidence. Similarly, the AMDRs represent expert opinion regarding the approximate intakes of energy-providing nutrients that are healthful in these North American populations. Thus these measures should be used with caution in other settings. Nutrient-based standards like the DRIs have also been developed by the World Health Organization/Food and Agricultural Organization of the United Nations and are available on the Web (http://www.who.int/nutrition/topics/nutrecomm/en/index.html). The European Food Safety Authority (EFSA) Panel on Dietetic Products, Nutrition and Allergies periodically publishes its recommendations in the EFSA Journal. Other countries have promulgated similar recommendations. The different standards have many similarities in their basic concepts, definitions, and nutrient recommendation levels, but there are some differences from the DRIs as a result of the functional criteria chosen, environmental differences, the timeliness of the evidence reviewed, and expert judgment.
The DRIs (e.g., the EAR, the UL, and energy needs) are estimates of physiologic requirements based on experimental evidence. Assuming that appropriate adjustments are made for age, sex, body size, and physical activity level, these estimates should be applicable to individuals in most parts of the world. However, the AIs are based on customary and adequate intakes in U.S. and Canadian populations, which appear to be compatible with good health, rather than on a large body of direct experimental evidence. Similarly, the AMDRs represent expert opinion regarding the approximate intakes of energy-providing nutrients that are healthful in these North American populations. Thus these measures should be used with caution in other settings. Nutrient-based standards like the DRIs have also been developed by the World Health Organization/Food and Agricultural Organization of the United Nations and are available on the Web (http://www.who.int/nutrition/topics/nutrecomm/en/index.html). The European Food Safety Authority (EFSA) Panel on Dietetic Products, Nutrition and Allergies periodically publishes its recommendations in the EFSA Journal. Other countries have promulgated similar recommendations. The different standards have many similarities in their basic concepts, definitions, and nutrient recommendation levels, but there are some differences from the DRIs as a result of the functional criteria chosen, environmental differences, the timeliness of the evidence reviewed, and expert judgment.
96e | Vitamin and Trace Mineral Deficiency and Excess |
Vitamins are required constituents of the human diet since they are synthesized inadequately or not at all in the human body. Only small amounts of these substances are needed to carry out essential biochemical reactions (e.g., by acting as coenzymes or prosthetic groups). Overt vitamin or trace mineral deficiencies are rare in Western countries because of a plentiful, varied, and inexpensive food supply; food fortification; and use of supplements. However, multiple nutrient deficiencies may appear together in persons who are chronically ill or alcoholic. After gastric bypass surgery, patients are at high risk for multiple nutrient deficiencies. Moreover, subclinical vitamin and trace mineral deficiencies, as diagnosed by laboratory testing, are quite common in the normal population, especially in the geriatric age group. Conversely, because of the widespread use of nutrient supplements, nutrient toxicities are gaining pathophysiologic and clinical importance.
![]() Victims of famine, emergency-affected and displaced populations, and refugees are at increased risk for protein-energy malnutrition and classic micronutrient deficiencies (vitamin A, iron, iodine) as well as for overt deficiencies in thiamine (beriberi), riboflavin, vitamin C (scurvy), and niacin (pellagra).
Victims of famine, emergency-affected and displaced populations, and refugees are at increased risk for protein-energy malnutrition and classic micronutrient deficiencies (vitamin A, iron, iodine) as well as for overt deficiencies in thiamine (beriberi), riboflavin, vitamin C (scurvy), and niacin (pellagra).
Body stores of vitamins and minerals vary tremendously. For example, stores of vitamin B12 and vitamin A are large, and an adult may not become deficient until ≥1 year after beginning to eat a deficient diet. However, folate and thiamine may become depleted within weeks among those eating a deficient diet. Therapeutic modalities can deplete essential nutrients from the body; for example, hemodialysis removes water-soluble vitamins, which must be replaced by supplementation.
Vitamins and trace minerals play several roles in diseases: (1) Deficiencies of vitamins and minerals may be caused by disease states such as malabsorption. (2) Either deficiency or excess of vitamins and minerals can cause disease in and of itself (e.g., vitamin A intoxication and liver disease). (3) Vitamins and minerals in high doses may be used as drugs (e.g., niacin for hypercholesterolemia). Since they are covered elsewhere, the hematologic-related vitamins and minerals (Chaps. 126 and 128) either are not considered or are considered only briefly in this chapter, as are the bone-related vitamins and minerals (vitamin D, calcium, phosphorus, magnesium; Chap. 423).
VITAMINS
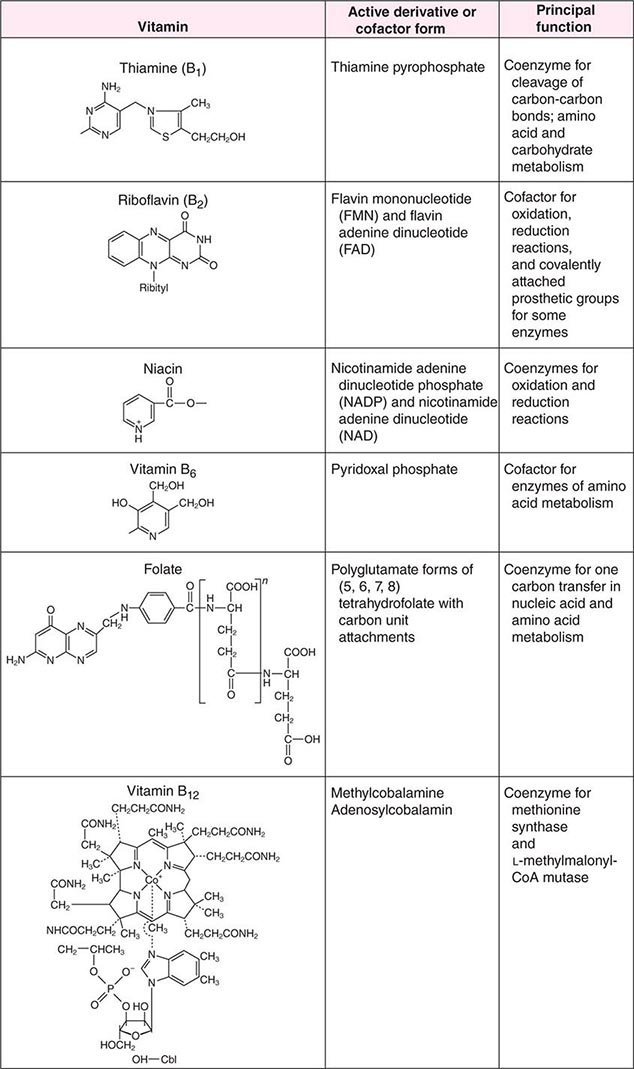
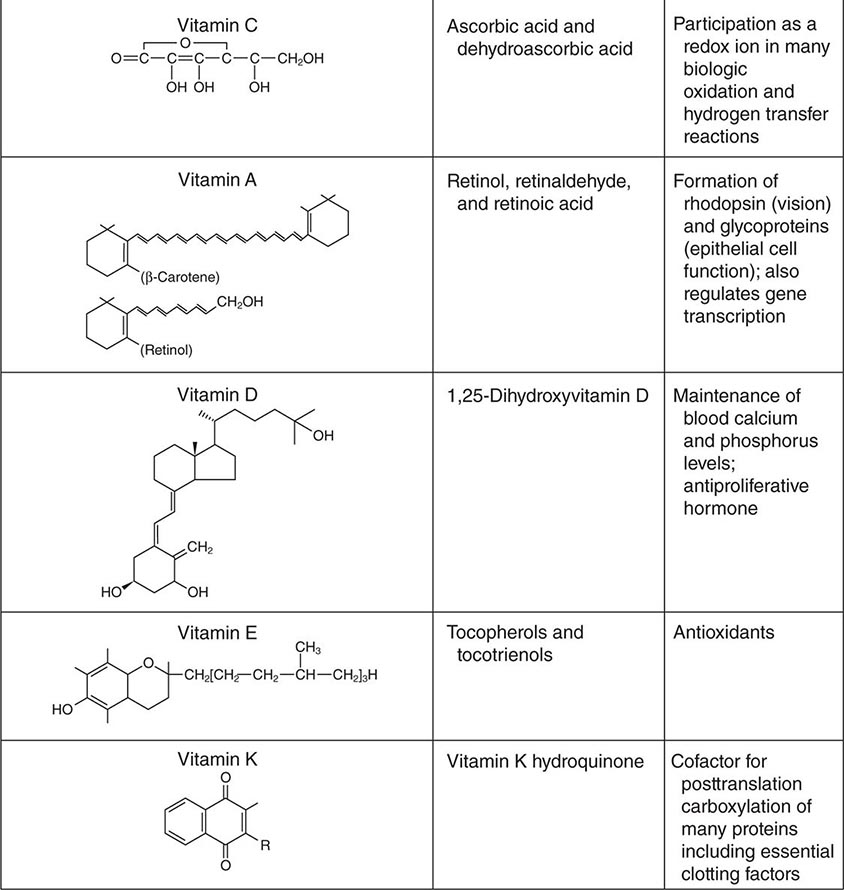
See also Table 96e-1 and Fig. 96e-1.
PRINCIPAL CLINICAL FINDINGS OF VITAMIN MALNUTRITION |
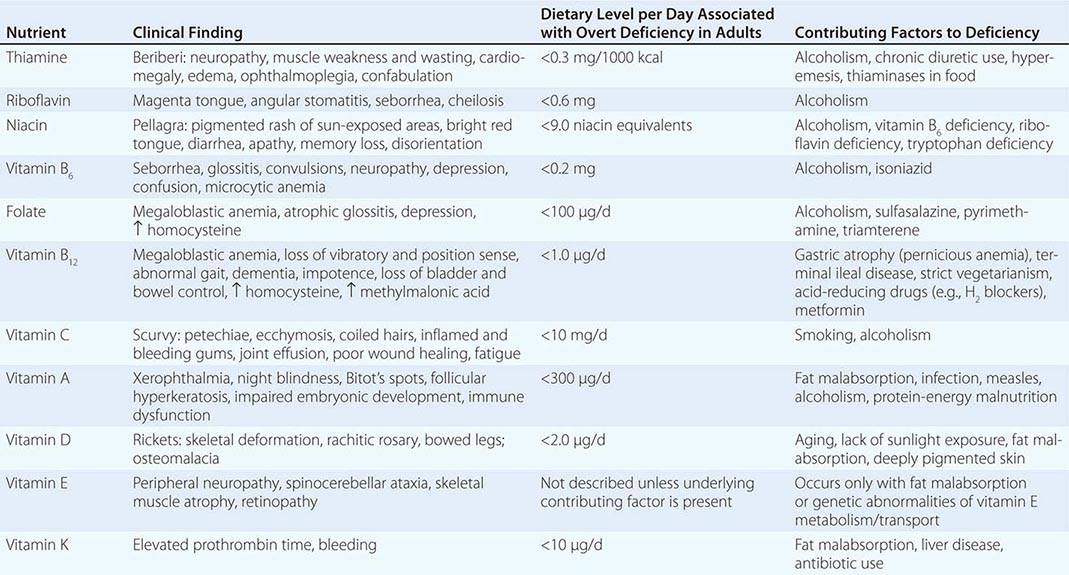
FIGURE 96e-1 Structures and principal functions of vitamins associated with human disorders.
THIAMINE (VITAMIN B1)
Thiamine was the first B vitamin to be identified and therefore is referred to as vitamin B1. Thiamine functions in the decarboxylation of α-ketoacids (e.g., pyruvate α-ketoglutarate) and branched-chain amino acids and thus is essential for energy generation. In addition, thiamine pyrophosphate acts as a coenzyme for a transketolase reaction that mediates the conversion of hexose and pentose phosphates. It has been postulated that thiamine plays a role in peripheral nerve conduction, although the exact chemical reactions underlying this function are not known.
Food Sources The median intake of thiamine in the United States from food alone is 2 mg/d. Primary food sources for thiamine include yeast, organ meat, pork, legumes, beef, whole grains, and nuts. Milled rice and grains contain little thiamine. Thiamine deficiency is therefore more common in cultures that rely heavily on a rice-based diet. Tea, coffee (regular and decaffeinated), raw fish, and shellfish contain thiaminases, which can destroy the vitamin. Thus, drinking large amounts of tea or coffee can theoretically lower thiamine body stores.
Deficiency Most dietary deficiency of thiamine worldwide is the result of poor dietary intake. In Western countries, the primary causes of thiamine deficiency are alcoholism and chronic illnesses such as cancer. Alcohol interferes directly with the absorption of thiamine and with the synthesis of thiamine pyrophosphate, and it increases urinary excretion. Thiamine should always be replenished when a patient with alcoholism is being refed, as carbohydrate repletion without adequate thiamine can precipitate acute thiamine deficiency with lactic acidosis. Other at-risk populations are women with prolonged hyperemesis gravidarum and anorexia, patients with overall poor nutritional status who are receiving parenteral glucose, patients who have had bariatric bypass surgery (bariatric Wernicke), and patients receiving chronic diuretic therapy (e.g., in hypertension or heart failure) due to increased urinary thiamine losses. Maternal thiamine deficiency can lead to infantile beriberi in breast-fed children. Thiamine deficiency could be an underlying factor in motor vehicle accidents and could be overlooked in the setting of head injury.
Thiamine deficiency in its early stage induces anorexia and nonspecific symptoms (e.g., irritability, decrease in short-term memory). Prolonged thiamine deficiency causes beriberi, which is classically categorized as wet or dry although there is considerable overlap between the two categories. In either form of beriberi, patients may complain of pain and paresthesia. Wet beriberi presents primarily with cardiovascular symptoms that are due to impaired myocardial energy metabolism and dysautonomia; it can occur after 3 months of a thiamine-deficient diet. Patients present with an enlarged heart, tachycardia, high-output congestive heart failure, peripheral edema, and peripheral neuritis. Patients with dry beriberi present with a symmetric peripheral neuropathy of the motor and sensory systems, with diminished reflexes. The neuropathy affects the legs most markedly, and patients have difficulty rising from a squatting position.
Alcoholic patients with chronic thiamine deficiency also may have central nervous system (CNS) manifestations known as Wernicke’s encephalopathy, which consists of horizontal nystagmus, ophthalmoplegia (due to weakness of one or more extraocular muscles), cerebellar ataxia, and mental impairment (Chap. 467). When there is an additional loss of memory and a confabulatory psychosis, the syndrome is known as Wernicke-Korsakoff syndrome. Despite the typical clinical picture and history, Wernicke-Korsakoff syndrome is underdiagnosed.
The laboratory diagnosis of thiamine deficiency usually is made by a functional enzymatic assay of transketolase activity measured before and after the addition of thiamine pyrophosphate. A >25% stimulation in response to the addition of thiamine pyrophosphate (i.e., an activity coefficient of 1.25) is interpreted as abnormal. Thiamine or the phosphorylated esters of thiamine in serum or blood also can be measured by high-performance liquid chromatography to detect deficiency.
Toxicity Although anaphylaxis has been reported after high intravenous doses of thiamine, no adverse effects have been recorded from either food or supplements at high doses. Thiamine supplements may be bought over the counter in doses of up to 50 mg/d.
RIBOFLAVIN (VITAMIN B2)
Riboflavin is important for the metabolism of fat, carbohydrate, and protein, acting as a respiratory coenzyme and an electron donor. Enzymes that contain flavin adenine dinucleotide (FAD) or flavin mononucleotide (FMN) as prosthetic groups are known as flavoenzymes (e.g., succinic acid dehydrogenase, monoamine oxidase, glutathione reductase). FAD is a cofactor for methyltetrahydrofolate reductase and therefore modulates homocysteine metabolism. The vitamin also plays a role in drug and steroid metabolism, including detoxification reactions.
Although much is known about the chemical and enzymatic reactions of riboflavin, the clinical manifestations of riboflavin deficiency are nonspecific and are similar to those of other deficiencies of B vitamins. Riboflavin deficiency is manifested principally by lesions of the mucocutaneous surfaces of the mouth and skin. In addition, corneal vascularization, anemia, and personality changes have been described with riboflavin deficiency.
Deficiency and Excess Riboflavin deficiency almost always is due to dietary deficiency. Milk, other dairy products, and enriched breads and cereals are the most important dietary sources of riboflavin in the United States, although lean meat, fish, eggs, broccoli, and legumes are also good sources. Riboflavin is extremely sensitive to light, and milk should be stored in containers that protect against photodegradation. Laboratory diagnosis of riboflavin deficiency can be made by determination of red blood cell or urinary riboflavin concentrations or by measurement of erythrocyte glutathione reductase activity, with and without added FAD. Because the capacity of the gastrointestinal tract to absorb riboflavin is limited (~20 mg after one oral dose), riboflavin toxicity has not been described.
NIACIN (VITAMIN B3)
The term niacin refers to nicotinic acid and nicotinamide and their biologically active derivatives. Nicotinic acid and nicotinamide serve as precursors of two coenzymes, nicotinamide adenine dinucleotide (NAD) and NAD phosphate (NADP), which are important in numerous oxidation and reduction reactions in the body. In addition, NAD and NADP are active in adenine diphosphate–ribose transfer reactions involved in DNA repair and calcium mobilization.
Metabolism and Requirements Nicotinic acid and nicotinamide are absorbed well from the stomach and small intestine. The bioavailability of niacin from beans, milk, meat, and eggs is high; bioavailability from cereal grains is lower. Since flour is enriched with “free” niacin (i.e., the non-coenzyme form), bioavailability is excellent. Median intakes of niacin in the United States considerably exceed the recommended dietary allowance (RDA).
The amino acid tryptophan can be converted to niacin with an efficiency of 60:1 by weight. Thus, the RDA for niacin is expressed in niacin equivalents. A lower-level conversion of tryptophan to niacin occurs in vitamin B6 and/or riboflavin deficiencies and in the presence of isoniazid. The urinary excretion products of niacin include 2-pyridone and 2-methyl nicotinamide, measurements of which are used in the diagnosis of niacin deficiency.
![]() Deficiency Niacin deficiency causes pellagra, which is found mostly among people eating corn-based diets in parts of China, Africa, and India. Pellagra in North America is found mainly among alcoholics; among patients with congenital defects of intestinal and kidney absorption of tryptophan (Hartnup disease; Chap. 434e); and among patients with carcinoid syndrome (Chap. 113), in which there is increased conversion of tryptophan to serotonin. The antituberculosis drug isoniazid is a structural analog of niacin and can precipitate pellagra. In the setting of famine or population displacement, pellagra results from the absolute lack of niacin but also from the deficiency of micronutrients required for the conversion of tryptophan to niacin (e.g., iron, riboflavin, and pyridoxine). The early symptoms of pellagra include loss of appetite, generalized weakness and irritability, abdominal pain, and vomiting. Bright red glossitis then ensues and is followed by a characteristic skin rash that is pigmented and scaling, particularly in skin areas exposed to sunlight. This rash is known as Casal’s necklace because it forms a ring around the neck; it is seen in advanced cases. Vaginitis and esophagitis also may occur. Diarrhea (due in part to proctitis and in part to malabsorption), depression, seizures, and dementia are also part of the pellagra syndrome. The primary manifestations of this syndrome are sometimes referred to as “the four D’s”: dermatitis, diarrhea, and dementia leading to death.
Deficiency Niacin deficiency causes pellagra, which is found mostly among people eating corn-based diets in parts of China, Africa, and India. Pellagra in North America is found mainly among alcoholics; among patients with congenital defects of intestinal and kidney absorption of tryptophan (Hartnup disease; Chap. 434e); and among patients with carcinoid syndrome (Chap. 113), in which there is increased conversion of tryptophan to serotonin. The antituberculosis drug isoniazid is a structural analog of niacin and can precipitate pellagra. In the setting of famine or population displacement, pellagra results from the absolute lack of niacin but also from the deficiency of micronutrients required for the conversion of tryptophan to niacin (e.g., iron, riboflavin, and pyridoxine). The early symptoms of pellagra include loss of appetite, generalized weakness and irritability, abdominal pain, and vomiting. Bright red glossitis then ensues and is followed by a characteristic skin rash that is pigmented and scaling, particularly in skin areas exposed to sunlight. This rash is known as Casal’s necklace because it forms a ring around the neck; it is seen in advanced cases. Vaginitis and esophagitis also may occur. Diarrhea (due in part to proctitis and in part to malabsorption), depression, seizures, and dementia are also part of the pellagra syndrome. The primary manifestations of this syndrome are sometimes referred to as “the four D’s”: dermatitis, diarrhea, and dementia leading to death.
Toxicity Prostaglandin-mediated flushing due to binding of the vitamin to a G protein–coupled receptor has been observed at daily nicotinic acid doses as low as 30 mg taken as a supplement or as therapy for dyslipidemia. There is no evidence of toxicity from niacin that is derived from food sources. Flushing always starts in the face and may be accompanied by skin dryness, itching, paresthesia, and headache. Pharmaceutical preparations of nicotinic acid combined with laropiprant, a selective prostaglandin D2 receptor 1 antagonist, or premedication with aspirin may alleviate these symptoms. Flushing is subject to tachyphylaxis and often improves with time. Nausea, vomiting, and abdominal pain also occur at similar doses of niacin. Hepatic toxicity is the most serious toxic reaction caused by sustained-release niacin and may present as jaundice with elevated aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels. A few cases of fulminant hepatitis requiring liver transplantation have been reported at doses of 3–9 g/d. Other toxic reactions include glucose intolerance, hyperuricemia, macular edema, and macular cysts. The combination of nicotinic acid preparations for dyslipidemia with 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors may increase the risk of rhabdomyolysis. The upper limit for daily niacin intake has been set at 35 mg. However, this upper limit does not pertain to the therapeutic use of niacin.
PYRIDOXINE (VITAMIN B6)
Vitamin B6 refers to a family of compounds that includes pyridoxine, pyridoxal, pyridoxamine, and their 5 ′-phosphate derivatives. 5 ′-Pyridoxal phosphate (PLP) is a cofactor for more than 100 enzymes involved in amino acid metabolism. Vitamin B6 also is involved in heme and neurotransmitter synthesis and in the metabolism of glycogen, lipids, steroids, sphingoid bases, and several vitamins, including the conversion of tryptophan to niacin.
Dietary Sources Plants contain vitamin B6 in the form of pyridoxine, whereas animal tissues contain PLP and pyridoxamine phosphate. The vitamin B6 contained in plants is less bioavailable than that in animal tissues. Rich food sources of vitamin B6 include legumes, nuts, wheat bran, and meat, although it is present in all food groups.
Deficiency Symptoms of vitamin B6 deficiency include epithelial changes, as seen frequently with other B vitamin deficiencies. In addition, severe vitamin B6 deficiency can lead to peripheral neuropathy, abnormal electroencephalograms, and personality changes that include depression and confusion. In infants, diarrhea, seizures, and anemia have been reported. Microcytic hypochromic anemia is due to diminished hemoglobin synthesis, since the first enzyme involved in heme biosynthesis (aminolevulinate synthase) requires PLP as a cofactor (Chap. 126). In some case reports, platelet dysfunction has been reported. Since vitamin B6 is necessary for the conversion of homocysteine to cystathionine, it is possible that chronic low-grade vitamin B6 deficiency may result in hyperhomocysteinemia and increased risk of cardiovascular disease (Chaps. 291e and 434e). Independent of homocysteine, low levels of circulating vitamin B6 have been associated with inflammation and elevated levels of C-reactive protein.
Certain medications, such as isoniazid, L-dopa, penicillamine, and cycloserine, interact with PLP due to a reaction with carbonyl groups. Pyridoxine should be given concurrently with isoniazid to avoid neuropathy. The increased ratio of AST to ALT seen in alcoholic liver disease reflects the relative vitamin B6 dependence of ALT. Vitamin B6 dependency syndromes that require pharmacologic doses of vitamin B6 are rare; they include cystathionine β-synthase deficiency, pyridoxine-responsive (primarily sideroblastic) anemias, and gyrate atrophy with chorioretinal degeneration due to decreased activity of the mitochondrial enzyme ornithine aminotransferase. In these situations, 100–200 mg/d of oral vitamin B6 is required for treatment.
High doses of vitamin B6 have been used to treat carpal tunnel syndrome, premenstrual syndrome, schizophrenia, autism, and diabetic neuropathy but have not been found to be effective.
The laboratory diagnosis of vitamin B6 deficiency is generally based on low plasma PLP values (<20 nmol/L). Vitamin B6 deficiency is treated with 50 mg/d; higher doses of 100–200 mg/d are given if the deficiency is related to medication use. Vitamin B6 should not be given with L-dopa, since the vitamin interferes with the action of this drug.
Toxicity The safe upper limit for vitamin B6 has been set at 100 mg/d, although no adverse effects have been associated with high intakes of vitamin B6 from food sources only. When toxicity occurs, it causes severe sensory neuropathy, leaving patients unable to walk. Some cases of photosensitivity and dermatitis have been reported.
FOLATE (VITAMIN B12)
See Chap. 128.
VITAMIN C
Both ascorbic acid and its oxidized product dehydroascorbic acid are biologically active. Actions of vitamin C include antioxidant activity, promotion of nonheme iron absorption, carnitine biosynthesis, conversion of dopamine to norepinephrine, and synthesis of many peptide hormones. Vitamin C is also important for connective tissue metabolism and cross-linking (proline hydroxylation), and it is a component of many drug-metabolizing enzyme systems, particularly the mixed-function oxidase systems.
Absorption and Dietary Sources Vitamin C is almost completely absorbed if <100 mg is administered in a single dose; however, only 50% or less is absorbed at doses >1 g. Enhanced degradation and fecal and urinary excretion of vitamin C occur at higher intake levels.
Good dietary sources of vitamin C include citrus fruits, green vegetables (especially broccoli), tomatoes, and potatoes. Consumption of five servings of fruits and vegetables a day provides vitamin C in excess of the RDA of 90 mg/d for men and 75 mg/d for women. In addition, ~40% of the U.S. population consumes vitamin C as a dietary supplement in which “natural forms” of the vitamin are no more bioavailable than synthetic forms. Smoking, hemodialysis, pregnancy, and stress (e.g., infection, trauma) appear to increase vitamin C requirements.
Deficiency Vitamin C deficiency causes scurvy. In the United States, this condition is seen primarily among the poor and the elderly, in alcoholics who consume <10 mg/d of vitamin C, and in individuals consuming macrobiotic diets. Vitamin C deficiency also can occur in young adults who eat severely unbalanced diets. In addition to generalized fatigue, symptoms of scurvy primarily reflect impaired formation of mature connective tissue and include bleeding into the skin (petechiae, ecchymoses, perifollicular hemorrhages); inflamed and bleeding gums; and manifestations of bleeding into joints, the peritoneal cavity, the pericardium, and the adrenal glands. In children, vitamin C deficiency may cause impaired bone growth. Laboratory diagnosis of vitamin C deficiency is based on low plasma or leukocyte levels.
Administration of vitamin C (200 mg/d) improves the symptoms of scurvy within several days. High-dose vitamin C supplementation (e.g., 1–2 g/d) may slightly decrease the symptoms and duration of upper respiratory tract infections. Vitamin C supplementation has also been reported to be useful in Chédiak-Higashi syndrome (Chap. 80) and osteogenesis imperfecta (Chap. 427). Diets high in vitamin C have been claimed to lower the incidence of certain cancers, particularly esophageal and gastric cancers. If proved, this effect may be due to the fact that vitamin C can prevent the conversion of nitrites and secondary amines to carcinogenic nitrosamines. However, an intervention study from China did not show vitamin C to be protective. A potential role for parenteral ascorbic acid in the treatment of advanced cancers has been suggested.
Toxicity Taking >2 g of vitamin C in a single dose may result in abdominal pain, diarrhea, and nausea. Since vitamin C may be metabolized to oxalate, it is feared that chronic high-dose vitamin C supplementation could result in an increased prevalence of kidney stones. However, except in patients with preexisting renal disease, this association has not been borne out in several trials. Nevertheless, it is reasonable to advise patients with a history of kidney stones not to take large doses of vitamin C. There is also an unproven but possible risk that chronic high doses of vitamin C could promote iron overload and iron toxicity. High doses of vitamin C can induce hemolysis in patients with glucose-6-phosphate dehydrogenase deficiency, and doses >1 g/d can cause false-negative guaiac reactions and interfere with tests for urinary glucose. High doses may interfere with the activity of certain drugs (e.g., bortezomib in myeloma patients).
BIOTIN
Biotin is a water-soluble vitamin that plays a role in gene expression, gluconeogenesis, and fatty acid synthesis and serves as a CO2 carrier on the surface of both cytosolic and mitochondrial carboxylase enzymes. The vitamin also functions in the catabolism of specific amino acids (e.g., leucine) and in gene regulation by histone biotinylation. Excellent food sources of biotin include organ meat such as liver or kidney, soy and other beans, yeast, and egg yolks; however, egg white contains the protein avidin, which strongly binds the vitamin and reduces its bioavailability.
Biotin deficiency due to low dietary intake is rare; rather, deficiency is due to inborn errors of metabolism. Biotin deficiency has been induced by experimental feeding of egg white diets and by biotin-free parenteral nutrition in patients with short bowels. In adults, biotin deficiency results in mental changes (depression, hallucinations), paresthesia, anorexia, and nausea. A scaling, seborrheic, and erythematous rash may occur around the eyes, nose, and mouth as well as on the extremities. In infants, biotin deficiency presents as hypotonia, lethargy, and apathy. In addition, infants may develop alopecia and a characteristic rash that includes the ears. The laboratory diagnosis of biotin deficiency can be established on the basis of a decreased concentration of urinary biotin (or its major metabolites), increased urinary excretion of 3-hydroxyisovaleric acid after a leucine challenge, or decreased activity of biotin-dependent enzymes in lymphocytes (e.g., propionyl-CoA carboxylase). Treatment requires pharmacologic doses of biotin–i.e., up to 10 mg/d. No toxicity is known.
PANTOTHENIC ACID (VITAMIN B5)
Pantothenic acid is a component of coenzyme A and phosphopantetheine, which are involved in fatty acid metabolism and the synthesis of cholesterol, steroid hormones, and all compounds formed from isoprenoid units. In addition, pantothenic acid is involved in the acetylation of proteins. The vitamin is excreted in the urine, and the laboratory diagnosis of deficiency is based on low urinary vitamin levels.
The vitamin is ubiquitous in the food supply. Liver, yeast, egg yolks, whole grains, and vegetables are particularly good sources. Human pantothenic acid deficiency has been demonstrated only by experimental feeding of diets low in pantothenic acid or by administration of a specific pantothenic acid antagonist. The symptoms of pantothenic acid deficiency are nonspecific and include gastrointestinal disturbance, depression, muscle cramps, paresthesia, ataxia, and hypoglycemia. Pantothenic acid deficiency is believed to have caused the “burning feet syndrome” seen in prisoners of war during World War II. No toxicity of this vitamin has been reported.
CHOLINE
Choline is a precursor for acetylcholine, phospholipids, and betaine. Choline is necessary for the structural integrity of cell membranes, cholinergic neurotransmission, lipid and cholesterol metabolism, methyl-group metabolism, and transmembrane signaling. Recently, a recommended adequate intake was set at 550 mg/d for men and 425 mg/d for women, although certain genetic polymorphisms can increase an individual’s requirement. Choline is thought to be a “conditionally essential” nutrient in that its de novo synthesis occurs in the liver and results in lesser-than-used amounts only under certain stress conditions (e.g., alcoholic liver disease). The dietary requirement for choline depends on the status of other nutrients involved in methyl-group metabolism (folate, vitamin B12, vitamin B6, and methionine) and thus varies widely. Choline is widely distributed in food (e.g., egg yolks, wheat germ, organ meat, milk) in the form of lecithin (phosphatidylcholine). Choline deficiency has occurred in patients receiving parenteral nutrition devoid of choline. Deficiency results in fatty liver, elevated aminotransferase levels, and skeletal muscle damage with high creatine phosphokinase values. The diagnosis of choline deficiency is currently based on low plasma levels, although nonspecific conditions (e.g., heavy exercise) may also suppress plasma levels.
Toxicity from choline results in hypotension, cholinergic sweating, diarrhea, salivation, and a fishy body odor. The upper limit for choline intake has been set at 3.5 g/d. Because of its ability to lower cholesterol and homocysteine levels, choline treatment has been suggested for patients with dementia and patients at high risk of cardiovascular disease. However, the benefits of such treatment have not been firmly documented. Choline- and betaine-restricted diets are of therapeutic value in trimethylaminuria (“fish odor syndrome”).
FLAVONOIDS
Flavonoids constitute a large family of polyphenols that contribute to the aroma, taste, and color of fruits and vegetables. Major groups of dietary flavonoids include anthocyanidins in berries; catechins in green tea and chocolate; flavonols (e.g., quercitin) in broccoli, kale, leeks, onions, and the skins of grapes and apples; and isoflavones (e.g., genistein) in legumes. Isoflavones have a low bioavailability and are partially metabolized by the intestinal flora. The dietary intake of flavonoids is estimated at 10–100 mg/d; this figure is almost certainly an underestimate attributable to a lack of information on their concentrations in many foods. Several flavonoids have antioxidant activity and affect cell signaling. From observational epidemiologic studies and limited clinical (human and animal) studies, flavonoids have been postulated to play a role in the prevention of several chronic diseases, including neurodegenerative disease, diabetes, and osteoporosis. The ultimate importance and usefulness of these compounds against human disease have not been demonstrated.
VITAMIN A
Vitamin A, in the strictest sense, refers to retinol. However, the oxidized metabolites retinaldehyde and retinoic acid are also biologically active compounds. The term retinoids includes all molecules (including synthetic molecules) that are chemically related to retinol. Retinaldehyde (11-cis) is the essential form of vitamin A that is required for normal vision, whereas retinoic acid is necessary for normal morphogenesis, growth, and cell differentiation. Retinoic acid does not function in vision and, in contrast to retinol, is not involved in reproduction. Vitamin A also plays a role in iron utilization, humoral immunity, T cell–mediated immunity, natural killer cell activity, and phagocytosis. Vitamin A is commercially available in esterified forms (e.g., acetate, palmitate), which are more stable than other forms.
There are more than 600 carotenoids in nature, ~50 of which can be metabolized to vitamin A. β-Carotene is the most prevalent carotenoid with provitamin A activity in the food supply. In humans, significant fractions of carotenoids are absorbed intact and are stored in liver and fat. It is estimated that ≥12 μg (range, 4–27 μg) of dietary all-trans β-carotene is equivalent to 1 μg of retinol activity, whereas the figure is ≥24 μg for other dietary provitamin A carotenoids (e.g., cryptoxanthin, α-carotene). The vitamin A equivalency for a β-carotene supplement in an oily solution is 2:1.
Metabolism The liver contains ~90% of the vitamin A reserves and secretes vitamin A in the form of retinol, which is bound to retinol-binding protein. Once binding has occurred, the retinol-binding protein complex interacts with a second protein, transthyretin. This trimolecular complex functions to prevent vitamin A from being filtered by the kidney glomerulus, thus protecting the body against the toxicity of retinol and allowing retinol to be taken up by specific cell-surface receptors that recognize retinol-binding protein. A certain amount of vitamin A enters peripheral cells even if it is not bound to retinol-binding protein. After retinol is internalized by the cell, it becomes bound to a series of cellular retinol-binding proteins, which function as sequestering and transporting agents as well as co-ligands for enzymatic reactions. Certain cells also contain retinoic acid–binding proteins, which have sequestering functions but also shuttle retinoic acid to the nucleus and enable its metabolism.
Retinoic acid is a ligand for certain nuclear receptors that act as transcription factors. Two families of receptors (retinoic acid receptors [RARs] and retinoid × receptors [RXRs]) are active in retinoid-mediated gene transcription. Retinoid receptors regulate transcription by binding as dimeric complexes to specific DNA sites—the retinoic acid response elements—in target genes (Chap. 400e). The receptors can either stimulate or repress gene expression in response to their ligands. RARs bind all-trans retinoic acid and 9-cis-retinoic acid, whereas RXRs bind only 9-cis-retinoic acid.
The retinoid receptors play an important role in controlling cell proliferation and differentiation. Retinoic acid is useful in the treatment of promyelocytic leukemia (Chap. 132) and also is used in the treatment of cystic acne because it inhibits keratinization, decreases sebum secretion, and possibly alters the inflammatory reaction (Chap. 71). RXRs dimerize with other nuclear receptors to function as coregulators of genes responsive to retinoids, thyroid hormone, and calcitriol. RXR agonists induce insulin sensitivity experimentally, perhaps because RXRs are cofactors for the peroxisome proliferator-activated receptors, which are targets for thiazolidinedione drugs such as rosiglitazone and troglitazone (Chap. 418).
Dietary Sources The retinol activity equivalent (RAE) is used to express the vitamin A value of food: 1 RAE is defined as 1 μg of retinol (0.003491 mmol), 12 μg of β-carotene, and 24 μg of other provitamin A carotenoids. In older literature, vitamin A often was expressed in international units (IU), with 1 μg of retinol equal to 3.33 IU of retinol and 20 IU of β-carotene, but these units are no longer in scientific use.
Liver, fish, and eggs are excellent food sources for preformed vitamin A; vegetable sources of provitamin A carotenoids include dark green and deeply colored fruits and vegetables. Moderate cooking of vegetables enhances carotenoid release for uptake in the gut. Carotenoid absorption is also aided by some fat in a meal. Infants are particularly susceptible to vitamin A deficiency because neither breast nor cow’s milk supplies enough vitamin A to prevent deficiency. In developing countries, chronic dietary deficiency is the main cause of vitamin A deficiency and is exacerbated by infection. In early childhood, low vitamin A status results from inadequate intakes of animal food sources and edible oils, both of which are expensive, coupled with seasonal unavailability of vegetables and fruits and lack of marketed fortified food products. Concurrent zinc deficiency can interfere with the mobilization of vitamin A from liver stores. Alcohol interferes with the conversion of retinol to retinaldehyde in the eye by competing for alcohol (retinol) dehydrogenase. Drugs that interfere with the absorption of vitamin A include mineral oil, neomycin, and cholestyramine.
![]() Deficiency Vitamin A deficiency is endemic in areas where diets are chronically poor, especially in southern Asia, sub-Saharan Africa, some parts of Latin America, and the western Pacific, including parts of China. Vitamin A status is usually assessed by measuring serum retinol (normal range, 1.05–3.50 μmol/L [30–100 μg/dL]) or blood-spot retinol or by tests of dark adaptation. Stable isotopic or invasive liver biopsy methods are available to estimate total body stores of vitamin A. As judged by deficient serum retinol (<0.70 μmol/L [20 μg/dL]), vitamin A deficiency worldwide is present in >90 million preschool-age children, among whom >4 million have an ocular manifestation of deficiency termed xerophthalmia. This condition includes milder stages of night blindness and conjunctival xerosis (dryness) with Bitot’s spots (white patches of keratinized epithelium appearing on the sclera) as well as rare, potentially blinding corneal ulceration and necrosis. Keratomalacia (softening of the cornea) leads to corneal scarring that blinds at least a quarter of a million children each year and is associated with fatality rates of 4–25%. However, vitamin A deficiency at any stage poses an increased risk of death from diarrhea, dysentery, measles, malaria, or respiratory disease. Vitamin A deficiency can compromise barrier, innate, and acquired immune defenses to infection. In areas where deficiency is widely prevalent, vitamin A supplementation can markedly reduce the risk of childhood mortality (by 23–34%, on average). About 10% of pregnant women in undernourished settings also develop night blindness (assessed by history) during the latter half of pregnancy, and this moderate vitamin A deficiency is associated with an increased risk of maternal infection and death.
Deficiency Vitamin A deficiency is endemic in areas where diets are chronically poor, especially in southern Asia, sub-Saharan Africa, some parts of Latin America, and the western Pacific, including parts of China. Vitamin A status is usually assessed by measuring serum retinol (normal range, 1.05–3.50 μmol/L [30–100 μg/dL]) or blood-spot retinol or by tests of dark adaptation. Stable isotopic or invasive liver biopsy methods are available to estimate total body stores of vitamin A. As judged by deficient serum retinol (<0.70 μmol/L [20 μg/dL]), vitamin A deficiency worldwide is present in >90 million preschool-age children, among whom >4 million have an ocular manifestation of deficiency termed xerophthalmia. This condition includes milder stages of night blindness and conjunctival xerosis (dryness) with Bitot’s spots (white patches of keratinized epithelium appearing on the sclera) as well as rare, potentially blinding corneal ulceration and necrosis. Keratomalacia (softening of the cornea) leads to corneal scarring that blinds at least a quarter of a million children each year and is associated with fatality rates of 4–25%. However, vitamin A deficiency at any stage poses an increased risk of death from diarrhea, dysentery, measles, malaria, or respiratory disease. Vitamin A deficiency can compromise barrier, innate, and acquired immune defenses to infection. In areas where deficiency is widely prevalent, vitamin A supplementation can markedly reduce the risk of childhood mortality (by 23–34%, on average). About 10% of pregnant women in undernourished settings also develop night blindness (assessed by history) during the latter half of pregnancy, and this moderate vitamin A deficiency is associated with an increased risk of maternal infection and death.