CHAPTER 15
Phenytoin and Fosphenytoin
CATHERINE A. MILLARES-SIPIN, PharmD, CGP, BCPS, BCACP
ANTONIA ALAFRIS, BS, PharmD, CGP
HENRY COHEN, MS, PharmD, FCCM, BCPP, CGP
OVERVIEW OF PHENYTOIN
Phenytoin (5,5-diphenylimidazolidine-2,4-dione, referred to as diphenylhydantoin) is an anticonvulsant medication that was first discovered in 1908 by Heinrich Biltz at the University of Kiel in Germany. In the 1920s, Arthur Dox, a chemist in Parke-Davis, compounded diphenylhydantoin in search of a hypnotic agent. Because the drug was not a hypnotic, he shelved it as “inactive.” It was not until November 1936 that Tracy Putnam discovered the anticonvulsant activity of diphenylhydantoin, and in June 1938, Parke, Davis, and Company marketed sodium diphenylhydantoin (Dilantin®). In the 1970s, the generic name of Dilantin® was shortened to phenytoin.1 Today, it is FDA-approved for the management of generalized tonic clonic (grand mal) seizures, complex partial seizures, and for the prevention of seizures after head trauma and neurosurgery.2 Phenytoin also has multiple unlabeled indications including the management of trigeminal neuralgia, syndrome of inappropriate antidiuretic hormone (SIADH), torsade de pointes, and arrhythmias (as a Class IB antiarrhythmic). Topical phenytoin has also been used to heal multiple acute and chronic wounds.3 As an anticonvulsant, phenytoin works by increasing the efflux and decreasing the influx of sodium ions in cell membranes of the motor cortex during the generation of nerve impulses.2
DOSING
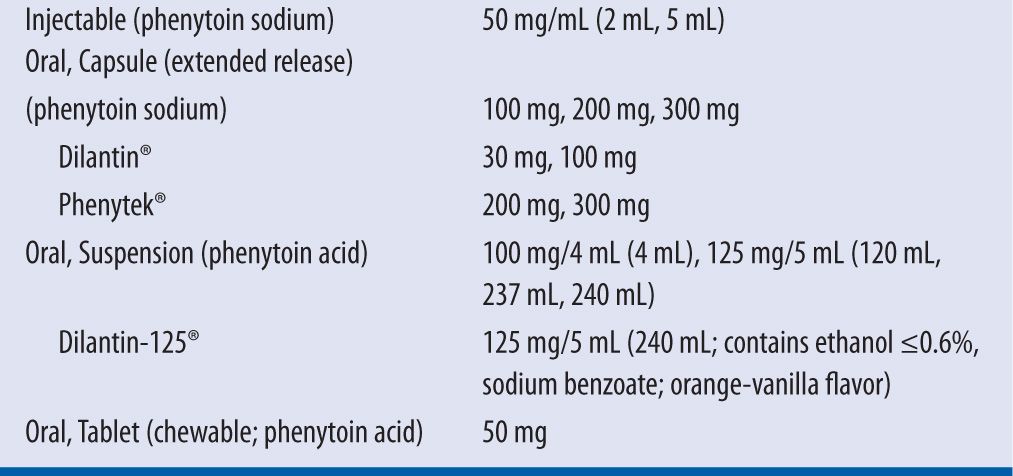
Phenytoin is available in multiple dosage forms including an injectable formulation, extended-release capsule, oral suspension, and chewable tablet (Table 15-1).2 Intramuscular (IM) administration of phenytoin is not recommended due to its erratic absorption and pain on injection. If IM administration is required, fosphenytoin is preferred. Intravenously (IV), phenytoin may be administered by IV push or IV piggyback using a 0.22 micron filter. The filter is used to remove crystals and minimize the incidence of phlebitis. The maximum rate of administration is 50 mg/min in adults and 0.5–1 mg/kg/min in neonates due to the risk of hypotension, as noted in a boxed warning in the drug package insert. In elderly or patients with preexisting cardiovascular conditions, phenytoin may be administered more slowly at 20 mg/minute.4 It must be noted that the oral suspension and chewable tablets contain 8 percent more phenytoin than the other formulations (92 mg of phenytoin base is equivalent to 100 mg of phenytoin sodium). Therefore, when patients are being switched from one dosage formulation to another, dosage adjustments and closer serum monitoring are recommended.2,4 The recommended dose for adult patients in status epilepticus is 15–20 mg/kg. For maintenance doses, adults may be loaded at 15–20 mg/kg. Due to the rate-limited gastrointestinal (GI) absorption of phenytoin, no more than 400 mg should be administered at a time. Hence, in order to ensure complete absorption and a decrease in GI side effects, a 1,000 mg loading dose may be administered in three divided doses at 400 mg, 300 mg, and 300 mg every two hours. A target level is achieved within 6–10 hours. The maintenance dose is 300 mg/day or 5–6 mg/kg/day in three divided doses or once to twice daily if the extended-release formulation is being used. For obese patients, it is recommended to receive loading doses based on adjusted body weight with a correction factor of 1.33 for a maximum loading dose of 2,000 mg. For maintenance dosing, the ideal body weight may be used and be adjusted according to therapeutic drug monitoring and clinical effectiveness. A serum phenytoin concentration of 10–20 mg/L is considered within therapeutic range. Concentrations between 5 and 10 mg/L may be therapeutic for some patients; however, concentrations less than 5 mg/L are not recommended. The target free phenytoin concentration is 1–2 mg/L.
| TABLE 15-1 | Dosage Formulations and Strengths of Phenytoin2 |
ADVERSE EVENTS
Phenytoin adverse effects can be common and chronic, and include hepatotoxicity, osteoporosis, megaloblastic anemia, gingival hyperplasia, hirsutism, and peripheral neuropathy. Phenytoin may cause gastrointestinal adverse effects such as nausea and vomiting; single doses above 100 mg increase the propensity of gastrointestinal intolerance. Phenytoin can cause central nervous system (CNS) adverse effects such as dizziness, confusion, drowsiness, and ataxia – although these usually occur at a greater frequency with high peak concentrations or toxic levels. Phenytoin-induced CNS adverse effects may be circumvented by administering a larger dose or the entire dose at bedtime. Phenytoin may cause cognitive dysfunction.
Phenytoin may cause a rash that presents as the antiepileptic hypersensitivity syndrome (AES). The onset of AES is generally within the first five weeks of initiating phenytoin therapy and presents with a symptom triad including rash, pruritus, and fever. AES presents with other nonspecific manifestations that can wax and wane including hepatotoxicity, blood dyscrasias, encephalitis, myositis, malaise, pulmonary infiltrates, and an acute respiratory distress syndrome. The rash may progress to life-threatening Steven–Johnsons syndrome or toxic epidermal necrolysis. Since it is difficult to discern if an isolated phenytoin-induced rash will be benign or progress to the AES, patients who develop rash should have phenytoin therapy discontinued. Patients who develop phenytoin-induced AES should avoid using other aromatic anticonvulsants that may cross-react, such as carbamazepine, oxcarbazepine, phenobarbital, lamotrigine, lacosamide, and zonisamide.
Acute phenytoin toxicity (levels above 20 mg/L) often exhibits concentration-dependent toxicities such as nystagmus and diplopia at levels between 20 and 30 mg/L; as the levels rise to 30–40 mg/L the nystagmus and diplopia dissipate and ataxia, nausea, and vomiting manifest. Intention tremor may persist at all phenytoin toxic levels. Some patients with phenytoin toxicity do present with cumulative symptomatology. Patients who present with chronic phenytoin toxicity may develop irreversible CNS effects such as dysarthria, ataxia, and encephalopathy. A list of adverse effects associated with phenytoin is depicted in Table 15-2.4,5 Hypotension is listed as a boxed warning in the package insert of phenytoin. It has been observed primarily in older adults and is associated with increased infusion rates. Monitoring of blood pressure and heart rate is recommended during IV administration and may be used to determine the safest rate for the patient. Due to the cardiotoxicity associated with phenytoin (e.g., bradycardia, hypotension, QRS prolongation, ventricular fibrillation), all patients receiving IV phenytoin must have continuous cardiac monitoring. Phenytoin is pregnancy category D because it crosses the placenta and has been associated with congenital malformations termed fetal hydantoin syndrome or fetal anticonvulsant syndrome. Isolated cases of malignancies and coagulation defects in the neonate have been reported. Phenytoin enters the breast milk and it is not recommended to be used by lactating women.
| TABLE 15-2 | Adverse Effects Associated with Phenytoin4,5 |

BIOAVAILABILITY
The bioavailability of phenytoin varies significantly among the different dosage forms.6 Phenytoin is poorly soluble in water, but it readily dissolves in an alkali environment. Due to the acidity of the stomach, phenytoin sodium changes to free phenytoin acid that precipitates after it dissolves. Hence, the rate and extent of absorption are highly dependent on the size of the phenytoin particles entering the intestine. For this reason, the different dosage forms of phenytoin have widely different bioavailabilities. No systematic difference in bioavailability has been noted between phenytoin sodium and phenytoin acid. In fact, clinicians should be careful when switching patients whose seizures are controlled from one phenytoin preparation to another because even small increases or decreases in bioavailability can significantly change the steady-state plasma concentration during chronic therapy.6-9 The evidence indicates that even changes in the excipient may result in phenytoin intoxication.7 The rate of absorption after a single oral dose of a capsule or tablet can be anywhere from 3 to 12 hours. However, in some patients, it may exceed 12 hours. The Food and Drug Administration recommends only the Dilantin Kapseals® to be administered once daily because many generic preparations are more rapidly absorbed and may produce fluctuations in the plasma phenytoin concentration.4 Numerous case studies report a decrease in phenytoin absorption when it is coadministered with enteral feedings.10 The exact mechanism is unknown; however, researchers speculate that phenytoin particles bind to certain components in the feeding formulas, such as calcium and dietary fiber. Others believe that phenytoin binds to the tube lumen, or the mechanism is pH dependent. Methods described to avoid this interaction include spacing the administration of the enteral feedings from that of phenytoin’s without compromising the patient’s dietary requirements. However, no consensus establishes the best method for avoiding this interaction.
VOLUME OF DISTRIBUTION
Phenytoin has an apparent volume of distribution of 0.6–0.7 L/kg adults and children and 1.2 L/kg in infants and neonates. Phenytoin distributes rapidly to the brain where plasma and brain concentrations achieve equilibrium within 20 minutes.10
PLASMA PROTEIN BINDING OF PHENYTOIN
Phenytoin is 92 percent bound to plasma albumin, and the free form (8%) is the pharmacologically active drug responsible for efficacy and toxicity.10 A smaller portion is bound to alpha-1-acid glycoprotein.11 When phenytoin levels are reported, they represent the unbound (free or active) and bound (inactive) phenytoin level, often referred to as phenytoin observed. In patients with normal albumin level and renal function, the fraction of unbound phenytoin (fup) is 0.1. Hence, a total phenytoin concentration of 10–20 mg/L represents a fup of 1–2 mg/L.
Conditions That Can Cause Hypoalbuminemia and the Effects on Free Phenytoin Concentration
Disease states or medications that can change the albumin concentration or phenytoin’s binding affinity (Ka) to albumin can ultimately alter the concentration of free, fraction unbound of phenytoin (fup). Disease states that can decrease the serum albumin concentration and eventually lead to increased free phenytoin concentrations include burns, hepatic cirrhosis, nephrotic syndrome, pregnancy, and cystic fibrosis.2,4,12 In patients with hypoalbuminemia, the observed total phenytoin concentration can appear normal or low, even though the free phenytoin level is increased because of less albumin to bind to. As the free form increases, the total phenytoin level remains unchanged.12 For example, a cachetic, hypoalbuminemic (albumin level <4.4 g/dL) patient has an observed total phenytoin level of 18 mg/L, which appears to be at target. However, the fraction of unbound phenytoin has increased from 0.1 to 0.2, resulting in a toxic free phenytoin level of 3.6 mg/L (normal free phenytoin is 1–2 mg/L).
The Sheiner-Tozer equation is a correction formula that uses the plasma albumin concentration to predict the free fraction of phenytoin using the total phenytoin observed4,13:
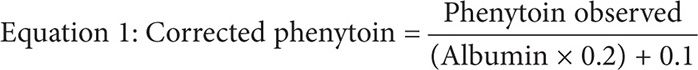
This equation can empirically adjust the observed total phenytoin concentration in cases of decreased albumin. However, temperature plays a critical role in the application of the Sheiner-Tozer equation.13 The formula was based on a free phenytoin assay, which was performed using a Centrifree micropartition filter for separation at a temperature of 37°C, resembling physiological body temperature. However, clinical laboratories routinely perform unbound phenytoin assays at room temperature of 25°C. In such cases, the Sheiner-Tozer equation must be changed to accommodate for the difference in room temperature by multiplying the albumin concentration by the constant 0.2514:
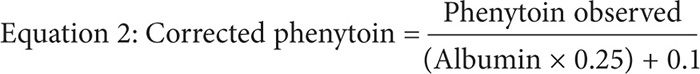
Conditions That Can Decrease Phenytoin’s Binding Affinity to Albumin and the Effects on Free Phenytoin Concentration
Certain disease states can decrease phenytoin’s binding affinity (Ka) to serum albumin. Such disease states include, severe jaundice, hyperbilirubinemia (total bilirubin >15 mg/dL), and renal insufficiency or failure with a calculated creatinine clearance (CrCl) <25 mL/min (fup increases two- to threefold in uremia).2,4,12,13,15,16 The binding affinity of phenytoin decreases in these situations either because of low albumin or because of alterations in the albumin molecule leading to decreased binding, or due to the accumulation of a major metabolite of phenytoin [5-(p-hydroxyphenyl)-5-phenylhydantoin, or p-HPPH], which displaces phenytoin from albumin.17 In addition, drugs like valproic acid, which has a high affinity for the same binding site as phenytoin, can easily displace phenytoin from albumin, resulting in increased amount of free phenytoin.18,19 Whichever the mechanism for the decreased binding may be, the observed phenytoin concentration in these situations is a poor predictor of the patient’s true phenytoin level.
Phenytoin’s binding affinity to albumin (Ka) is less likely to be affected if the calculated creatinine CrCl is >25 mL/min. However, once the CrCl is 10–25 mL/min, Ka is likely to be decreased to an unknown extent. Patients with end-stage renal disease (ESRD), or CrCl <10 mL/min, have both decreased albumin level and Ka. In such situations, the Sheiner-Tozer equation must be changed where the albumin is multiplied by the constant 0.120,21:
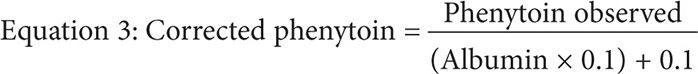
CASE STUDIES
CASE 1: CALCULATING FOR THE CORRECTED PHENYTOIN LEVEL IN PATIENTS WITH HYPOALBUMINEMIA WHEN THE SERUM PHENYTOIN TEST IS PERFORMED AT 37°C
SP is a 72-year-old female (62 kg, 5′6″) with an observed phenytoin level of 6.2 mg/L at 37°C. Her recent albumin level was 2 g/dL, and her serum creatinine level was 0.6 mg/dL. Calculate for the corrected phenytoin level.
Step 1: Calculate her creatinine clearance. Since this patient is above the age of 65 y/o, round up the serum creatinine from 0.6 mg/dL to 1 mg/dL when calculating for her creatinine clearance.

Step 2: Calculate for the corrected phenytoin level. Because her creatinine clearance is >25 mL/min and the test is performed at 37°C, use Equation 1.

CASE 2: CALCULATING FOR THE CORRECTED PHENYTOIN LEVEL IN PATIENTS WITH HYPOALBUMINEMIA
A 26-year-old (52 kg, 5′5″) white female is receiving phenytoin 100 mg po tid for partial epilepsy. She has a steady-state phenytoin concentration of 14 mg/L. She is seizure free but has been complaining of daytime drowsiness and dizziness. An SMA-18 panel includes the following laboratory values: creatinine 0.5 mg/dL and albumin 2.8 g/dL. What is the corrected steady-state phenytoin concentration?
Step 1: It is not necessary to calculate the creatinine clearance in this case. This patient is 26 years old with a normal serum creatinine and, intuitively, will have a projected creatinine clearance above 25 mL/min.
Step 2. Calculate for the corrected phenytoin level. Although the temperature in which phenytoin was assayed is not mentioned, one can assume that phenytoin serum samples are maintained and assayed at room temperature at 25°C. Because her creatinine clearance is >25 mL/min and we are assuming that the phenytoin samples were maintained and assayed at room temperature at 25°C, use Equation 2.
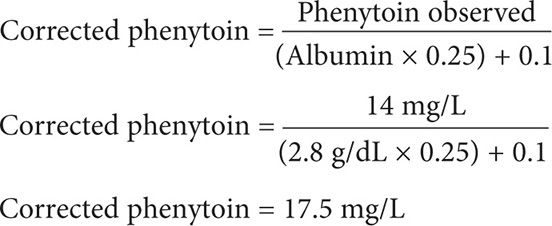
This patient has a corrected serum phenytoin concentration of 17.5 mg/L. Although this patient’s corrected serum phenytoin concentration is within the target range of 10–20 mg/dL, she is experiencing phenytoin-induced CNS-adverse effects. The patient should be monitored for other CNS and non-CNS signs and symptoms of phenytoin toxicity. In order to mitigate the phenytoin-induced CNS-adverse effects, the medical team may consider administering the entire phenytoin dose at bedtime, or a reduced phenytoin daily dose.
CASE 3: CALCULATING FOR THE CORRECTED PHENYTOIN LEVEL IN PATIENTS WITH HYPOALBUMINEMIA WHEN THE SERUM PHENYTOIN TEST IS PERFORMED AT 25°C
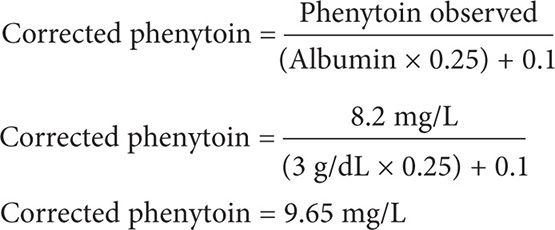
TD is an 80-year-old male (59 kg, 5′2″) with an observed phenytoin level of 8.2 mg/L at 25°C. His recent albumin level was 3 g/dL, and his serum creatinine level was 0.8 mg/dL. Calculate for the corrected phenytoin level.
Step 1: Calculate his creatinine clearance.

Step 2: Calculate for the corrected phenytoin level. Because his creatinine clearance is >25 mL/min and the test is performed at 25°C, use Equation 2.
CASE 4: CALCULATING FOR THE CORRECTED PHENYTOIN LEVEL IN PATIENTS WITH HYPOALBUMINEMIA
A 67-year-old (50 kg, 5′2″) black female is receiving phenytoin 100 mg po tid for partial epilepsy. She has a steady-state phenytoin concentration of 6 mg/L and is seizure free. An SMA-18 panel includes the following laboratory values: creatinine 1 mg/dL and albumin 1.7 g/dL. What is the corrected steady-state phenytoin concentration?
Step 1: Calculate her creatinine clearance.
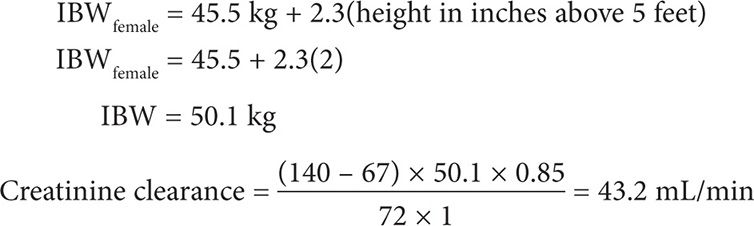
Step 2. Calculate for the corrected phenytoin level. Although the temperature in which phenytoin was assayed is not mentioned, one can assume that phenytoin serum samples are maintained and assayed at room temperature at 25°C. Because her creatinine clearance is >25 mL/min and we are assuming that the phenytoin samples were maintained and assayed at room temperature at 25°C, use Equation 2.

This patient has a corrected serum phenytoin concentration of 11.4 mg/L. Because she is seizure free and her corrected serum phenytoin concentration is within the target range of 10–20 mg/L, no phenytoin dosage adjustments or heightened monitoring for toxicity is warranted.
CASE 5: CALCULATING FOR THE CORRECTED PHENYTOIN LEVEL IN PATIENTS WITH END-STAGE RENAL DISEASE
SM is an 88-year-old female with history of ESRD and receiving dialysis every Monday, Wednesday, and Friday. She has been receiving phenytoin suspension 125 mg every 12 hours for 8 months for seizure prophylaxis. Her observed phenytoin level was 5 mg/L and albumin was 3.6 g/dL. Calculate for the corrected phenytoin level assuming the phenytoin assay was performed at 37° C.
Step 1: No need to calculate for her creatinine clearance. For ESRD patients, always assume that the CrCl is going to be <10 mL/min.
Step 2: Calculate for the corrected phenytoin concentration using Equation 3.

CASE 6: CALCULATING FOR THE CORRECTED PHENYTOIN LEVEL IN PATIENTS WITH END-STAGE RENAL DISEASE AND HYPOALBUMINEMIA
A 28-year-old (85 kg, 6′1″) black male is receiving phenytoin capsule 100 mg po twice daily and at bedtime for epilepsy. He has a steady-state phenytoin concentration of 12 mg/L. He has a history of chronic kidney disease with a creatinine clearance of 8 mL/min. An SMA-18 panel includes the following laboratory values: BUN = 66 mg/dL, albumin 3 g/dL, and globulin 2.7 g/dL. What is the corrected steady-state phenytoin concentration?
Step 1: It is not necessary to calculate the creatinine clearance in this case; this patient has CKD with a CrCl of 8 mL/min.
Step 2: Calculate for the corrected phenytoin concentration using Equation 3. Be careful to use the serum albumin in the equation and not the serum globulin, since phenytoin is a weak acid and is highly bound to the albumin portion [not globulin] of the plasma protein binding sites.
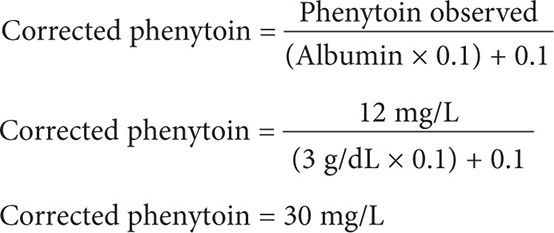
This patient has a toxic corrected serum phenytoin concentration of 30 mg/L. The patient should be monitored for signs and symptoms of phenytoin toxicity, the phenytoin dose should be held and a new reduced dosing regimen should be considered.
CASE 7: CALCULATING FOR THE CORRECTED PHENYTOIN LEVEL IN PATIENTS WITH END-STAGE RENAL DISEASE AND HYPOALBUMINEMIA
A 65-year-old (55 kg, 5′7″) white male is receiving phenytoin capsule 100 mg po bid for epilepsy. He has a steady-state phenytoin concentration of 9 mg/L. He has a history of chronic kidney disease with a creatinine clearance of 10 mL/min. An SMA-18 panel includes the following laboratory values: BUN = 66 mg/dL, albumin 1.5 g/dL, and globulin 1.9 g/dL. What is the corrected steady-state phenytoin concentration?
Step 1: It is not necessary to calculate the creatinine clearance in this case; this patient has CKD with a CrCl of 10 mL/min.
Step 2: Calculate for the corrected phenytoin concentration using Equation 3. Be careful to use the serum albumin in the equation and not the serum globulin, since phenytoin is a weak acid and is highly bound to the albumin portion [not globulin] of the plasma protein binding sites.
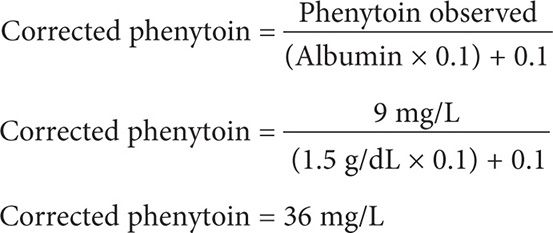
This patient has a toxic corrected serum phenytoin concentration of 36 mg/L. The patient should be monitored for signs and symptoms of phenytoin toxicity, the phenytoin dose should be held and a new reduced dosing regimen should be considered.
CASE 8: CALCULATING FOR THE CORRECTED PHENYTOIN LEVEL IN PATIENTS WITH END-STAGE RENAL DISEASE AND A NORMAL ALBUMIN
A 47-year-old (65 kg, 5′10″) Hispanic male is receiving phenytoin 100 mg po tid for epilepsy. He has a steady-state phenytoin concentration of 15 mg/L. He has a history of chronic kidney disease with a creatinine clearance of 5 mL/min. An SMA-18 panel includes the following laboratory values: albumin 4.6 g/dL and globulin 1.9 g/dL. What is the corrected steady-state phenytoin concentration?
Step 1: It is not necessary to calculate the creatinine clearance in this case; this patient has CKD with a CrCl of 5 mL/min.
Step 2: Calculate for the corrected phenytoin concentration using Equation 3. Be careful to use the serum albumin in the equation and not the serum globulin, since phenytoin is a weak acid and is highly bound to the albumin portion [not globulin] of the plasma protein binding sites. Although the patient’s albumin is 4.6 g/dL, be sure to use an albumin of 4.4 g/dL as the Sheiner Tozer equation is only designed to correct for hypoalbuminemia at less than 4.4 g/dL.
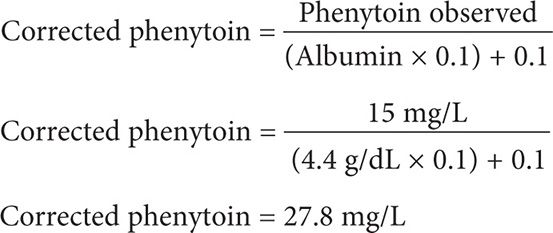
This patient has a toxic corrected serum phenytoin concentration of 27.8 mg/L. The patient should be monitored for signs and symptoms of phenytoin toxicity, and the phenytoin dose should be held and a new reduced dosing regimen should be considered.
CASE 9: CALCULATING FOR THE CORRECTED PHENYTOIN LEVEL IN PATIENTS WITH END-STAGE RENAL DISEASE AND A NORMAL ALBUMIN
A 36-year-old (75 kg, 5′11″) white male is receiving phenytoin 100 mg po qid for epilepsy. He has a steady-state phenytoin concentration of 22 mg/L. He has a history of chronic kidney disease with a creatinine clearance of 8 mL/min. An SMA-18 panel includes the following laboratory values: albumin 5.2 g/dL and globulin 3 g/dL. What is the corrected steady-state phenytoin concentration?
Step 1: It is not necessary to calculate the creatinine clearance in this case; this patient has CKD with a CrCl of 8 mL/min.
Step 2: Calculate for the corrected phenytoin concentration using Equation 3. Be careful to use the serum albumin in the equation and not the serum globulin, since phenytoin is a weak acid and is highly bound to the albumin portion [not globulin] of the plasma protein binding sites. Although the patient’s albumin is 5.2 g/dL, be sure to use an albumin 4.4 g/dL as the Sheiner Tozer equation is only designed to correct for hypoalbuminemia at less than 4.4 g/dL.
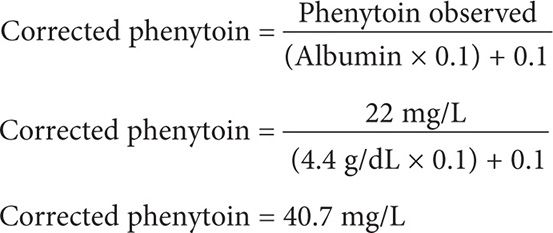
This patient has a toxic corrected serum phenytoin concentration of 40.7 mg/L. The patient should be monitored for signs and symptoms of phenytoin toxicity, the phenytoin dose should be held and a new reduced dosing regimen should be designed.
CASE 10: CALCULATING FOR THE CORRECTED PHENYTOIN LEVEL IN PATIENTS WITH RENAL FAILURE
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


