INTRODUCTION
This chapter presents endocrine pharmacology relevant to both the male and female reproductive tracts. Although men and women differ in their hormonal profiles, androgens and estrogens are both under the control of the anterior pituitary gland gonadotropins, luteinizing hormone (LH) and follicle-stimulating hormone (FSH), and ultimately regulated by hypothalamic release of gonadotropin-releasing hormone (GnRH). Female hormone patterns are temporally more complex and cyclic than male patterns: hormonal control of the menstrual cycle is an illustrative example of how sex hormones are integrated into a complex physiologic system. Understanding the menstrual cycle also provides a basis for understanding the pharmacology of contraception.
A number of diseases are treated pharmacologically via modification of reproductive hormone activity; these range from infertility and endometriosis to breast and prostate cancer. Key concepts in this chapter include (1) the interactions between estrogen and the pituitary gland, (2) the effects of GnRH release frequency on gonadotropin release, (3) the tissue selectivity of estrogen receptor agonists and antagonists, and (4) the various strategies used to antagonize the effects of endogenous sex hormones, from suppression of the hypothalamic-pituitary-reproduction axis to antagonism at the target tissue receptor.
 Amy J first notices that her hair is thinning during her teenage years. Even though she loses some hair on her scalp, Ms. J notices excessive hair growth on her face; she sometimes has to shave to remove inappropriate hair growth. At age 24, she goes to her doctor complaining of both her hair problem and the fact that her periods are irregular. On further questioning, the doctor discovers that the longest interval between her menstrual cycles has been 6 months and the shortest 22 days. When Ms. J does have periods, they are heavy and last for more than her previous average of 5 days. The increased hair growth on her face, extremities, abdomen, and breasts had begun around age 15. Ms. J also reports a problem with being overweight since high school, although in middle school, she had been extremely active in soccer, field hockey, and swimming. The doctor orders several tests and finds that Ms. J has mildly elevated free and total testosterone levels and an increased ratio of plasma LH to FSH.
Amy J first notices that her hair is thinning during her teenage years. Even though she loses some hair on her scalp, Ms. J notices excessive hair growth on her face; she sometimes has to shave to remove inappropriate hair growth. At age 24, she goes to her doctor complaining of both her hair problem and the fact that her periods are irregular. On further questioning, the doctor discovers that the longest interval between her menstrual cycles has been 6 months and the shortest 22 days. When Ms. J does have periods, they are heavy and last for more than her previous average of 5 days. The increased hair growth on her face, extremities, abdomen, and breasts had begun around age 15. Ms. J also reports a problem with being overweight since high school, although in middle school, she had been extremely active in soccer, field hockey, and swimming. The doctor orders several tests and finds that Ms. J has mildly elevated free and total testosterone levels and an increased ratio of plasma LH to FSH.
Based on these findings, the doctor tells Ms. J that she probably has a disorder called polycystic ovarian syndrome (PCOS). He recommends combination oral contraceptives to regularize her menstrual cycles. He also prescribes spironolactone to ameliorate her problems with hair growth and balding.
Questions
1. What is the pathophysiologic link between excessive hair growth and infertility in polycystic ovarian syndrome?
2. Why was spironolactone prescribed to treat Ms. J’s hair problem?
3. How do oral contraceptives act, and how would they help regulate Ms. J’s menstrual cycles?
 PHYSIOLOGY OF REPRODUCTIVE HORMONES
PHYSIOLOGY OF REPRODUCTIVE HORMONES
Synthesis of Progestins, Androgens, and Estrogens
The synthesis of progestins, androgens, and estrogens is closely intertwined. All three groups are steroid hormones derived from the metabolism of cholesterol. The synthesis of these hormones is similar to that of adrenal sex hormones, which is discussed in Chapter 29, Pharmacology of the Adrenal Cortex.
The terminology progestins, androgens, and estrogens denotes a number of related hormones rather than a single molecule in each group (Fig. 30-1). The progestins consist of progesterone, a common precursor to testosterone and estrogen synthesis (see also Fig. 29-2), and a number of synthetically altered progesterone derivatives used for therapeutic purposes. Progestins generally exert antiproliferative effects on the female endometrium by promoting the endometrial lining to secrete rather than proliferate (see below). Progesterone is also required for the maintenance of pregnancy. Androgens, all of which have masculinizing properties, include dehydroepiandrosterone (DHEA), androstenedione, testosterone, and dihydrotestosterone (DHT); among the androgens, testosterone is considered the classic circulating androgen and DHT the classic intracellular androgen. Androgens are required for conversion to a male phenotype during development and for male sexual maturation. Estrogens refer to a number of substances that share a common feminizing activity. 17β-Estradiol is the most potent naturally occurring estrogen, while estrone and estriol are less potent.
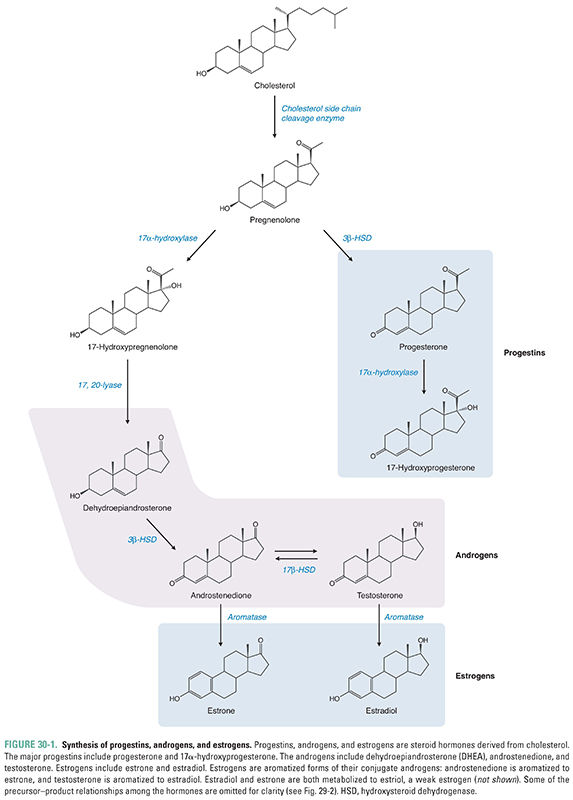
Note that all estrogens are derived from the aromatization of precursor androgens (Fig. 30-1). The ovary and placenta most actively synthesize the aromatase enzyme that converts androgens to estrogens, but other nonreproductive tissues such as adipose tissue, hypothalamic neurons, and muscle can also aromatize androgens to estrogen. After menopause, the majority of circulating estrogen is derived from adipose tissue. This is also the main source of circulating estrogens in men.
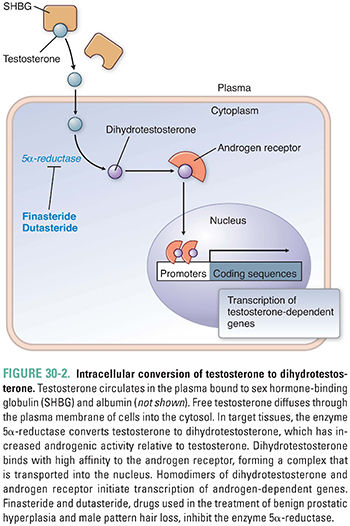
Progestins, androgens, and estrogens are all hormones that bind to a related superfamily of nuclear hormone receptors; glucocorticoids, mineralocorticoids, vitamin D, and thyroid hormone also bind to the same superfamily of receptors. Once synthesized, these hormones diffuse into the plasma, where they bind tightly to carrier proteins such as sex hormone-binding globulin (SHBG) and albumin. Only the unbound fraction of hormone is able to diffuse into cells and bind to an intracellular receptor. Interestingly, testosterone is essentially a prohormone. Testosterone binds to the androgen receptor but with only modest affinity. As a result, testosterone has only modest androgenic activity. Instead, testosterone is converted in target tissues to the more active dihydrotestosterone (Fig. 30-2), which binds to the androgen receptor with an affinity tenfold higher than that of testosterone. The formation of dihydrotestosterone from testosterone is catalyzed by the enzyme 5α-reductase. There are at least two subtypes of 5α-reductase. Differential tissue expression of these enzymes provides some pharmacologic specificity for the 5α-reductase inhibitors. The importance of dihydrotestosterone as the most active androgen is highlighted in individuals with inherited deficiencies of 5α-reductase. Males lacking this enzyme are phenotypically female because they are unable to convert testosterone to dihydrotestosterone and are thus unable to activate a program of male differentiation during development.
The estrogen receptor (ER) is the best studied of the sex hormone receptors and serves as an example for all three receptor types (i.e., estrogen receptor, androgen receptor, and progesterone receptor). Because progestins, androgens, and estrogens are lipophilic steroid hormones, the fraction of hormone that remains unbound to plasma proteins can freely diffuse across the plasma membrane into the cytosol of cells. Once inside the cell, the hormone ligand binds to its specific intracellular receptor, which subsequently dimerizes. For example, association of estrogen with the estrogen receptor causes dimerization of two estrogen–estrogen receptor complexes, and the dimer then binds to estrogen response elements (EREs) in promoter regions of DNA. This binding to EREs, together with the recruitment of coactivators or corepressors, enhances or inhibits the transcription of specific genes and thereby causes the physiologic effects of the hormone.
There are two subtypes of estrogen receptors—ERα and ERβ. In addition, it is now recognized that many estrogen receptor actions involve association of the receptor with other transcription cofactors. In other words, dimerization of the estrogen receptor and subsequent binding of the dimer to EREs are insufficient to explain the complex and varied actions of estrogen in different tissues. The specific transcription factors that are recruited by the estrogen receptor appear to be tissue-dependent and ligand-dependent and probably account for some of the target specificity of estrogen action. Although the subtypes and molecular associations of the androgen and progesterone receptors have not been studied as thoroughly as those of the estrogen receptor, it is likely that the same complexities exist for these receptors. The recognition that differential binding of modular transcription factors to ERs could alter estrogenic effects will likely prove a burgeoning area of pharmacologic research in the near future, as pharmaceutical researchers continue to develop receptor agonists and antagonists with selective actions in specific tissues. Selective estrogen receptor modulators (SERMs, see below) are the first drugs to take advantage of the tissue selectivity of sex hormone receptor function.
In some cells, a membrane-bound G protein-coupled estrogen receptor (GPER) is also activated by estradiol, resulting in activation of adenylyl cyclase (Gαs effect), mobilization of intracellular calcium (Gβγ effect), and transactivation of the epidermal growth factor receptor (Gβγ effect). The role of GPER activation in the physiology and pathophysiology of estrogen action is an area of active investigation.
Hypothalamic-Pituitary-Reproduction Axis
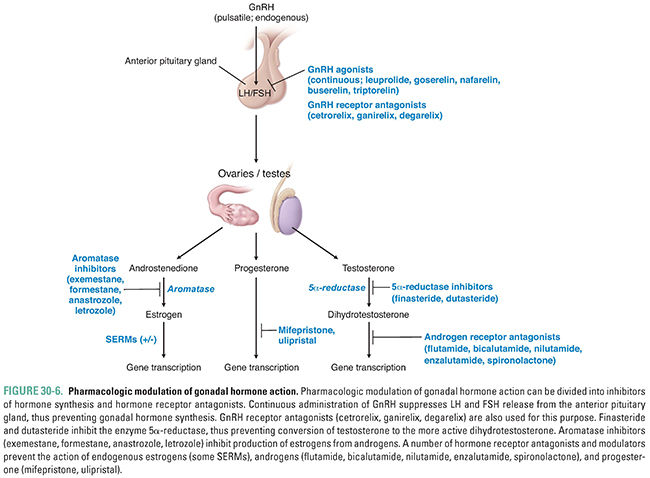
The hypothalamic-pituitary-reproduction axis regulates sex hormone synthesis. Gonadotropin-releasing hormone (GnRH) resides at the top of this three-tiered hierarchy. The hypothalamus secretes GnRH in pulses (Fig. 30-3). GnRH travels via the hypothalamic–pituitary portal system to stimulate gonadotroph cells of the anterior pituitary gland. Stimulation of gonadotroph cells via a G protein-coupled cell surface receptor increases the synthesis and secretion of LH and FSH, which are jointly referred to as the gonadotropins.
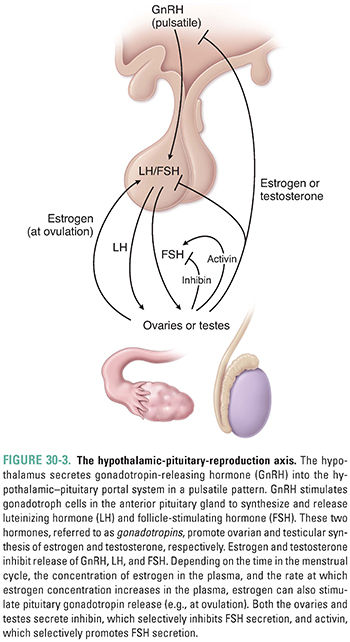
Although one cell type produces both LH and FSH, the synthesis and release of these two hormones are controlled independently. Current research suggests that the rate of GnRH secretion may preferentially alter the secretion patterns of LH and FSH. Pulsatile secretion of GnRH is critical for the proper functioning of the hypothalamic-pituitary-reproduction axis. When GnRH is administered continuously, gonadotroph release of LH and FSH is suppressed rather than stimulated. This effect has the important pharmacologic consequence that pulsatile administration of exogenous GnRH stimulates gonadotropin release, whereas continuous GnRH administration inhibits LH and FSH release and thereby blocks target cell function.
LH and FSH have analogous but somewhat different effects in males and females. The pertinent target cells in the male are the Leydig and Sertoli cells of the testis, while the thecal and granulosa cells of the ovary mediate gonadotropin function in the female (Fig. 30-4). In each case, a two-cell system is coordinated to mediate sex hormone actions. In the male, LH stimulates testicular Leydig cells to increase the synthesis of testosterone, which then diffuses into neighboring Sertoli cells. In the Sertoli cell, FSH stimulation increases the production of androgen binding protein (ABP), which is important for maintaining the high testicular concentrations of testosterone necessary for spermatogenesis. In addition, FSH stimulates the Sertoli cell to produce other proteins necessary for sperm maturation. In the female, LH stimulates the thecal cells to synthesize the androgen androstenedione, which is then aromatized to estrone and estradiol in the granulosa cells under the influence of FSH.
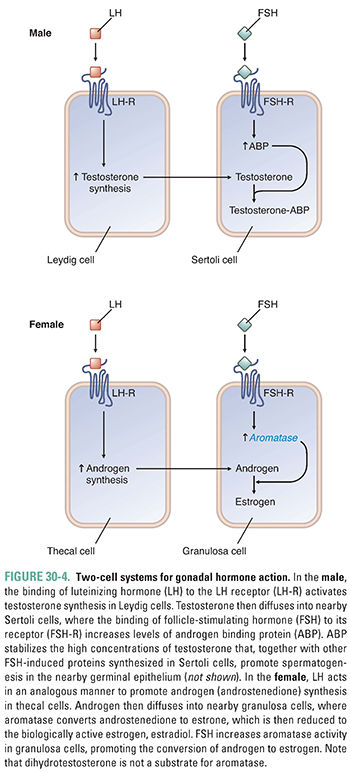
Both Sertoli cells and granulosa cells synthesize and secrete the regulatory proteins inhibin A, inhibin B, and activin. Inhibins secreted by the gonad act on the anterior pituitary gland to inhibit the release of FSH, while activin stimulates FSH release. Neither the inhibins nor activin has an effect on anterior pituitary gland LH release (Fig. 30-3). The role of these regulatory proteins in controlling hormone action is still not completely understood. In the male, testosterone is also an important negative regulator of pituitary gland and hypothalamic hormone release. The role of estrogen in the female is more complex and can involve either positive or negative feedback depending on the prevailing hormonal milieu; this topic is addressed below as part of the menstrual cycle discussion. In the female, the combination of estradiol and progesterone synergistically suppresses GnRH, LH, and FSH secretion by actions at both the hypothalamus and pituitary gland.
Integration of Endocrine Control: The Menstrual Cycle
The female menstrual cycle is governed by the cycling of hormones with an approximate periodicity of 28 days (normal range, 24–35 days). This cycle begins at the onset of puberty and continues uninterrupted (with the exception of pregnancy) until menopause (Fig. 30-5). The start of the cycle, cycle day 1, is arbitrarily defined as the first day of menstruation. Ovulation occurs at the midportion (about day 14) of each cycle. The portion of the menstrual cycle before ovulation is often referred to as the follicular or proliferative phase; during this time, the developing ovarian follicle produces most of the gonadal hormones, which stimulate cellular proliferation of the endometrium. Subsequent to ovulation, the corpus luteum produces progesterone, and the endometrium becomes secretory rather than proliferative. The second half of the menstrual cycle is thus often referred to as the luteal or secretory phase, depending on whether the ovary or the endometrium is considered as the frame of reference.
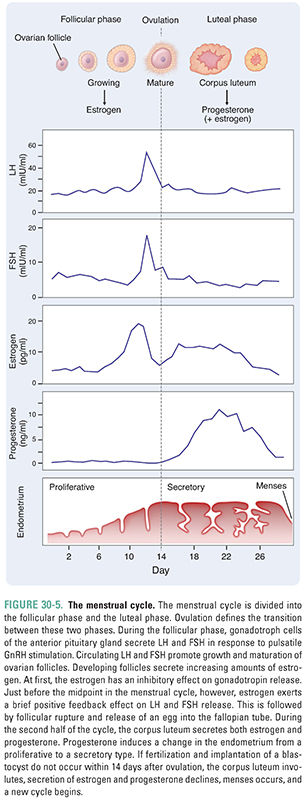
At the start of the menstrual cycle, there is low production of estrogen and inhibin A. As a result, the anterior pituitary gland secretes increasing amounts of FSH and LH. These hormones stimulate the maturation of four to six follicles, each of which contains an ovum arrested in the first stage of meiosis. Maturing follicles secrete increasing concentrations of estrogen, inhibin A, and inhibin B. Estrogen causes the follicles to increase the expression of LH and FSH receptors on thecal and granulosa cells, respectively. Receptor up-regulation increases the follicular response to pituitary gland gonadotropins and allows one follicle to secrete increasing quantities of estrogen. The increased plasma estrogen and inhibin levels partially suppress pituitary gland LH and FSH release. In turn, the decreased gonadotropin levels cause other follicles to become atretic, so that usually, only one follicle matures. At the same time, increased estrogen levels stimulate the uterine endometrium to proliferate rapidly.
As the dominant follicle continues to grow, it secretes high, sustained levels of estrogen. Although the mechanism is still not completely understood, the combination of high estrogen levels and the rapid rate of increase of estrogen levels causes a brief positive feedback effect on gonadotroph release of gonadotropins, stimulating rather than inhibiting release of LH and FSH. The resulting midcycle surge of LH and FSH stimulates the dominant follicle to swell and to increase the activity of its proteolytic enzymes. Approximately 40 hours after the onset of the LH surge, the follicle ruptures and ovulation occurs. The ovum is released into the peritoneal cavity and is then taken up by a fallopian tube, where it begins its route toward the uterus. If the oocyte becomes fertilized in the fallopian tube, it reaches the uterus approximately 4 days after ovulation and implants into the endometrium approximately 5–6 days after ovulation.
The cellular remains of the ruptured ovarian follicle become the corpus luteum. Cells of the corpus luteum secrete estrogen and progesterone, not just estrogen. The presence of progesterone in the second half of the menstrual cycle causes the endometrium to switch from a proliferative to a secretory state. The endometrium begins synthesizing proteins necessary for implantation of a fertilized egg. The blood supply to the endometrium also increases to provide increased nutrients if pregnancy ensues.
The corpus luteum has a lifespan of approximately 14 days. If fertilization and implantation of a viable blastocyst do not occur within 14 days of ovulation, the corpus luteum becomes atretic and ceases its production of estrogen and progesterone. Without the trophic effects of estrogen and progesterone, the endometrial lining sheds and menstruation begins. In the absence of estrogen and progesterone, the inhibition of gonadotrophs is removed, and production of FSH and LH increases. This stimulates the development of new ovarian follicles and the beginning of another menstrual cycle.
If fertilization does occur, implantation within the uterine lining causes the blastocyst to secrete human chorionic gonadotropin (hCG). The presence of hCG stimulates the corpus luteum to remain viable and continue secreting progesterone. Because hCG is one of the first proteins produced by the embryo that is unique to pregnancy, pregnancy tests assay for the presence of hCG. hCG production decreases after 10–12 weeks of pregnancy, when the placenta begins to secrete progesterone autonomously. Special considerations attending the use of drugs in pregnancy are discussed in Box 6-1.
Pathophysiologic processes in the reproductive tract reflect one of three general mechanisms of dysregulation (Table 30-1). The first is disruption of the hypothalamic-pituitary-reproduction axis, which causes a number of underlying disorders that can lead to infertility. The second is inappropriate growth of estrogen-dependent or testosterone-dependent tissue. This can lead to breast cancer or prostate cancer, as well as to benign but clinically important conditions such as endometriosis or endometrial hyperplasia. Finally, decreased estrogen secretion, as in menopause, or decreased androgen secretion, as in some aging men, is associated with a number of undesirable health consequences.
Disruption of the Hypothalamic-Pituitary-Reproduction Axis
The hypothalamic-pituitary-reproduction axis is normally tightly regulated via feedback inhibition or stimulation of hormone activity, with the goal of producing a successful menstrual cycle every month. When this axis is disrupted, infertility can result. Common causes of infertility due to disruption of sex hormone production include polycystic ovarian syndrome and prolactinomas.
Polycystic ovarian syndrome (PCOS) is a complex syndrome characterized by anovulation or oligo-ovulation and by increased levels of plasma androgen. PCOS is a common problem affecting between 3% and 5% of women of reproductive age. The diagnosis is typically clinical, as in the case of Ms. J, and based on the concurrent findings of oligo-ovulation and hirsutism (excessive hair growth). Although multiple etiologies are likely to be responsible for PCOS, all of the etiologies result in increased androgen secretion and suppression of normal ovulatory cycles. The increased androgen secretion results in masculinization; as seen in Ms. J’s case, male pattern baldness and inappropriate facial hair growth are common. Many women with PCOS are treated with both an estrogen–progestin contraceptive to suppress ovarian production of testosterone and an antiandrogen, such as spironolactone (see below), to abrogate the masculinizing effects of increased circulating testosterone.
Three primary hypotheses have been advanced to explain the development of PCOS. The first, referred to as the LH hypothesis, is based on the observation that many women with PCOS have an increased frequency and amplitude of pituitary LH pulses. In fact, 90% of women with PCOS have increased circulating LH. Increased LH activity stimulates thecal cells of the ovary to synthesize increased amounts of androgens, including androstenedione and testosterone. In addition, the increased LH and androgen levels prevent normal follicle growth, in turn preventing follicle secretion of large amounts of estrogen. The absence of an estrogen “trigger” prevents the LH surge and ovulation. As seen in the introductory case, patients with PCOS menstruate irregularly, and the menstrual periods that they do have tend to have heavy flow.
The second hypothesis, referred to as the insulin theory, is based on the observation that many women with PCOS are obese and insulin resistant and secrete increased insulin. Increased insulin decreases the production of sex hormone-binding globulin (SHBG), which results in a higher concentration of free testosterone and therefore greater androgenic effects on peripheral tissues. It has also been observed that insulin can directly synergize with LH to increase androgen production by thecal cells. Interestingly, in women with PCOS, medications that specifically treat insulin resistance, such as metformin, may result in regular ovulatory menses and normalization of testosterone levels.
The third hypothesis is the ovarian hypothesis. This explanation posits dysregulation of sex steroid synthesis at the level of the thecal cell. For example, an abnormal increase in the activity of the oxidative enzymes responsible for androgen synthesis could lead to greater thecal cell production of androgens in response to any given stimulus. It is important to note that these hypotheses are not mutually exclusive and that PCOS could result from a combination of two or three mechanisms. When the cellular mechanisms underlying this disease are better elucidated, new pharmacologic therapies can be developed to treat the etiology of the disease rather than its effects.
Prolactinomas are another common cause of infertility among women of reproductive age. These clonal, benign tumors of lactotrophs in the anterior pituitary gland can cause infertility through two parallel pathways. First, increased prolactin levels suppress estrogen synthesis, both by antagonizing the hypothalamic release of GnRH and by decreasing gonadotroph sensitivity to GnRH. This antagonism decreases LH and FSH release and thereby decreases end-organ stimulation by the hypothalamic-pituitary-reproduction axis. The second mechanism, common to all pituitary gland tumors, is a crowding-out or mass effect. Because the pituitary gland is enclosed in the bony sella turcica, lactotroph proliferation in the anterior pituitary gland leads to crowding of other cell types and thereby inhibits the function of nearby gonadotroph cells. Prolactin-secreting tumors typically remain responsive to the inhibitory effect of dopamine agonists. In most cases, chronic administration of dopamine agonists such as cabergoline or bromocriptine suppresses prolactin secretion and causes the tumor cells to shrink, thereby decreasing the size of the tumor and restoring normal gonadotroph function and ovulation.
Inappropriate Growth of Hormone-Dependent Tissues
The growth of breast tissues is dependent on many hormones, including estrogen, progesterone, androgens, prolactin, and insulin-like growth factors. Many (but not all) breast cancers express the estrogen receptor (ER), and the growth of such cancers is often stimulated by endogenous levels of estrogen and inhibited by antiestrogens. When a breast carcinoma is found to express the ER, an estrogen receptor antagonist (either a pure antagonist such as fulvestrant or a selective estrogen receptor modulator such as tamoxifen; see below) or an estrogen synthesis inhibitor (an aromatase inhibitor such as anastrozole, letrozole, exemestane, or formestane) is commonly administered to slow tumor growth. Prostate growth is androgen-dependent and requires the local conversion of testosterone to dihydrotestosterone by type II 5α-reductase in stromal cells of the prostate. Both enzyme inhibition (finasteride or dutasteride) and receptor antagonist (flutamide, bicalutamide, nilutamide, or enzalutamide) strategies are used to treat conditions in which the growth of prostate tissue is dysregulated, such as benign prostatic hyperplasia and metastatic prostate cancer (see below).
Endometriosis is the growth of endometrial tissue outside the uterus. The fact that endometriosis is usually found in areas surrounding the fallopian tube (ovaries, rectovaginal pouch, and uterine ligaments) has led to the hypothesis that endometriosis could result from retrograde migration of endometrial tissue via the fallopian tubes during menstruation. Other etiologies are possible, however, including metaplastic tissue growth from the peritoneum or spread of endometrial cells to extrauterine sites via lymphatic ducts. There is also evidence of increased aromatase activity in endometrial tissue from such patients. Because foci of endometriosis respond to estrogen stimulation, endometriosis grows and regresses with the menstrual cycle. This can lead to severe pain, abnormal bleeding, and the formation of adhesions in the peritoneal cavity. In turn, adhesion formation can lead to infertility. Because endometriosis is usually estrogen-dependent, treatment with long half-life GnRH agonists often achieves regression of the disease.
Decreased Estrogen or Androgen Secretion
The effects of decreased sex hormone production vary depending on the age of the patient at the onset of symptoms. Hypogonadism results if sex hormone production is impaired before adolescence. Patients with hypogonadism do not undergo sexual maturation, but proper hormone replacement can, in many cases, allow the development of secondary sexual characteristics.
Menopause is a normal physiologic response to exhaustion of the ovarian follicles. Throughout a woman’s lifetime, follicles are arrested in meiosis. Only a small percentage of follicles mature during the menstrual cycle; the rest eventually become atretic. Menstrual cycles cease when all of the follicles are depleted from the ovaries. Follicle depletion leads to a decrease in estrogen and inhibins (because developing follicles are the main estrogen and inhibin source in premenopausal women) and an increase in LH and FSH (because estrogen and inhibins suppress gonadotropin release). After menopause, androstenedione continues to be converted to estrone by aromatase in peripheral (mainly adipose) tissues. However, estrone is a less potent estrogen than estradiol. Because of the relative lack of estrogen after menopause, many women experience hot flashes, vaginal dryness, and decreased libido. Postmenopausal women are also at risk for osteoporosis. The role of estrogen in the maintenance of bone mass is discussed in further detail in Chapter 32, Pharmacology of Bone Mineral Homeostasis.
Men do not experience a sudden decrease in sex hormones in a manner analogous to the female menopause, but androgen secretion does decline gradually with age. Although controversy currently exists over the role of androgen therapy in normal elderly men, androgen replacement is indicated in cases of adult hypogonadism where both low testosterone levels and symptoms of hypogonadism are present.
 PHARMACOLOGIC CLASSES AND AGENTS
PHARMACOLOGIC CLASSES AND AGENTS
Pharmacologic agents have been developed to target most of the steps in gonadal physiology and pathophysiology. The relevant drug classes include modulators of anterior pituitary gland gonadotroph activity and specific antagonists of peripheral hormone action. In addition, sex hormones are often used as replacement therapy or to modify gonadotropin release (Fig. 30-6).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


 PATHOPHYSIOLOGY
PATHOPHYSIOLOGY