Peter R. Martin and Sachin Patel
Variables Affecting the Development of Substance Use Disorders |
Role of Personality Characteristics and Co-Occurring Disorders in Substance Use Disorders |
This chapter considers pharmacologic agents implicated in substance use disorders and the relevant brain processes involved in the clinical progression of these disorders. While the pharmacology of these agents is important to understanding their effects on behavior and their abuse liability, personality characteristics, as well as the presence of co-occurring psychiatric and medical conditions, may also contribute to the risk of developing substance use disorders. Understanding substance use disorders as biopsychosocial syndromes, rather than simply the pharmacologic consequences of chronic alcohol/drug use, has led to recognition of the central role that learning plays in substance use disorders and the potential for an integrated pharmacopsychosocial approach to treatment.
Most individuals with substance use disorders also have a second diagnosable psychiatric condition, but it is not easy to determine whether psychiatric symptoms are a cause or consequence of alcohol/drug use. For example, although alcohol is widely used to self-medicate depression and/or anxiety, it may be difficult to determine whether such psychiatric symptoms in alcoholics are the cause of drinking or its effect, because the actions of alcohol per se, as well as alcohol withdrawal and dependence, can also result in significant anxiety or depression.
Genetic determinants of the psychopharmacologic actions of abused drugs are increasingly recognized. Nonetheless, environmental variables have a significant influence on the development of substance use. For example, societal attitudes toward substance use often influence the likelihood that a substance will be taken in the first place. The availability and cost of a substance are also affected by its legal and tax status. The availability of other, nondrug alternatives may be a key factor in determining the likelihood that substance use disorders emerge for a given agent.
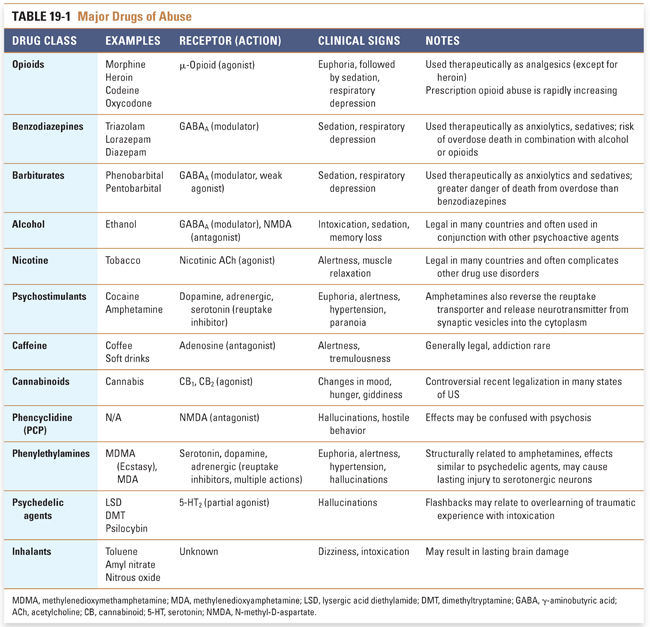
This chapter describes the mechanisms of action of selected representative substances of abuse, and the mechanisms of other important substances of abuse are summarized in Table 19-1. Since addiction is a disorder of brain reward pathways, learning, and motivated behavior, the use of medications in an integrated pharmacopsychosocial approach to treat substance use disorders is also discussed.
 CA, a 33-year-old man, is brought to the emergency department with chills, severe nausea, vomiting, diarrhea, muscle aches, and anxiety. Mr. A explains that he is currently shooting morphine or heroin about 3 days per week and using marijuana or cocaine “whenever.” He feels he wants to die. On examination, he has enlarged pupils, temperature is 103°F, blood pressure is 170/95 mm Hg, and heart rate is 108 beats/min. He is irritable and has abdominal cramping, hyperalgesia, and photophobia. Mr. A is given clonidine, with loperamide, ibuprofen, and promethazine as needed for diarrhea, pain, and nausea/vomiting, respectively. The severity of withdrawal does not diminish significantly until he is given sublingual buprenorphine/naloxone every 8 hours. Over the next week, Mr. A’s buprenorphine/naloxone is titrated to a once-daily dose, with progressive diminution of withdrawal symptoms and drug craving.
CA, a 33-year-old man, is brought to the emergency department with chills, severe nausea, vomiting, diarrhea, muscle aches, and anxiety. Mr. A explains that he is currently shooting morphine or heroin about 3 days per week and using marijuana or cocaine “whenever.” He feels he wants to die. On examination, he has enlarged pupils, temperature is 103°F, blood pressure is 170/95 mm Hg, and heart rate is 108 beats/min. He is irritable and has abdominal cramping, hyperalgesia, and photophobia. Mr. A is given clonidine, with loperamide, ibuprofen, and promethazine as needed for diarrhea, pain, and nausea/vomiting, respectively. The severity of withdrawal does not diminish significantly until he is given sublingual buprenorphine/naloxone every 8 hours. Over the next week, Mr. A’s buprenorphine/naloxone is titrated to a once-daily dose, with progressive diminution of withdrawal symptoms and drug craving.
Mr. A is discharged to a 28-day intensive outpatient treatment program during which he continues buprenorphine/naloxone. He agrees to attend daily mutual support self-help meetings (Alcoholics or Narcotics Anonymous), where he tells the tale of his addiction. He began diet pills in his teens for weight control and drinking as a response to physical abuse by his alcoholic father. He was prescribed acetaminophen and codeine for pain after minor surgery; thereafter, he started “street” pain pills and “doctor shopping” for back pain and depression, and he even had healthy teeth extracted to get opioids from the dentist. He was distraught when a close relative died, and he switched to intravenous opioids, including fentanyl patches that he ate or injected. He had three inpatient treatments, always relapsing after discharge to increasingly higher drug doses. He also tried methadone maintenance treatment for 6 months with some benefit but was unable to completely stop using “street drugs” and “never felt normal on methadone.”
Mr. A continues to be abstinent from illicit opioids after 10 years on sublingual buprenorphine/naloxone. He visits his psychiatrist monthly for psychotherapy and adheres to his treatment contract of monthly attendance at group therapy and weekly Narcotics Anonymous (NA) or Alcoholics Anonymous (AA) meetings. He consistently provides drug-free urine tests. He has been promoted at work, has negotiated a loan for purchase of a house, and seems relatively content having gained considerable understanding about management of his drug use disorder. Recently, he has begun discussing tapering and eventually discontinuing buprenorphine/naloxone maintenance.
Questions
1. What caused Mr. A’s physical symptoms and signs (i.e., chills, nausea, vomiting, fever, enlarged pupils, and hypertension) on his initial visit to the emergency department?
2. How could Mr. A’s pain be managed if he were to need surgery while taking buprenorphine/naloxone?
3. How can mutual support programs such as AA or NA help treat addiction, and how should such programs be complemented by medical oversight?
4. What was the rationale for initiating clonidine treatment for Mr. A?
5. Why did Mr. A’s initial symptoms and long-term opioid craving abate with the combination of buprenorphine/naloxone? How long should maintenance be continued?
The empirically based nomenclature promulgated by the American Psychiatric Association (APA) in the Diagnostic and Statistical Manual (DSM, Box 19-1) defines substance use disorder (used interchangeably in this chapter with addiction) as “a problematic pattern of use” leading to “significant impairment or distress.” This definition avoids value judgment and is generalizable across cultures. Psychosocial features of substance use disorders are similar for diverse psychopharmacologic agents with abuse liability and are likely more important in the development and maintenance of pathologic drug use than the unique pharmacologic profile of any given drug. Diagnostic features are conceptualized as clinical clusters “of cognitive, behavioral, and physiological symptoms indicating that the individual continues using the substance despite significant substance-related problems:” loss of control, salience to the behavioral repertoire, and neuroadaptation. However, the fundamental element is drug-seeking, the sine qua non of addiction. In the introductory case, Mr. A felt that he had little else than drugs in his life and could not stop using without help, and it is likely that he was addicted to opioids (and to the other drugs he was using).
BOX 19-1 Criteria for Substance Use Disorders (Addiction) from the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) |
A problematic pattern of substance use leading to clinically significant impairment or distress, as manifested by at least two of the following, occurring within a 12-month period: 1. The substance is often taken in larger amounts or over a longer period than was intended. 2. There is a persistent desire or unsuccessful efforts to cut down or control substance use. 3. A great deal of time is spent in activities necessary to obtain the substance (e.g., visiting multiple doctors or driving long distances), to use the substance (e.g., chain-smoking), or to recover from its effects. 4. Craving, or a strong desire or urge to use the substance. 5. Recurrent substance use resulting in a failure to fulfill major role obligations at work, school, or home. 6. Continued substance use despite having persistent or recurrent social or interpersonal problems caused or exacerbated by the effects of the substance. 7. Important social, occupational, or recreational activities are given up or reduced because of substance use. 8. Recurrent substance use in situations in which it is physically hazardous. 9. Substance use is continued despite knowledge of having a persistent or recurrent physical or psychological problem that is likely to have been caused or exacerbated by the substance (e.g., current cocaine use despite recognition of cocaine-induced depression, or continued drinking despite recognition that an ulcer was made worse by alcohol consumption). 10. Tolerance, as defined by either of the following: a. A need for markedly increased amounts of the substance to achieve intoxication or desired effect. b. A markedly diminished effect with continued use of the same amount of substance. 11. Withdrawal, as manifested by either of the following: a. The characteristic withdrawal syndrome for the substance (as defined by the APA criteria for withdrawal for a specific substance). b. The same (or closely related) substance is taken to relieve or avoid withdrawal symptoms. The presence of 2–3 of the above symptoms is specified as “Mild” substance use disorder; 4–5 symptoms, “Moderate” substance use disorder; and 6 or more symptoms, “Severe” substance use disorder. Reprinted with permission from the American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington, VA: American Psychiatric Association; 2013 |
The terms tolerance, dependence, and withdrawal may be defined based on clinically apparent physiologic changes as well as more subtle alterations in brain reward neurocircuitry. Tolerance refers to the decreased effect of a substance that develops with continued use, in other words, the dose–response curve shifts to the right as larger doses are needed to produce the same response, as happened in the case of Mr. A. The drug toxicity profile and the drug lethality profile often do not shift in the same way or to the same degree as the psychopharmacologic effect(s) for which the drug is primarily self-administered. Thus, when taking heroin, it is likely that Mr. A experienced adverse effects of constipation and pupillary constriction at a dose insufficient to get “high.” Additionally, since the brain respiratory centers often do not develop tolerance to increased doses of heroin, a lethal overdose is more likely as a person takes higher doses of this drug. The opposite effect, termed sensitization (also called inverse tolerance), refers to a shift of the dose–response curve to the left, so that repeated administrations of a drug result in a greater effect of a given dose, and a lower dose is required to achieve the same effect. Interestingly, tolerance and sensitization to different pharmacologic actions of a drug may occur concurrently. Thus, upon repeated administration of central nervous system (CNS) depressants like alcohol, stimulant effects (e.g., disinhibition) demonstrate sensitization, whereas depressant actions (e.g., sleep) acquire tolerance.
Dependence can be defined only indirectly by (1) tolerance, (2) the emergence of a withdrawal syndrome upon drug discontinuation or administration of a specific antagonist, (3) drug “craving,” or (4) drug-seeking behavior manifested as a result of conditioned stimuli after withdrawal has abated. In Mr. A’s case, his initial symptoms in the emergency department were caused by heroin withdrawal, a manifestation of physical dependence that could be alleviated by any μ-opioid receptor agonist. Physical dependence is sometimes distinguished from psychological dependence, or the continued craving for drug and proclivity to return to out-of-control opioid use even after acute withdrawal symptoms have abated. Physical dependence results from many of the same mechanisms that produce tolerance. As with tolerance, homeostatic set-points are altered to compensate for the presence of the drug. If drug use is discontinued, the altered set-points produce effects opposite to those manifested in the presence of the drug. The altered set-points also activate autonomic nervous system stress responses, which partially explains the benefits of drugs such as clonidine in treating withdrawal. For example, abrupt withdrawal from a CNS depressant involves hyperarousal, whereas withdrawal from a stimulant involves depression and lethargy; discontinuing a drug of either class results in a nonspecific increase in autonomic activity. Psychological dependence involves resetting the reward system of the brain as a result of repeated drug use. Thus, even after drug use has ceased, brain reward mechanisms may be altered so that affective and neuroendocrine disturbances and drug craving persist and the individual is prone to relapse. There is significant neurobiological overlap between “psychological” and “physical” components of dependence, and some have questioned the real utility of such a distinction. In any event, the recently published DSM-5 eschews the term “dependence” because the clinical and pharmacologic meanings of this term can be confusing. The newly adopted diagnostic term is “substance use disorder,” which is meant unequivocally to serve as a clinical construct, whereas dependence retains its pharmacologic meaning only (Box 19-1).
Tolerance and dependence typically coexist, but the presence of either does not necessarily imply pathologic drug use. For example, a patient given an opioid for surgical pain will likely develop tolerance to the drug and require progressively larger doses for analgesia; moreover, should drug administration cease or an opioid antagonist be given, the patient will likely develop a withdrawal syndrome. However, it will likely be possible to taper and eventually eliminate the analgesic once the surgical basis of pain abates. In this case, if the patient does not manifest drug-seeking behavior, a pathologic condition (substance use disorder) is not present despite the presence of tolerance and dependence. This point underlines the vital role of physicians in (1) effectively treating postsurgical pain without providing open-ended opioid prescriptions and (2) directly addressing drug-seeking should it arise.
 MECHANISMS OF TOLERANCE, DEPENDENCE, AND WITHDRAWAL
MECHANISMS OF TOLERANCE, DEPENDENCE, AND WITHDRAWAL
Acquired tolerance results when repeated administration of a drug shifts the dose–response curve of the drug to the right, so that a larger dose of the drug is required to produce the same effect. Innate tolerance refers to preexisting interindividual variations in sensitivity to the drug (i.e., variations that are present before the first administration of the drug). Innate differences in sensitivity can arise from genetic variation of receptors at which the drug acts or differences among individuals in drug absorption, metabolism, or excretion. As with any multifactorial trait, genetic variability is strongly influenced by the environment. An example of innate tolerance is observed with alcohol: those with low innate sensitivity as young adults are at higher risk for alcoholism later in life.
Acquired tolerance includes pharmacokinetic, pharmacodynamic, and learned components. Pharmacokinetic tolerance develops when the capacity to metabolize or excrete the drug increases as a result of drug exposure. Increased metabolism is typically attributable to induction of metabolic enzymes such as the cytochrome P450s (see Chapter 4, Drug Metabolism). In such cases, pharmacokinetic tolerance results in a lower concentration of drug at its site of action for any given dose.
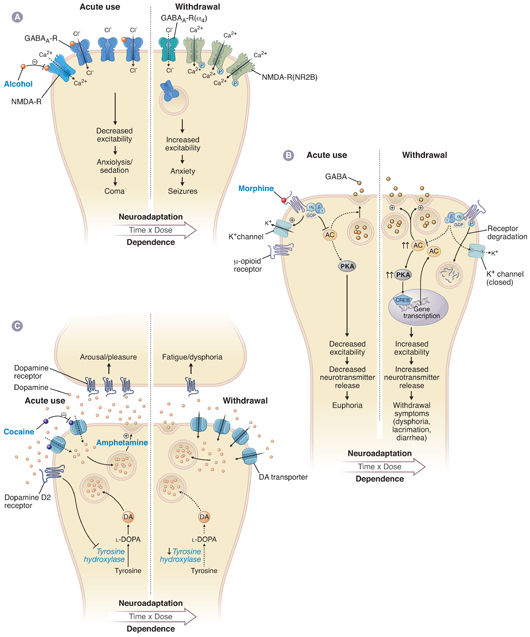
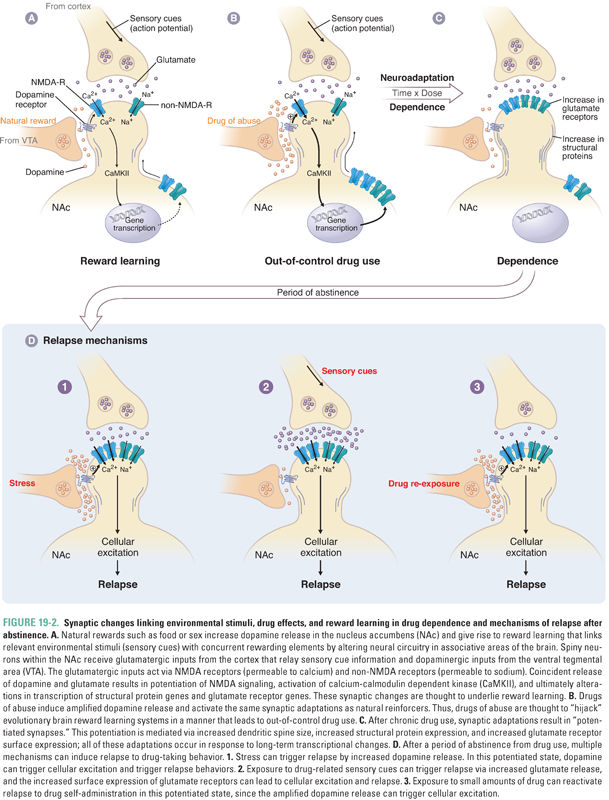
Pharmacodynamic tolerance is caused by neuronal adaptations resulting in reduced response to the same concentration of drug at its site of action in the nervous system. Short-term exposure to a drug can induce neuroadaptive changes in neurotransmitter release and clearance from the synapse, a decrease in the number of neurotransmitter receptors, altered conductance of ion channels, or modified signal transduction (Fig. 19-1). Longer term administration of the drug can cause neuroadaptive changes in the expression of genes relevant to the pharmacologic action of the drug; these changes are closely linked with adaptations in the brain that are thought to be involved in learning and memory formation (Fig. 19-2). Indeed, persistent adaptations to drug use both modify existing synapses and create new synapses, effectively “rewiring” the brain. Such long-lasting molecular and cellular adaptations are likely to explain the cravings and relapses that can occur long after drug use has been discontinued.

Pharmacodynamic tolerance is closely related to another form of tolerance, termed learned tolerance. In behavioral tolerance, a form of learned tolerance, drug use results in compensatory changes in behavior that are not directly related to the pharmacologic action of the drug but rather to accommodation to drug effects through learning acquired while the person is in the intoxicated “state” or in the environment in which the intoxication occurred. Conditioned tolerance occurs when environmental cues associated with exposure to a drug induce preemptive, reflexive compensatory changes, called a conditioned opponent response. This mechanism of conditioning is an unconscious phenomenon but is often the basis for relapse in addicts. For example, seeing paraphernalia associated with use of a drug such as cocaine (which produces tachycardia) may elicit preemptive bradycardia and, thus, craving for the drug.
Dependence is typically associated with tolerance, and it results from mechanisms closely related to those that produce pharmacodynamic and learned tolerance. Substance use disorders are clinical manifestations of dependence and result from the need for the drug to be present in the brain to maintain “near-normal” functioning. If the drug is eliminated from the body so that it no longer occupies its site of action, the adaptations that produced dependence are unmasked and manifested as an acute withdrawal syndrome that lasts until the system re-equilibrates to the absence of drug (days). Subsequently, a protracted withdrawal syndrome, characterized by craving for the drug (i.e., an intense preoccupation with obtaining the drug), may emerge and continue indefinitely (years). Protracted withdrawal is also associated with subtle dysregulation of learning, drives/motivations, reward, and the potential for relapse. This syndrome should be distinguished from premorbid risk factors for addiction that do not resolve with abstinence and from brain injury that is sustained as a result of drug use.
Like tolerance, dependence is associated with changes in cellular signaling pathways (Fig. 19-1). For example, up-regulation of the cAMP pathway by a drug contributes to acute withdrawal upon discontinuation of the drug because up-regulated adenylyl cyclase causes a “supranormal” response in neurons when physiologic levels of neurotransmitter stimulate the cAMP-coupled receptor. Conversely, a drug that produces dependence by decreasing receptor number or receptor sensitivity renders the down-regulated receptors understimulated by physiologic levels of neurotransmitter after drug discontinuation.
The effects of alcohol illustrate that excitatory and inhibitory mechanisms can act in a synergistic fashion on opposing neurotransmitter systems. Acute alcohol intake causes sedation by facilitating the inhibitory activity of GABA at its receptors and inhibiting the excitatory activity of glutamate at its receptors. Over time, the GABA receptors are down-regulated and their subunit structure is modified through a variety of molecular mechanisms, thus decreasing the level of inhibition to counter the sedative effects of alcohol. Simultaneously, the NMDA receptors are up-regulated, also decreasing the level of inhibition due to alcohol. If the alcohol is abruptly removed, the decreased GABAergic inhibition and enhanced glutamatergic excitation result in a state of central nervous system hyperactivity, which causes the signs and symptoms of alcohol withdrawal. The balance between these inhibitory (GABAergic) and excitatory (glutamatergic) pathways may explain the alternating sedation and hyperactivity characteristic of alcohol intoxication and withdrawal, respectively.
Because dependence can occur without tolerance and vice versa, it is clear that learning-related changes, not necessarily due to the pharmacologic actions of a drug, are also involved. In the 1950s, Olds and Milner implanted electrodes in various regions of the rat brain to systematically determine which neuroanatomic areas could reinforce self-stimulation. (Self-stimulation consisted of a short pulse of nondestructive electric current that was delivered in the brain at the site of the electrode upon the animal’s pressing of a lever.) The medial forebrain bundle and ventral tegmental area (VTA) in the midbrain were found to be particularly effective sites. These sites have been termed pleasure centers, or the foci of reward in the brain. A subset of dopaminergic neurons projects directly from the VTA to the nucleus accumbens (NAc) via the medial forebrain bundle. It is believed that these neurons are crucial for the brain reward pathway, which reinforces motivated behavior and facilitates learning and memory via links to the hippocampus, amygdala, and prefrontal cortex. Severing this pathway, or blocking dopamine receptors in the NAc with a dopamine receptor antagonist (such as haloperidol; see Chapter 14, Pharmacology of Dopaminergic Neurotransmission), decreases electrical self-stimulation of the VTA. Moreover, release of dopamine in the NAc can be detected in vivo using the technique of microdialysis, whereby a cannula is inserted into a specific brain region in order to determine the concentrations of neurotransmitters. These measurements show that increases in concentrations of dopamine are associated with drug self-administration by laboratory animals and that dopaminergic synapses in the NAc are active during electrical stimulation of the brain reward pathway, supporting the hypothesis that NAc dopamine is necessary for reward. Drugs capable of causing dependence are readily self-administered by animals directly into the VTA, NAc, or the cortical or subcortical areas that innervate these two areas, often at the cost even of eating food (Fig. 19-3).
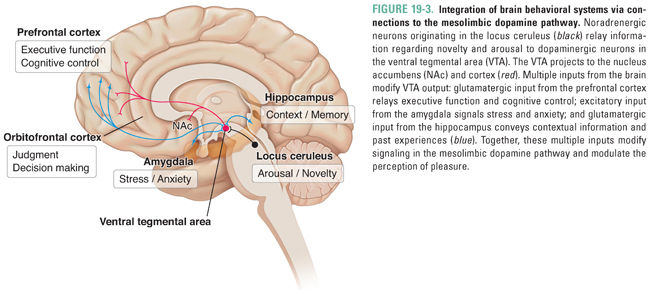
Although the dopaminergic pathway mediates reward, dopamine may also increase the salience of stimuli, alert the organism to the importance of stimuli, and guide motor activity to seek rewarding stimuli. As discussed above, the dopamine pathway is activated by all drugs of abuse. Importantly, behaviors that are necessary for survival of the species (e.g., feeding, reproduction, and exploration) also result in dopamine release in the NAc but to a much smaller degree, suggesting that drugs of abuse may pharmacologically “hijack” the normal evolutionary functions of reward pathways. With repeated experiences via conditioning (i.e., the association of an element of the environment with the reward through rewiring of brain circuits), this dopamine pathway is also activated during anticipation of the reward, as can be demonstrated in humans using functional neuroimaging techniques such as positron emission tomography (PET) and functional magnetic resonance imaging (fMRI) when addicts are exposed to drug-related sensory cues. Although the dopaminergic neurons that link the VTA and the NAc serve as the final common pathway of reward, these neurons receive inputs from a number of brain regions (cortex, hippocampus, thalamus, amygdala, and raphe nuclei) that modify reward and thereby mediate reward-associated learning (Figs. 19-3 and 19-4).
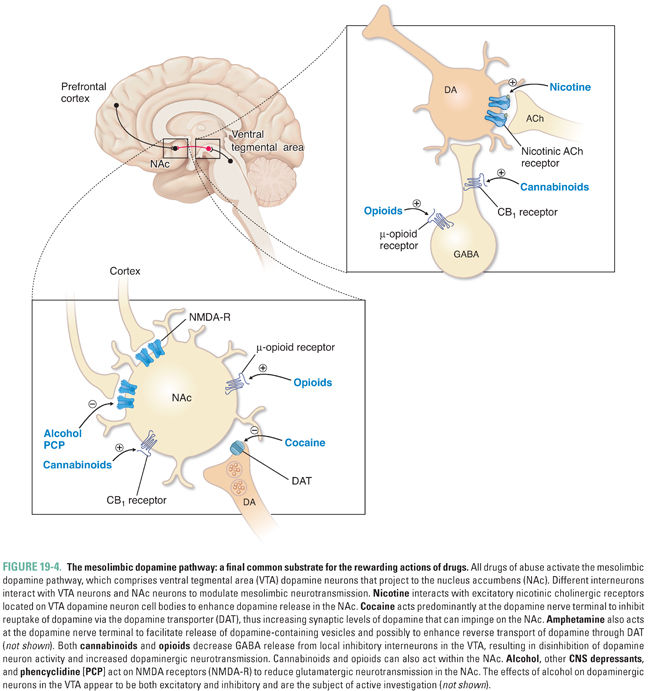
Since withdrawal from certain drugs of abuse can be aversive, avoiding acute withdrawal was for many years thought to be the primary motivation for continued abuse. However, this explanation is not consistent with the observations that the effects of addiction are felt long after the physical symptoms of withdrawal have abated; withdrawal can occur without concomitant drug-seeking, as is often the case after treatment for acute pain; and drugs such as stimulants, hallucinogens, and cannabinoids cause significant dependence without a striking acute withdrawal syndrome. Years after an addict has discontinued use of a substance, he or she can experience intense cravings and, thus, is prone to relapse. The likelihood of relapse is especially strong in situations in which individuals simultaneously encounter both stress and the context in which the drug was previously used. In part, this is due to the interplay between reward and memory circuitry in the brain that, under normal circumstances, assigns emotional value to certain memories. Hence, the motivational underpinnings of drug-seeking are tied to both socioenvironmental stimuli and subjective effects of the drug, each of which can have both rewarding and aversive linkages with previous experiences via learning. This is a more complex explanation than the “simple” avoidance of acute withdrawal.
 MECHANISMS OF SUBSTANCE USE DISORDERS
MECHANISMS OF SUBSTANCE USE DISORDERS
The drug-seeking activity characteristic of substance use disorders results from the interplay of learning, reward mechanisms, and individual propensity toward the development of addiction.
Learning and Development of Substance Use Disorders
Recognition that chronic drug self-administration results in long-lasting changes in the experience of reward has led to our understanding that the relevant neural circuits can never return to their predrug state. The term allostasis describes this enduring, progressively evolving adaptive process in brain reward pathways upon repeated exposure to abused drugs. Allostasis means that the baseline to which the brain returns upon discontinuing drug use can change even after acute withdrawal has abated. (This is in contrast to homeostasis, which is defined as the process whereby a system repeatedly re-equilibrates to the same baseline.) Accordingly, even when the drug is no longer present in the brain, the addict cannot experience positive emotions in the way he or she did prior to beginning drug use (termed anhedonia); the unsuccessful attempt to recapture the previous “near-normal” state fuels drug-seeking. Human and animal studies have found evidence for long-term neuroadaptation in altered neurotransmitter levels (e.g., dopamine and serotonin depletion after chronic alcohol or stimulant use), changes in neurotransmitter receptors, altered signal transduction pathways, changes in gene expression, and altered synaptic configuration and function. Clinically, abstinent patients report not only craving but also dysphoria, sleep disturbances, and increased stress reactivity (e.g., panic attacks), which can last for weeks, months, or years after detoxification.
A common misconception is that addicts are pleasure seekers and that their focus on drugs represents withdrawal from life into irresponsible hedonism. Current thinking about addiction recognizes the heterogeneity of the addictive process. For some individuals, reward factors (positive reinforcement) may predominate, and getting high or feeling euphoric motivates drug use. For others, relief factors (negative reinforcement) predominate, such as drinking to reduce stress or to reduce the dysphoria of protracted withdrawal. A large proportion of addicts self-medicate to reduce distress associated with co-occurring psychiatric and medical disorders. Furthermore, the motivations to use early in the course of substance use disorder may differ substantially from motivations as the illness progresses (Fig. 19-5). As a result of allostasis, positive reinforcement is rare in the later stages of the illness. For example, drinking in one’s teens to relieve shyness may progress to drinking for euphoria and disinhibition. Ultimately, after years of drinking to intoxication, the middle-aged person may drink to prevent withdrawal-associated depression and anxiety, or perhaps to alleviate chronic pain. Drug use in each of these situations is linked via learning to elements of the environment associated with drug use or to memories and emotions, each of which can trigger craving and drug-seeking.
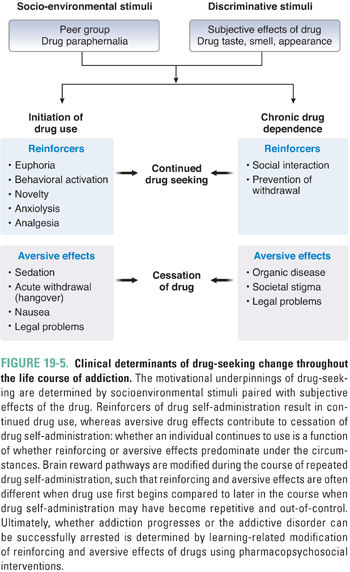
The essence of substance use disorder is drug-seeking behavior, whereby an individual cannot control the urge to obtain and use a psychoactive substance despite recognized negative consequences and at the exclusion of other needs that typically constitute a balanced life. Studies in laboratory animals suggest that drug-seeking behavior is the result of dysfunctional “reward learning” (i.e., the processes that guide the organism to fulfill needs or goals have gone awry). Thus, if the organism initiates an action that results in a goal or “reward” (e.g., self-administration of a psychoactive agent), and if the organism “learns” that its action resulted in the reward, the likelihood of engaging in that behavior is enhanced. For example, if a person uses cocaine for the first time and finds it pleasurable or that it alleviates depressive symptoms from which the individual is suffering, obtaining and using cocaine are reinforced. The intense experience of cocaine, relative to natural rewards such as food and sex, results in a preferential expenditure of energy to obtain cocaine over other rewards. Thus, cocaine has effectively “hijacked” reward-learning systems, biasing future behavior in favor of obtaining cocaine over natural rewards. Reexposure to environmental or affective states that are associated with cocaine use serve as cues to increase drug-seeking behavior. For example, reexposure to drug paraphernalia can induce intense craving, drug-seeking behavior, and relapse in cocaine addicts.
Variables Affecting the Development of Substance Use Disorders
The development of substance use disorder is dependent on the nature of the drug; genetic, acquired, psychological, and social traits of the drug user; and environmental factors.
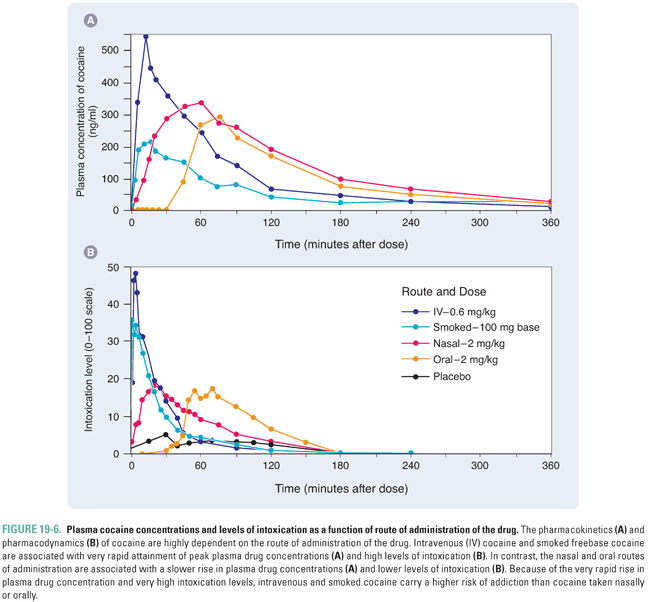
The ability of a drug to activate reward mechanisms is strongly correlated with its ability to cause addiction. Pharmacokinetic properties of the drug can significantly influence its effects on the brain. In general, the more rapid the rise in drug concentrations at the target neurons, the greater the activation of reward pathways. For example, many drugs of abuse are highly lipophilic and can easily permeate the blood–brain barrier. In addition, direct injection or rapid absorption of drug through a large surface area (e.g., through the lungs via smoking) is more highly reinforcing than slower absorption through the intestinal or nasal mucosa. Furthermore, rapidly eliminated drugs are more addictive than slowly eliminated drugs, since slow clearance of a drug maintains the drug concentration at the site of action for a longer duration, diminishing the severity of acute withdrawal.
The importance of pharmacokinetic effects is demonstrated by the potential for abuse of various forms of cocaine (Fig. 19-6), and these principles are readily applicable to other drugs of abuse. The use of coca leaves as a chew or in teas is widely practiced among people living in the Andean mountains: this has a relatively low potential for addiction because of the slow rate of rise and low peak concentration of drug attained by absorption through the buccal or intestinal mucosa. The rapid absorption of extracted cocaine through the nasal mucosa is substantially more reinforcing. The most reinforcing and addictive forms of cocaine are intravenous injections and inhalation of smoked freebase (crack cocaine), both of which result in a very rapid rise in plasma concentration and a high peak concentration of drug.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





