28
Pharmacologic Management of Parkinsonism & Other Movement Disorders
CASE STUDY
A 64-year-old architect complains of left-hand tremor at rest, which interferes with his writing and drawing. He also notes a stooped posture, a tendency to drag his left leg when walking, and slight unsteadiness on turning. He remains independent in all activities of daily living. Examination reveals hypomimia (flat facies), hypophonia, a rest tremor of the left arm and leg, mild rigidity in all limbs, and impaired rapid alternating movements in the left limbs. Neurologic and general examinations are otherwise normal. What is the likely diagnosis and prognosis? He is started on a dopamine agonist, which he seems to tolerate well, and the dose is gradually built up to the therapeutic range. About a year later, he and his wife return for follow-up. It now becomes apparent that he is spending large sums of money, which he cannot afford, on gambling and refuses to stop, despite his wife’s entreaties. To what is his condition due and how should it be managed?
Several types of abnormal movement are recognized. Tremor consists of a rhythmic oscillatory movement around a joint and is best characterized by its relation to activity. Tremor at rest is characteristic of parkinsonism, when it is often associated with rigidity and an impairment of voluntary activity. Tremor may occur during maintenance of sustained posture (postural tremor) or during movement (intention tremor). A conspicuous postural tremor is the cardinal feature of benign essential or familial tremor. Intention tremor occurs in patients with a lesion of the brainstem or cerebellum, especially when the superior cerebellar peduncle is involved; it may also occur as a manifestation of toxicity from alcohol or certain other drugs.
Chorea consists of irregular, unpredictable, involuntary muscle jerks that occur in different parts of the body and impair voluntary activity. In some instances, the proximal muscles of the limbs are most severely affected, and because the abnormal movements are then particularly violent, the term ballismus has been used to describe them. Chorea may be hereditary or may occur as a complication of a number of general medical disorders and of therapy with certain drugs.
Abnormal movements may be slow and writhing in character (athetosis) and in some instances are so sustained that they are more properly regarded as abnormal postures (dystonia). Athetosis or dystonia may occur with perinatal brain damage, with focal or generalized cerebral lesions, as an acute complication of certain drugs, as an accompaniment of diverse neurologic disorders, or as an isolated inherited phenomenon of uncertain cause known as idiopathic torsion dystonia or dystonia musculorum deformans. Various genetic loci have been reported depending on the age of onset, mode of inheritance, and response to dopaminergic therapy. The physiologic basis is uncertain, and treatment is unsatisfactory.
Tics are sudden coordinated abnormal movements that tend to occur repetitively, particularly about the face and head, especially in children, and can be suppressed voluntarily for short periods of time. Common tics include repetitive sniffing or shoulder shrugging. Tics may be single or multiple and transient or chronic. Gilles de la Tourette’s syndrome is characterized by chronic multiple tics; its pharmacologic management is discussed at the end of this chapter.
Many of the movement disorders have been attributed to disturbances of the basal ganglia. The basic circuitry of the basal ganglia involves three interacting neuronal loops that include the cortex and thalamus as well as the basal ganglia themselves (Figure 28–1). However, the precise function of these anatomic structures is not yet fully understood, and it is not possible to relate individual symptoms to involvement at specific sites.
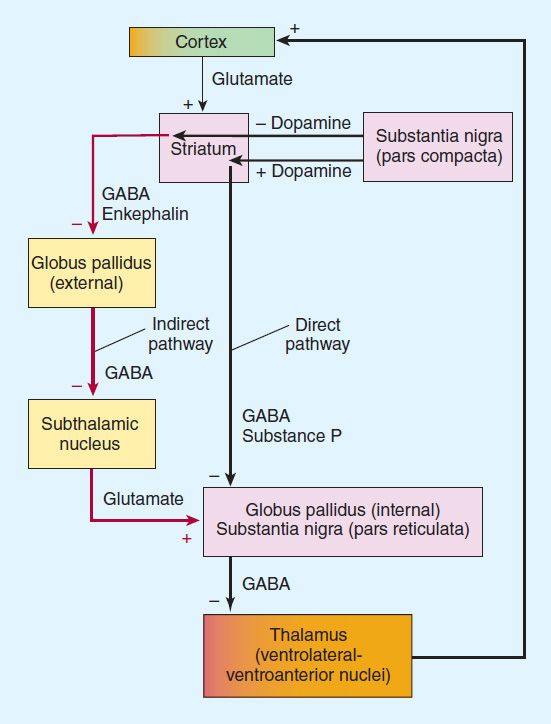
FIGURE 28–1 Functional circuitry between the cortex, basal ganglia, and thalamus. The major neurotransmitters are indicated. In Parkinson’s disease, there is degeneration of the pars compacta of the substantia nigra, leading to overactivity in the indirect pathway (red) and increased glutamatergic activity by the subthalamic nucleus.
 PARKINSONISM
PARKINSONISM
Parkinsonism is characterized by a combination of rigidity, bradykinesia, tremor, and postural instability that can occur for a variety of reasons but is usually idiopathic (Parkinson’s disease or paralysis agitans). Cognitive decline occurs in many patients as the disease advances. Other non-motor symptoms—which are receiving increasing attention—are affective disorders (anxiety or depression), personality changes, abnormalities of autonomic function (sphincter or sexual functions; choking; sweating abnormalities; and disturbances of blood pressure regulation), sleep disorders, and sensory complaints or pain. The disease is generally progressive, leading to increasing disability unless effective treatment is provided.
Pathogenesis
The pathogenesis of parkinsonism seems to relate to a combination of impaired degradation of proteins, intracellular protein accumulation and aggregation, oxidative stress, mitochondrial damage, inflammatory cascades, and apoptosis. Studies in twins suggest that genetic factors are important, especially when the disease occurs in patients under age 50. Recognized genetic abnormalities account for 10–15% of cases. Mutations of the α-synuclein gene at 4q21 or duplication and triplication of the normal synuclein gene are associated with Parkinson’s disease, which is now widely recognized as a synucleinopathy. Mutations of the leucine-rich repeat kinase 2 (LRRK2) gene at 12cen, and the UCHL1 gene may also cause autosomal dominant parkinsonism. Mutations in the parkin gene (6q25.2–q27) cause early onset, autosomal recessive, familial parkinsonism, or sporadic juvenile-onset parkinsonism. Several other genes or chromosomal regions have been associated with familial forms of the disease. Environmental or endogenous toxins may also be important in the etiology of the disease. Epidemiologic studies reveal that cigarette smoking, coffee, anti-inflammatory drug use, and high serum uric acid levels are protective, whereas the incidence of the disease is increased in those working in teaching, health care, or farming, and in those with lead or manganese exposure or with vitamin D deficiency.
The finding of Lewy bodies (intracellular inclusion bodies containing α-synuclein) in fetal dopaminergic cells transplanted into the brain of parkinsonian patients some years previously has provided some support for suggestions that Parkinson’s disease may represent a prion disease.
Staining for α-synuclein has revealed that pathology is more widespread than previously recognized, developing initially in the olfactory nucleus and lower brainstem (stage 1 of Braak scale), then the higher brainstem (stage 2), the substantia nigra (stage 3), the mesocortex and thalamus (stage 4), and finally the entire neocortex (stage 5). The motor features of Parkinson’s disease develop at stage 3 on the Braak scale.
The normally high concentration of dopamine in the basal ganglia of the brain is reduced in parkinsonism, and pharmacologic attempts to restore dopaminergic activity with levodopa and dopamine agonists alleviate many of the motor features of the disorder. An alternative but complementary approach has been to restore the normal balance of cholinergic and dopaminergic influences on the basal ganglia with antimuscarinic drugs. The pathophysiologic basis for these therapies is that in idiopathic parkinsonism, there is a loss of dopaminergic neurons in the substantia nigra that normally inhibit the output of GABAergic cells in the corpus striatum (Figure 28–2). Drugs that induce parkinsonian syndromes either are dopamine receptor antagonists (eg, antipsychotic agents; see Chapter 29) or lead to the destruction of the dopaminergic nigrostriatal neurons (eg, 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine [MPTP]; see below). Various other neurotransmitters, such as norepinephrine, are also depleted in the brain in parkinsonism, but these deficiencies are of uncertain clinical relevance.
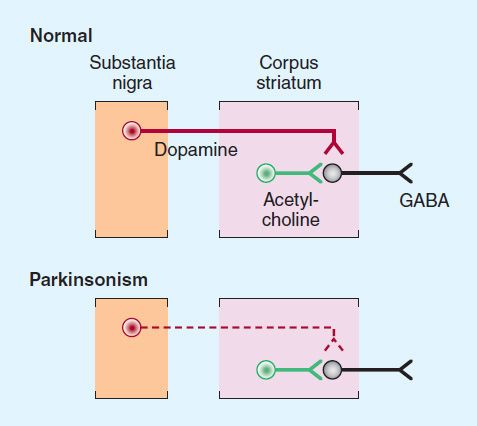
FIGURE 28–2 Schematic representation of the sequence of neurons involved in parkinsonism. Top: Dopaminergic neurons (red) originating in the substantia nigra normally inhibit the GABAergic output from the striatum, whereas cholinergic neurons (green) exert an excitatory effect. Bottom: In parkinsonism, there is a selective loss of dopaminergic neurons (dashed, red).
LEVODOPA
Dopamine does not cross the blood-brain barrier and if given into the peripheral circulation has no therapeutic effect in parkinsonism. However, (−)-3-(3,4-dihydroxyphenyl)-L-alanine (levodopa), the immediate metabolic precursor of dopamine, does enter the brain (via an L-amino acid transporter, LAT), where it is decarboxylated to dopamine (see Figure 6–5). Several noncatecholamine dopamine receptor agonists have also been developed and may lead to clinical benefit, as discussed in the text that follows.
Dopamine receptors are discussed in detail in Chapters 21 and 29. They exist in five subtypes. D1 and D5 receptors are classified as the D1 receptor family based on genetic and biochemical factors; D2, D3, and D4 are grouped as belonging to the D2 receptor family. Dopamine receptors of the D1 type are located in the pars compacta of the substantia nigra and presynaptically on striatal axons coming from cortical neurons and from dopaminergic cells in the substantia nigra. The D2 receptors are located postsynaptically on striatal neurons and presynaptically on axons in the substantia nigra belonging to neurons in the basal ganglia. The benefits of dopaminergic antiparkinsonism drugs appear to depend mostly on stimulation of the D2 receptors. However, D1- receptor stimulation may also be required for maximal benefit and one of the newer drugs is D3 selective. Dopamine agonist or partial agonist ergot derivatives such as lergotrile and bromocriptine that are powerful stimulators of the D2 receptors have antiparkinsonism properties, whereas certain dopamine blockers that are selective D2 antagonists can induce parkinsonism.
Chemistry
Dopa is the amino acid precursor of dopamine and norepinephrine (discussed in Chapter 6). Its structure is shown in Figure 28–3. Levodopa is the levorotatory stereoisomer of dopa.
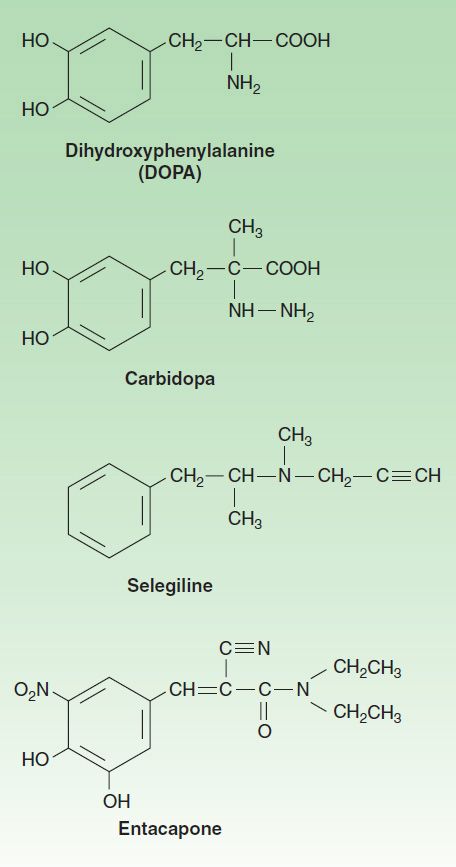
FIGURE 28–3 Some drugs used in the treatment of parkinsonism.
Pharmacokinetics
Levodopa is rapidly absorbed from the small intestine, but its absorption depends on the rate of gastric emptying and the pH of the gastric contents. Ingestion of food delays the appearance of levodopa in the plasma. Moreover, certain amino acids from ingested food can compete with the drug for absorption from the gut and for transport from the blood to the brain. Plasma concentrations usually peak between 1 and 2 hours after an oral dose, and the plasma half-life is usually between 1 and 3 hours, although it varies considerably among individuals. About two thirds of the dose appears in the urine as metabolites within 8 hours of an oral dose, the main metabolic products being 3-methoxy-4-hydroxyphenyl acetic acid (homovanillic acid, HVA) and dihydroxyphenylacetic acid (DOPAC). Unfortunately, only about 1–3% of administered levodopa actually enters the brain unaltered; the remainder is metabolized extracerebrally, predominantly by decarboxylation to dopamine, which does not penetrate the blood-brain barrier. Accordingly, levodopa must be given in large amounts when used alone. However, when given in combination with a dopa decarboxylase inhibitor that does not penetrate the blood-brain barrier, the peripheral metabolism of levodopa is reduced, plasma levels of levodopa are higher, plasma half-life is longer, and more dopa is available for entry into the brain (Figure 28–4). Indeed, concomitant administration of a peripheral dopa decarboxylase inhibitor such as carbidopa may reduce the daily requirements of levodopa by approximately 75%.
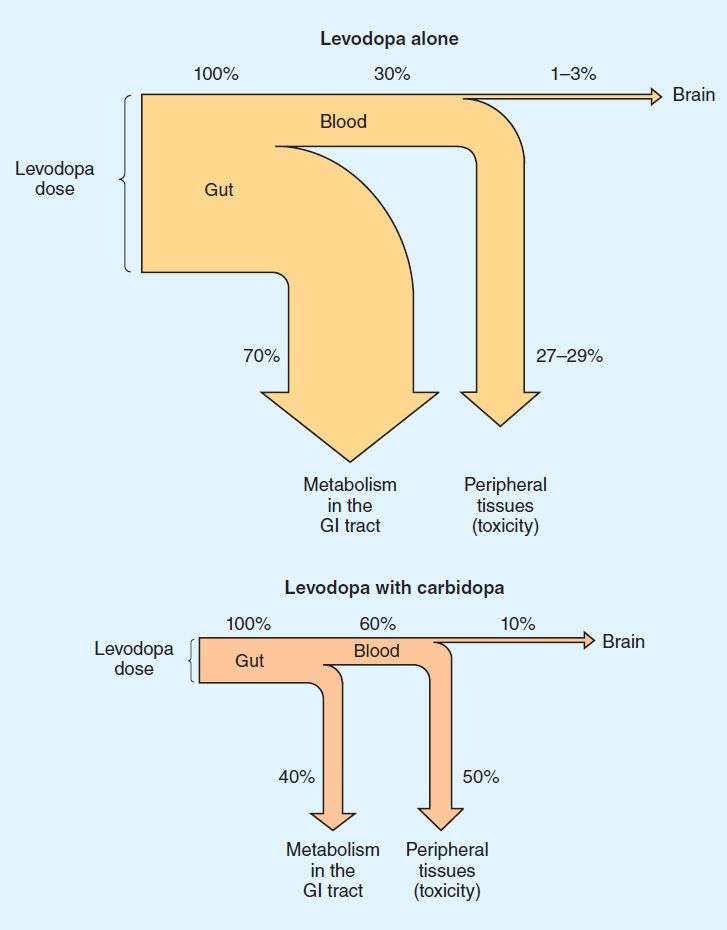
FIGURE 28–4 Fate of orally administered levodopa and the effect of carbidopa, estimated from animal data. The width of each pathway indicates the absolute amount of the drug at each site, whereas the percentages shown denote the relative proportion of the administered dose. The benefits of co-administration of carbidopa include reduction of the amount of levodopa required for benefit and of the absolute amount diverted to peripheral tissues and an increase in the fraction of the dose that reaches the brain. GI, gastrointestinal. (Data from Nutt JG, Fellman JH: Pharmacokinetics of levodopa. Clin Neuropharmacol 1984;7:35.)
Clinical Use
The best results of levodopa treatment are obtained in the first few years of treatment. This is sometimes because the daily dose of levodopa must be reduced over time to avoid adverse effects at doses that were well tolerated initially. Some patients become less responsive to levodopa, perhaps because of loss of dopaminergic nigrostriatal nerve terminals or some pathologic process directly involving striatal dopamine receptors. For such reasons, the benefits of levodopa treatment often begin to diminish after about 3 or 4 years of therapy, regardless of the initial therapeutic response. Although levodopa therapy does not stop the progression of parkinsonism, its early initiation lowers the mortality rate. However, long-term therapy may lead to a number of problems in management such as the on-off phenomenon discussed below. The most appropriate time to introduce levodopa therapy must therefore be determined individually.
When levodopa is used, it is generally given in combination with carbidopa (Figure 28–3), a peripheral dopa decarboxylase inhibitor, which reduces peripheral conversion to dopamine. Combination treatment is started with a small dose, eg, carbidopa 25 mg, levodopa 100 mg three times daily, and gradually increased. It should be taken 30–60 minutes before meals. Most patients ultimately require carbidopa 25 mg, levodopa 250 mg three or four times daily. It is generally preferable to keep treatment with this agent at a low level (eg, carbidopa-levodopa 25/100 three times daily) when possible, and if necessary, to add a dopamine agonist, to reduce the risk of development of response fluctuations. A controlled-release formulation of carbidopa-levodopa is available and may be helpful in patients with established response fluctuations or as a means of reducing dosing frequency. A formulation of carbidopa-levodopa (10/100, 25/100, 25/250) that disintegrates in the mouth and is swallowed with the saliva (Parcopa) is available commercially and is best taken about 1 hour before meals. The combination (Stalevo) of levodopa, carbidopa, and a catechol-O-methyltransferase (COMT) inhibitor (entacapone) is discussed in a later section. Finally, therapy by infusion of levodopa-carbidopa into the duodenum or upper jejunum appears to be safe and is superior to a number of oral combination therapies in patients with response fluctuations. This is an approved therapy in Europe and Canada for treating advanced levodopa-responsive parkinsonism but is not yet available in the USA. A permanent access tube is inserted via a percutaneous endoscopic gastrostomy in patients who have responded well to carbidopa-levodopa gel administered through a nasoduodenal tube. A morning bolus (100–300 mg of levodopa) is delivered via a portable infusion pump, followed by a continuous maintenance dose (40–120 mg/h), with supplemental bolus doses as required.
Levodopa can ameliorate many of the clinical motor features of parkinsonism, but it is particularly effective in relieving bradykinesia and any disabilities resulting from it. When it is first introduced, about one third of patients respond very well and one third less well. Most of the remainder either are unable to tolerate the medication or simply do not respond at all, especially if they do not have classic Parkinson’s disease.
Adverse Effects
A. Gastrointestinal Effects
When levodopa is given without a peripheral decarboxylase inhibitor, anorexia and nausea and vomiting occur in about 80% of patients. These adverse effects can be minimized by taking the drug in divided doses, with or immediately after meals, and by increasing the total daily dose very slowly. Antacids taken 30–60 minutes before levodopa may also be beneficial. The vomiting has been attributed to stimulation of the chemoreceptor trigger zone located in the brainstem but outside the blood-brain barrier. Fortunately, tolerance to this emetic effect develops in many patients. Antiemetics such as phenothiazines should be avoided because they reduce the antiparkinsonism effects of levodopa and may exacerbate the disease.
When levodopa is given in combination with carbidopa, adverse gastrointestinal effects are much less frequent and troublesome, occurring in less than 20% of cases, so that patients can tolerate proportionately higher doses.
B. Cardiovascular Effects
A variety of cardiac arrhythmias have been described in patients receiving levodopa, including tachycardia, ventricular extrasystoles and, rarely, atrial fibrillation. This effect has been attributed to increased catecholamine formation peripherally. The incidence of such arrhythmias is low, even in the presence of established cardiac disease, and may be reduced still further if the levodopa is taken in combination with a peripheral decarboxylase inhibitor.
Postural hypotension is common, but often asymptomatic, and tends to diminish with continuing treatment. Hypertension may also occur, especially in the presence of nonselective monoamine oxidase inhibitors or sympathomimetics or when massive doses of levodopa are being taken.
C. Behavioral Effects
A wide variety of adverse mental effects have been reported, including depression, anxiety, agitation, insomnia, somnolence, confusion, delusions, hallucinations, nightmares, euphoria, and other changes in mood or personality. Such adverse effects are more common in patients taking levodopa in combination with a decarboxylase inhibitor rather than levodopa alone, presumably because higher levels are reached in the brain. They may be precipitated by intercurrent illness or operation. It may be necessary to reduce or withdraw the medication. Several atypical antipsychotic agents that have low affinity for dopamine D2 receptors (clozapine, olanzapine, quetiapine, and risperidone; see Chapter 29) are now available and may be particularly helpful in counteracting such behavioral complications.
D. Dyskinesias and Response Fluctuations
Dyskinesias occur in up to 80% of patients receiving levodopa therapy for more than 10 years. The character of dopa dyskinesias varies between patients but tends to remain constant in individual patients. Choreoathetosis of the face and distal extremities is the most common presentation. The development of dyskinesias is dose related, but there is considerable individual variation in the dose required to produce them. A number of compounds are being studied as possible antidyskinetic agents, but these studies are still at an early stage.
Certain fluctuations in clinical response to levodopa occur with increasing frequency as treatment continues. In some patients, these fluctuations relate to the timing of levodopa intake (wearing-off reactions or end-of-dose akinesia). In other instances, fluctuations in clinical state are unrelated to the timing of doses (on-off phenomenon). In the on-off phenomenon, off-periods of marked akinesia alternate over the course of a few hours with on-periods of improved mobility but often marked dyskinesia. For patients with severe off-periods who are unresponsive to other measures, subcutaneously injected apomorphine may provide temporary benefit. The phenomenon is most likely to occur in patients who responded well to treatment initially. The exact mechanism is unknown. The dyskinesias may relate to an unequal distribution of striatal dopamine. Dopaminergic denervation plus chronic pulsatile stimulation of dopamine receptors with levodopa has been associated with development of dyskinesias. A lower incidence of dyskinesias occurs when levodopa is administered continuously (eg, intraduodenally or intrajejunally), and with drug delivery systems that enable a more continuous delivery of dopaminergic medication.
E. Miscellaneous Adverse Effects
Mydriasis may occur and may precipitate an attack of acute glaucoma in some patients. Other reported but rare adverse effects include various blood dyscrasias; a positive Coombs’ test with evidence of hemolysis; hot flushes; aggravation or precipitation of gout; abnormalities of smell or taste; brownish discoloration of saliva, urine, or vaginal secretions; priapism; and mild—usually transient—elevations of blood urea nitrogen and of serum transaminases, alkaline phosphatase, and bilirubin.
Drug Holidays
A drug holiday (discontinuance of the drug for 3–21 days) may temporarily improve responsiveness to levodopa and alleviate some of its adverse effects but is usually of little help in the management of the on-off phenomenon. Furthermore, a drug holiday carries the risks of aspiration pneumonia, venous thrombosis, pulmonary embolism, and depression resulting from the immobility accompanying severe parkinsonism. For these reasons and because of the temporary nature of any benefit, drug holidays are not recommended.
Drug Interactions
Pharmacologic doses of pyridoxine (vitamin B6) enhance the extracerebral metabolism of levodopa and may therefore prevent its therapeutic effect unless a peripheral decarboxylase inhibitor is also taken. Levodopa should not be given to patients taking monoamine oxidase A inhibitors or within 2 weeks of their discontinuance because such a combination can lead to hypertensive crises.
Contraindications
Levodopa should not be given to psychotic patients because it may exacerbate the mental disturbance. It is also contraindicated in patients with angle-closure glaucoma, but those with chronic open-angle glaucoma may be given levodopa if intraocular pressure is well controlled and can be monitored. It is best given combined with carbidopa to patients with cardiac disease; even so, the risk of cardiac dysrhythmia is slight. Patients with active peptic ulcer must also be managed carefully, since gastrointestinal bleeding has occasionally occurred with levodopa. Because levodopa is a precursor of skin melanin and conceivably may activate malignant melanoma, it should be used with particular care in patients with a history of melanoma or with suspicious undiagnosed skin lesions; such patients should be monitored by a dermatologist regularly.
DOPAMINE RECEPTOR AGONISTS
Drugs acting directly on postsynaptic dopamine receptors may have a beneficial effect in addition to that of levodopa (Figure 28–5). Unlike levodopa, they do not require enzymatic conversion to an active metabolite, act directly on the postsynaptic dopamine receptors, have no potentially toxic metabolites, and do not compete with other substances for active transport into the blood and across the blood-brain barrier. Moreover, drugs selectively affecting certain (but not all) dopamine receptors may have more limited adverse effects than levodopa. A number of dopamine agonists have antiparkinsonism activity. The older dopamine agonists (bromocriptine and pergolide) are ergot (ergoline) derivatives (see Chapter 16), and are rarely—if ever—used to treat parkinsonism. Their side effects are of more concern than those of the newer agents (pramipexole and ropinirole). However, various impulse control disorders (such as gambling disorders, compulsive shopping, or hypersexuality) may be enhanced by activation of D2 or D3 dopamine receptors in the mesocorticolimbic system in certain individuals. These may occur with one dopamine agonist and not another. They are not dose-dependent, but in some patients a dose reduction may ameliorate them. The prevalence of impulse control disorders varies in different reports but may be as high as 15–25% in parkinsonian patients treated with these agents. Risk factors include a history of drug use or a family history of gambling disorders.
There is no evidence that one agonist is superior to another; individual patients, however, may respond to one but not another of these agents. Moreover, their duration of action varies and is lengthened by extended-release preparations. Apomorphine is a potent dopamine agonist but is discussed separately in a later section in this chapter because it is used primarily as a rescue drug for patients with disabling response fluctuations to levodopa.
Dopamine agonists have an important role as first-line therapy for Parkinson’s disease, and their use is associated with a lower incidence of the response fluctuations and dyskinesias that occur with long-term levodopa therapy. In consequence, dopaminergic therapy is often initiated with a dopamine agonist. Alternatively, a low dose of carbidopa plus levodopa (eg, Sinemet-25/100 three times daily) is introduced, and a dopamine agonist is then added. In either case, the dose of the dopamine agonist is built up gradually depending on response and tolerance. Dopamine agonists may also be given to patients with parkinsonism who are taking levodopa and who have end-of-dose akinesia or on-off phenomenon or are becoming resistant to treatment with levodopa. In such circumstances, it is generally necessary to lower the dose of levodopa to prevent intolerable adverse effects. The response to a dopamine agonist is generally disappointing in patients who have never responded to levodopa.
Bromocriptine
Bromocriptine is a D2 agonist; its structure is shown in Table 16–6. This drug has been widely used to treat Parkinson’s disease in the past but is now rarely used for this purpose, having been superseded by the newer dopamine agonists. The usual daily dose of bromocriptine for parkinsonism varies between 7.5 and 30 mg. To minimize adverse effects, the dose is built up slowly over 2 or 3 months depending on response or the development of adverse reactions.
Pergolide
Pergolide, another ergot derivative, directly stimulates both D1 and D2 receptors. It too has been widely used for parkinsonism but is no longer available in the United States because its use has been associated with the development of valvular heart disease. It is nevertheless still used in certain countries.
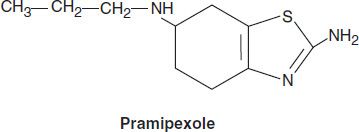
Pramipexole
Pramipexole is not an ergot derivative, but it has preferential affinity for the D3 family of receptors. It is effective as monotherapy for mild parkinsonism and is also helpful in patients with advanced disease, permitting the dose of levodopa to be reduced and smoothing out response fluctuations. Pramipexole may ameliorate affective symptoms. A possible neuroprotective effect has been suggested by its ability to scavenge hydrogen peroxide and enhance neurotrophic activity in mesencephalic dopaminergic cell cultures.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


