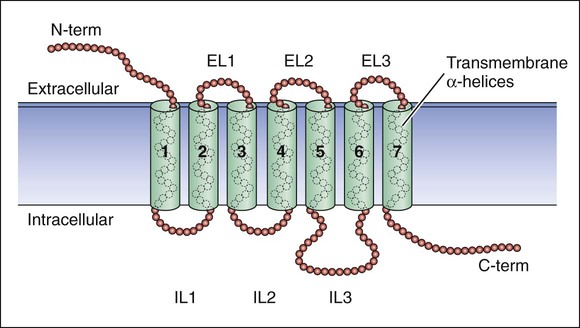
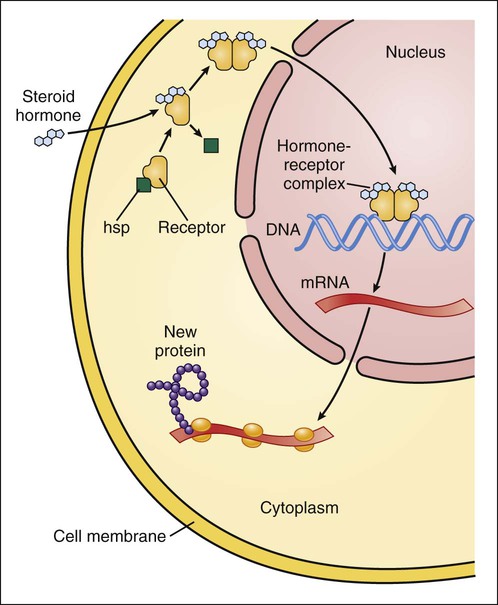
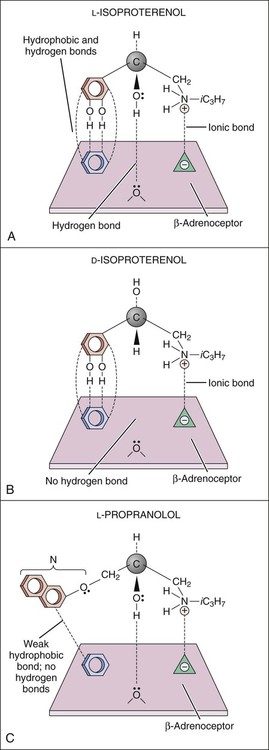
Drugs produce their effects by interacting with specific cell molecules called receptors. By far, most ligands (drugs or neurotransmitters) bind to protein molecules, although some agents act directly on DNA or membrane lipids (Table 3-1). TABLE 3-1 The largest family of receptors for pharmaceutical agents is G protein–coupled receptors (GPCRs). These membrane-spanning proteins consist of four extracellular, seven transmembrane, and four intracellular domains (Fig. 3-1). Extracellular domains and, to some extent, transmembrane regions determine ligand binding and selectivity. Intracellular loops, especially the third one, mediate the receptor interaction with its effector molecule, a guanine nucleotide binding protein (G protein). Steroid hormone receptors are intracellular proteins that translocate to the nucleus on ligand (steroid) binding. In the nucleus, the steroid-receptor complex alters the transcription rate of specific genes (Fig. 3-2). DNA is also a receptor site for ligands that bind directly to nucleic acids, most notably the antineoplastic agents. Other macromolecules that serve as receptors include the various lipids and phospholipids that make up the membrane. Some of the effects of general anesthetics and alcohol are caused by interaction with membrane lipids. To initiate a cellular response, a drug must first bind to a receptor. In most cases, drugs bind to their receptor by forming hydrogen, ionic, or hydrophobic (van der Waals) bonds with a receptor site (Fig. 3-3). These weak bonds are reversible and enable the drug to dissociate from the receptor as the tissue concentration of the drug declines. The binding of drugs to receptors often exhibits stereospecificity, so that only one of the stereoisomers (enantiomers) will form a three-point attachment with the receptor. In a few cases, drugs form relatively permanent covalent bonds with a specific receptor. This occurs, for example, with antineoplastic drugs that bind to DNA and with drugs that irreversibly inhibit the enzyme cholinesterase. Signal transduction describes the pathway from ligand binding to conformational changes in the receptor, receptor interaction with an effector molecule (if present), and other downstream molecules called second messengers. This cascade of receptor-mediated biochemical events ultimately leads to a physiologic effect (Table 3-2). TABLE 3-2 Examples of Receptors and Signal Transduction Pathways
Pharmacodynamics
Nature of Drug Receptors
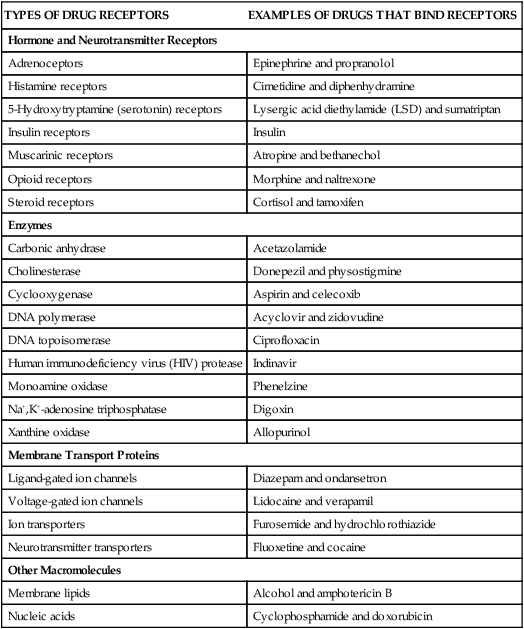
TYPES OF DRUG RECEPTORS
EXAMPLES OF DRUGS THAT BIND RECEPTORS
Hormone and Neurotransmitter Receptors
Adrenoceptors
Epinephrine and propranolol
Histamine receptors
Cimetidine and diphenhydramine
5-Hydroxytryptamine (serotonin) receptors
Lysergic acid diethylamide (LSD) and sumatriptan
Insulin receptors
Insulin
Muscarinic receptors
Atropine and bethanechol
Opioid receptors
Morphine and naltrexone
Steroid receptors
Cortisol and tamoxifen
Enzymes
Carbonic anhydrase
Acetazolamide
Cholinesterase
Donepezil and physostigmine
Cyclooxygenase
Aspirin and celecoxib
DNA polymerase
Acyclovir and zidovudine
DNA topoisomerase
Ciprofloxacin
Human immunodeficiency virus (HIV) protease
Indinavir
Monoamine oxidase
Phenelzine
Na+,K+-adenosine triphosphatase
Digoxin
Xanthine oxidase
Allopurinol
Membrane Transport Proteins
Ligand-gated ion channels
Diazepam and ondansetron
Voltage-gated ion channels
Lidocaine and verapamil
Ion transporters
Furosemide and hydrochlorothiazide
Neurotransmitter transporters
Fluoxetine and cocaine
Other Macromolecules
Membrane lipids
Alcohol and amphotericin B
Nucleic acids
Cyclophosphamide and doxorubicin
Types of Drug Receptors
Drug-Receptor Interactions
Receptor Binding and Affinity
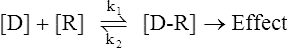
Signal Transduction
FAMILY AND TYPE OF RECEPTOR
MECHANISM OF SIGNAL TRANSDUCTION
EXAMPLE OF EFFECT IN TISSUE OR CELL
G Protein–Coupled Receptors
α1-Adrenoceptor
Activation of phospholipase C
Vasoconstriction
α2-Adrenoceptor
Inhibition of adenylyl cyclase
Release of norepinephrine decreased
β-Adrenoceptor
Stimulation of adenylyl cyclase
Heart rate increased
Muscarinic receptor
Activation of phospholipase C
Glandular secretion increased
Ligand-Gated Ion Channels
GABAA receptors
Chloride ion flux
Hyperpolarization of neuron
Nicotinic receptors
Sodium ion flux
Skeletal muscle contraction
Membrane-Bound Enzymes
Atrial natriuretic factor receptors
Stimulation of guanylyl cyclase
Sodium excretion increased
Insulin receptors
Activation of tyrosine kinase
Glucose uptake stimulated
Nuclear Receptors
Steroid receptors
Activation of gene transcription
Reduced cytokine production
Thyroid hormone receptors
Activation of gene transcription
Oxygen consumption increased ![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Pharmacodynamics
Only gold members can continue reading. Log In or Register to continue