Noninfectious Causes
Foreign material that is usually recognizable on microscopic examination can elicit a granulomatous reaction on the peritoneum. Starch granules from surgical gloves (7), douche fluid, and lubricants typically incite granulomatous and fibrosing peritonitis; in occasional cases, the inflammatory reaction may be of tuberculoid type with caseous necrosis (8). The periodic acid-Schiff (PAS)–positive starch granules exhibit a characteristic Maltese cross configuration under polarized light. Talc was once an important cause of granulomatous and fibrosing peritonitis because of its use as a lubricant on surgical gloves (9). Talc-induced peritonitis has also been described in drug abusers (10). Other iatrogenic causes of granulomatous peritonitis include cellulose and cotton fibers from surgical pads and drapes (11), hemostatic materials (12,13), and oily substances such as hysterosalpingographic contrast medium, mineral oil, and paraffin (14–16). The last three substances are associated with a lipogranulomatous reaction. A foreign body reaction to Surgicel that resulted in a pelvic mass that mimicked recurrent ovarian cancer has also been described (12).
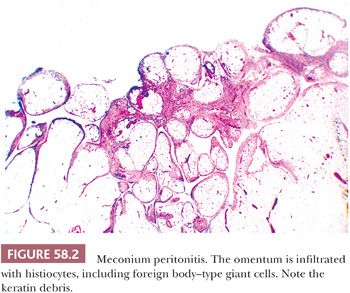
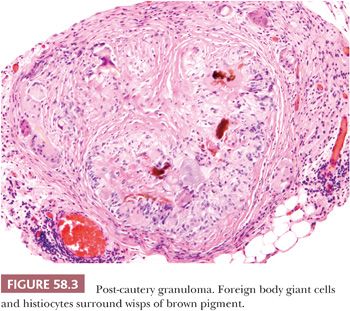
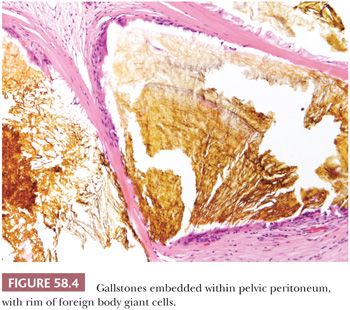
Escaped bowel contents, including vegetable matter, food-derived starch (17), and barium sulfate (18), can produce a peritoneal foreign body reaction. Sebaceous material and keratin from ruptured dermoid cysts typically evoke an intense granulomatous, lipogranulomatous, and fibrosing peritoneal inflammatory reaction that may mimic a neoplasm at operation (19). Use of an endoscopic retrieval bag in the laparoscopic removal of dermoid cysts can reduce the risk of intraoperative spillage and subsequent granulomatous peritonitis (20). Granulomatous inflammation to keratin exfoliated from uterine and ovarian endometrioid carcinomas with squamous differentiation is discussed later (see “Tumor-like Lesions”). Leakage of amniotic fluid antenatally or its spillage at cesarean section can result in contamination of the peritoneal cavity by vernix caseosa (lanugo hair, squamous cells, keratin) and meconium (bile and pancreatic and intestinal secretions), resulting in a granulomatous peritonitis (Fig. 58.2) (21,22). Meconium peritonitis, which is caused by bowel perforation in utero, can also be a problem in newborn infants; in boys, the process may involve the tunica vaginalis and may result in a tumor-like scrotal mass (23). Calcification, rather than granulomatous inflammation, may dominate the microscopic picture. Chronic bile peritonitis may be associated with granulomatous inflammation and fibrosis; cholesterol crystals and bile pigment may be identifiable within giant cells (24). Granulomatous peritonitis has also been described secondary to Crohn disease (25), sarcoidosis (26), and Whipple disease (27). Post-cautery granulomas can also involve the peritoneum, recognizable by characteristic strands of brown or black pigment (Fig. 58.3). Necrotizing peritoneal granulomas have been described following diathermy ablation of endometriosis (28). Necrotic pseudoxanthomatous nodules of endometriosis can resemble necrotic granulomas (29) and are described on page 2688. Spillage of bile or gallstones during laparoscopic cholecystectomy may also be a source of peritoneal granulomatous inflammation (30–33). Subsequent implantation of gallstones on peritoneal surfaces including the ovaries (“ovarian cholelithiasis”) may result in abdominal pain, be associated with foreign body granulomatous reaction and fibrosis, or act as a nidus for infection (31). At laparoscopy, the reddish-brown, pigmented lesions can mimic endometriosis (30). On microscopy, yellowish-brown bile pigment and cholesterol crystals can be identified, often with a rim of foreign body giant cells (Fig. 58.4). Positive Fouchet staining for bile, with knowledge of the history of a previous cholecystectomy, will facilitate the correct diagnosis.
NONGRANULOMATOUS HISTIOCYTIC LESIONS
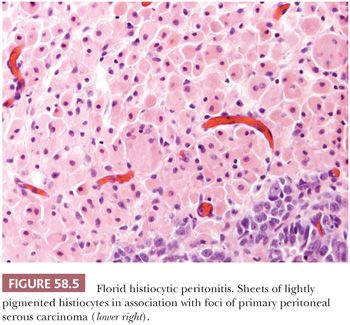
The peritoneum is occasionally involved by histiocytic infiltrates rather than discrete granulomas. Ceroid-rich histiocytes involving the peritoneum and omentum may be secondary to endometriosis (29) or in association with a peritoneal decidual reaction (34). Florid histiocytic peritonitis may also rarely occur in association with peritoneal or ovarian cancers including serous carcinoma (Fig. 58.5)(35). The differential diagnosis of histiocytic peritonitis includes peritoneal melanosis (see “Tumor-like Lesions”). Aggregates of nonpigmented histiocytes, sometimes admixed with hyperplastic mesothelial cells, may appear as small, grossly visible nodules at operation (36). We are aware of a histiocytic peritoneal nodule from a patient with a granulosa cell tumor in which the histiocytes were initially misinterpreted microscopically as metastatic granulosa cell tumor. Ruffolo and Suster (37) described a diffuse histiocytic proliferation of the pelvic peritoneum that was associated with endocervicosis. The histiocytes in histiocytic nodules can be distinguished from any admixed mesothelial cells or from pure proliferations of hyperplastic mesothelial cells by the differing immunoprofiles of the two cell types (35,36).
Mucicarminophilic histiocytosis is characterized by histiocytes that contain polyvinylpyrrolidone (PVP), a substance that has been used as a blood substitute (38). These cells can be found in many sites both within and outside the female genital tract, including the pelvic lymph nodes and the omentum. The histiocytes have vacuolated basophilic to lavender cytoplasm and an eccentric nucleus, an appearance that may suggest the diagnosis of signet ring cell adenocarcinoma. The histiocytes are mucicarminophilic, but, in contrast to neoplastic signet ring cells, they are PAS negative; other stains are also helpful in the differential diagnosis (38). Mucicarminophilic peritoneal histiocytes potentially mimicking a signet ring cell adenocarcinoma can also be encountered as a reaction to oxidized regenerated cellulose, a hemostatic agent (39).
PERITONEAL FIBROSIS
Reactive peritoneal fibrosis, often accompanied by fibrous adhesions, is a common sequela of peritoneal inflammation and a frequent complication of a surgical procedure (40). In occasional cases, differentiating between markedly reactive peritoneal fibrosis and a desmoplastic mesothelioma may be difficult, particularly in a small biopsy specimen. Features favoring a diagnosis of mesothelioma include nuclear atypia, necrosis, the presence of organized patterns of collagen deposition (fascicular, storiform), and infiltration of normal tissues (41).
Localized hyaline plaques are a common incidental finding on the splenic capsule, and they are probably related to splenic congestion (42). Fibrous thickening of the peritoneum has been described in patients with hepatic cirrhosis and ascites (43). Hyalinized silicotic nodules involving the peritoneum have been described in patients with silicosis (44).
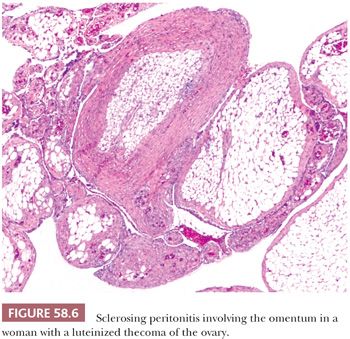
Sclerosing peritonitis is a rare, potentially fatal disorder characterized by a fibrous peritoneal thickening. Concato (45) described pearly white thickening of the peritoneum, either in the form of discrete plaques or continuous sheets involving the hepatic, splenic, and diaphragmatic peritoneum. More recent reports have described a similar lesion that encases the small bowel (“abdominal cocoon”), causing bowel obstruction. It occurs in an idiopathic form, typically affecting adolescent girls (46), or it may be secondary to practolol therapy (47), chronic ambulatory peritoneal dialysis (48), sarcoidosis (49), the use of a peritoneovenous (LeVeen) or ventriculoperitoneal shunts (50,51), lupus erythematosus, or ovarian fibrothecomatous tumors or tumor-like lesions (Fig. 58.6) (52,53). In the last situation, the peritoneal lesions may be misinterpreted intraoperatively or even on microscopic examination as metastatic tumor. Sclerosing peritonitis should be distinguished from peritoneal encapsulation, a congenital malformation in which an accessory peritoneal membrane encases loops of small bowel in a saclike structure (54).
RARE TYPES OF PERITONITIS
Rarely, eosinophilic peritonitis is seen in cases of eosinophilic gastroenteritis and the hypereosinophilic syndrome (55). Isolated cases of eosinophilic ascites have been associated with childhood atopy, peritoneal dialysis, vasculitis, lymphoma or metastatic carcinoma, and ruptured hydatid cysts (55). Rare cases of peritonitis may be secondary to peritoneal involvement by collagen vascular diseases, including systemic lupus erythematosus and Degos disease.
TUMOR-LIKE LESIONS
MESOTHELIAL HYPERPLASIA
Hyperplasia of mesothelial cells is a common response to inflammation and chronic effusions (Figs. 58.7 through 58.10). The lesions may be noted at operation as solitary or multiple small nodules, but, more commonly, they are incidental findings on microscopic examination (56–60). Mesothelial hyperplasia often involves the adnexal areas in cases of chronic salpingitis and endometriosis (59), and it is occasionally encountered, particularly in the omentum, in association with ovarian tumors (57). The process can also occur on the surface of the ovary or within the walls of endometriotic cysts or cystic borderline epithelial tumor; in such cases, it may be misinterpreted as an invasive tumor (Fig. 58.10) (57). Mesothelial hyperplasia may be confined to a hernia sac; in such cases, it may be due to trauma or incarceration (58), or, rarely, it can involve a laparoscopic site. Hyperplastic mesothelial cells are occasionally an incidental microscopic finding within the pelvic and intra-abdominal lymph nodes, and, in such cases, they are usually associated with and are probably secondary to mesothelial hyperplasia of the peritoneum (Fig. 58.11) (60).
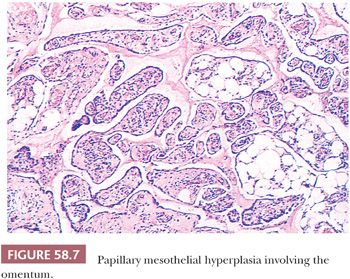
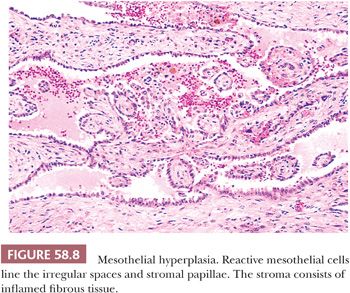
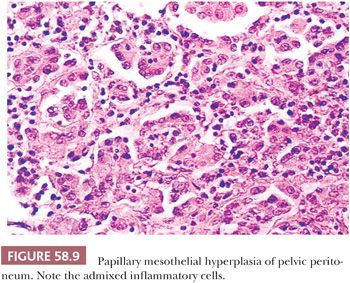
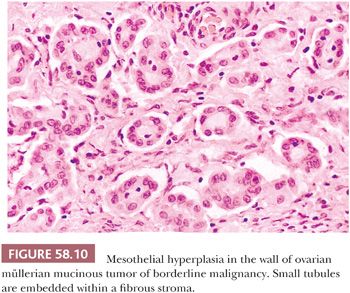
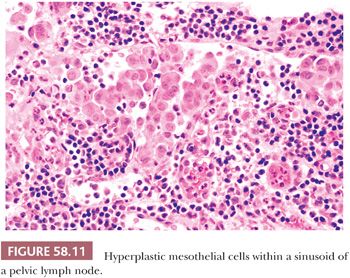
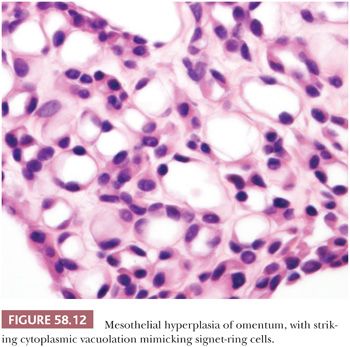
In florid examples, solid, trabecular, tubular, papillary, or tubulopapillary patterns (Figs. 58.7 through 58.10) and limited degrees of extension of the mesothelial cells into the underlying tissues or entrapment by reactive fibrous tissue may be seen. The cells are often focally disposed in linear, sometimes parallel, thin layers, separated by fibrin or fibrous tissue. When lymph nodes are involved, hyperplastic mesothelial cells typically occupy subcapsular and intranodal sinusoids (Fig. 58.11) (60). Hyperplastic mesothelial cells may have cytoplasmic vacuoles containing acid mucin (predominantly hyaluronic acid) or, less commonly, may exhibit marked cytoplasmic clearing mimicking signet ring cells (Fig. 58.12) (41). On occasion, reactive mesothelial cells may have abundant eosinophilic cytoplasm and resemble decidual cells (61). Mild to moderate nuclear pleomorphism, mitotic figures, and occasional multinucleated cells may be seen. Psammoma bodies are encountered in occasional cases, and, rarely, eosinophilic strap-shaped cells resembling rhabdomyoblasts have been described (58).
Differential Diagnosis
The major differential diagnosis of mesothelial hyperplasia is that of peritoneal malignant mesothelioma (PMM). The presence of grossly visible nodules, necrosis, conspicuous large cytoplasmic vacuoles, severe nuclear pleomorphism, and invasion favor PMM over mesothelial hyperplasia (41,62,63). Some of these features, however, such as severe nuclear atypia, are often not present, or they may only be present focally within a PMM. Although epithelial membrane antigen (EMA) and p53 have both been proposed as immunohistochemical (IHC) markers of mesothelial malignancy, Churg et al. (62) concluded that, in their experience, these stains are not helpful in individual cases. Attanoos et al. (63) have found that, in contrast to the cells of malignant mesotheliomas, reactive mesothelial cells are typically immunoreactive for desmin. In some cases, the distinction between a hyperplastic and malignant mesothelial lesion may be difficult or impossible, particularly in a biopsy specimen. An apparently benign, otherwise typical mesothelial proliferation may occasionally precede the appearance of a malignant mesothelioma (41). Positivity for GLUT-1 and oncofetal protein IMP3 are in favor of mesothelioma, as reactive mesothelial cells are usually negative for these markers (64–66). Other investigators have found GLUT-1 to be highly specific for mesothelioma but with relatively low sensitivity (65). Deletion of p16, detected by fluorescence in situ hybridization, has been identified in 59% and 0% of malignant mesothelioma and mesothelial hyperplasias, respectively (64).
The differential diagnosis of mesothelial hyperplasia also includes metastatic adenocarcinomas and serous borderline tumors (SBTs) and carcinomas of primary peritoneal or ovarian origin. Similarly, intranodal hyperplastic mesothelial cells should not be confused with nodal involvement by metastatic SBT or a primary intranodal SBT arising from intranodal endosalpingiosis (see “Endosalpingiosis” under “Peritoneal Serous Lesions”). The findings of columnar cells with or without cilia, intracellular or extracellular neutral mucin, and numerous psammoma bodies favor a serous tumor, although psammoma bodies may occur on a reactive basis in cases of mesothelial hyperplasia. IHC markers for epithelial differentiation (see “Malignant Mesothelioma”) may also be of value in the differential diagnosis. Immunoreactivity for desmin, if it is strong and diffuse (63), or a CD44-positive/E-cadherin–negative phonotype favors reactive mesothelial cells versus those that are carcinomatous in cytologic cell block preparations (67).
PERITONEAL INCLUSION CYSTS
Peritoneal inclusion cysts typically occur in the peritoneal cavity of women in the reproductive age group (68–77). Rarely, they may occur in males, and similar lesions have been described in the pleural cavity (56). Some are incidental findings at laparotomy in the form of single or multiple small, thin-walled, translucent, unilocular cysts that may be attached or that may lie free in the peritoneal cavity. Occasionally, they may involve the round ligament (potentially simulating an inguinal hernia) (71), the spleen (72), the liver (73), or kidney (74). The cysts have a smooth lining; contents that vary from yellow and watery to gelatinous; and a single layer of typically flattened, benign-appearing mesothelial cells, typically posing no diagnostic problems. Although most of these unilocular mesothelial cysts are probably reactive in origin, some of those located in the mesocolon, mesentery of the small intestine, retroperitoneum, kidney, and spleen may be developmental.
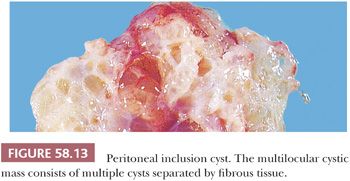
Multilocular cystic masses that may measure up to 20 cm in diameter (Fig. 58.13) and that are lined by mesothelial cells have been referred to as multilocular peritoneal inclusion cysts (MPICs) (68,70), benign cystic mesotheliomas (69), inflammatory cysts of the peritoneum, or postoperative peritoneal cysts. MPICs are usually associated with clinical manifestations, most commonly lower abdominal pain, a palpable mass, or both. They are typically attached to pelvic organs, and they may simulate a cystic ovarian tumor on clinical examination, at laparotomy, or even on pathologic examination; the upper abdominal cavity, the retroperitoneum, or a hernia sac may also be involved. Unlike the smaller unilocular cysts, the septa and walls of MPICs may contain abundant fibrous tissue. Their contents may resemble those of the unilocular cysts, or they may be serosanguineous or bloody.
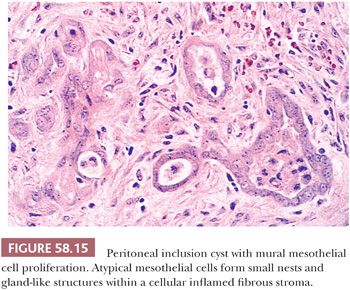
On microscopic examination, MPICs are typically lined by a single layer of flat to cuboidal, occasionally hobnail-shaped mesothelial cells with generally bland nuclear features, although the presence of some reactive atypia is not infrequent (Fig. 58.14A). The lining cells occasionally form small papillae and cribriform patterns or undergo squamous metaplasia (Fig. 58.14B) (69,70). In some cases, mural proliferations of typical or atypical mesothelial cells arranged singly, as gland-like structures or nests (Fig. 58.15), or in adenomatoid tumor-like patterns may be encountered, creating an infiltrative appearance that should not be confused with a malignant tumor (68–70). Occasional vacuolated mesothelial cells in the stroma may simulate signet ring cells (70). Intracellular and extracellular hyaline bodies have been encountered in rare cases (75). The septa typically consist of a loose, fibrovascular connective tissue with a sparse inflammatory infiltrate that may occasionally include foamy histiocytes. In some cases, numerous acute and chronic inflammatory cells, abundant fibrin, broad bands of granulation and fibrous tissue, and evidence of recent and remote hemorrhage are found in the cyst walls.
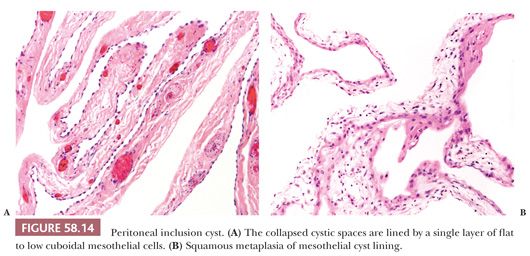
A history of a prior abdominal operation, pelvic inflammatory disease, endometriosis, or combinations thereof was present in 84% of patients in one series (70), suggesting a role for inflammation in the pathogenesis of the cysts. An inflammatory pathogenesis is also supported by the occurrence of cases in which the dividing line between florid adhesions associated with inflammation and an MPIC may be difficult. With rare exceptions, no association with asbestos exposure has been demonstrated. A hormonal basis for some MPICs has been suggested by their regression with the use of long-acting gonadotropin-releasing hormone agonists (76) or tamoxifen (77). Follow-up examinations have not disclosed a malignant behavior in cases that we consider MPICs, but, in as many as half of the cases, the lesions have recurred one or more times from months to many years postoperatively, likely as a result of newly formed postoperative adhesions (70). We thus prefer the designation multilocular peritoneal inclusion cyst to that of “benign cystic mesothelioma” until convincing evidence is found for their neoplastic nature.
Differential Diagnosis
MPICs are most often confused with multilocular cystic lymphangiomas. In contrast to MPICs, the latter typically occur in children (more frequently in boys) and they are usually extrapelvic, usually being localized to the mesentery of the small intestine, omentum, mesocolon, or retroperitoneum. Their contents may be chylous, and histologic examination often reveals mural lymphoid aggregates and smooth muscle, which are rare findings in MPICs. In problematic cases, IHC stains may be useful to distinguish between endothelial and mesothelial cells. Another lesion in the differential diagnosis of MPICs is the rare multicystic adenomatoid tumor (78). In contrast to MPICs, the latter typically involve the myometrium, contain foci of typical adenomatoid tumor, and lack prominent numbers of inflammatory cells.
CALCIFYING FIBROUS PSEUDOTUMOR
This rare lesion is usually an incidental finding on the visceral peritoneum of the small bowel, stomach, or mesentery of adults (79); it can be misinterpreted intraoperatively as metastatic carcinoma. The process usually forms a well circumscribed solid mass (usually <4 cm in diameter), often with a gritty sectioned surface. Microscopic examination reveals dense hyalinized collagen that often appears as concentric whorls, with occasional fibroblasts, lymphocytes, plasma cells, and dystrophic and psammomatous calcification. The spindle cells are typically CD34 positive and anaplastic lymphoma kinase (ALK) negative, the latter in contrast to positive ALK immunoreactivity characteristic of inflammatory myofibroblastic tumor. Calcifying fibrous pseudotumor was initially thought to be reactive, but similarly named lesions that arise in soft tissue appear to be neoplastic, and evidence suggests that these entities may be potentially recurrent.
REACTIVE NODULAR FIBROUS PSEUDOTUMOR
Yantiss et al. (80) have applied this term to lesions that involved the gastrointestinal tract or mesentery in adults. They were single or multiple and ranged up to 6 cm. Some infiltrated the bowel wall, but all had a benign course. Microscopic examination revealed low to moderately cellular proliferation of fibroblasts, collagen, and sparse mononuclear inflammatory cells. The cells were variably immunoreactive for vimentin, CD117, actins (muscle-specific actin, smooth muscle actin), and desmin but not CD34 or ALK-1.
SPLENOSIS
Splenosis, which results from the implantation of splenic tissue, is typically an incidental finding at laparotomy months to years after splenectomy for traumatic rupture of the spleen (81). A few to innumerable red-blue peritoneal nodules that range from punctate to 7 cm in diameter are scattered widely throughout the abdominal, and less commonly the pelvic, cavity. The intraoperative appearance may mimic endometriosis, benign or malignant vascular tumors, or metastatic cancer.
TROPHOBLASTIC IMPLANTS
Implants of trophoblast on the pelvic or omental peritoneum can complicate the operative treatment of tubal pregnancy (82). The implants are more likely to occur in cases managed by laparoscopy (vs. laparotomy) and are more likely to arise after salpingotomy (vs. salpingectomy). The clinical presentation in such cases includes an initial decline in the serum human chorionic gonadotropin (hCG) due to removal of the ectopic pregnancy, followed by a rising level; abdominal pain; and, in some cases, intra-abdominal hemorrhage. In some cases, multiple red-black peritoneal nodules have been found at laparotomy. Microscopic examination of the implants reveals viable trophoblastic tissue that may include chorionic villi.
Some peritoneal lesions are composed exclusively or predominantly of intermediate trophoblast, resembling placental site nodules (83). Some of these may represent implants following the treatment of a tubal pregnancy or the residue of a peritoneal implantation site.
MELANOSIS
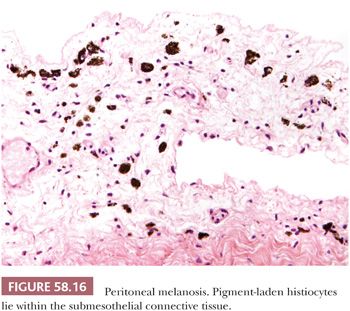
The reported cases of peritoneal melanosis have all been associated with ovarian dermoid cysts; in three cases, the cysts had ruptured preoperatively (84). At laparotomy, focal or diffuse, tan to black, peritoneal staining or similarly pigmented tumor-like nodules are encountered within the pelvis, in the omentum, and, in some cases, within the contents and linings of the dermoid cysts. On histologic examination, the pigmented lesions consist of pigment-laden histiocytes within a fibrous stroma (Fig. 58.16). Most of the dermoid cysts contained prominent areas of gastric mucosa. Although the pigment has been suggested to be melanin, Jaworski et al. (84) found a mixture of iron, but not hemosiderin, and other substances (lipid, carbohydrate, protein) in their case, and they suggested that the acid produced by the gastric mucosa in the dermoid cysts led to peptic ulceration and hemorrhage. They postulated that the pigment is derived from the breakdown of heme within an acid and lipid environment. Ultrastructural detection of intrahistiocytic melanosomes has been described in a case of peritoneal melanosis associated with ovarian mucinous cystadenoma and colonic adenocarcinoma (85). Peritoneal melanosis is easily distinguished from metastatic malignant melanoma by the bland nuclear features and the mitotic inactivity of the pigmented histiocytes. Notably, co-existence of these two lesions has been reported (86).
PERITONEAL KERATIN GRANULOMAS
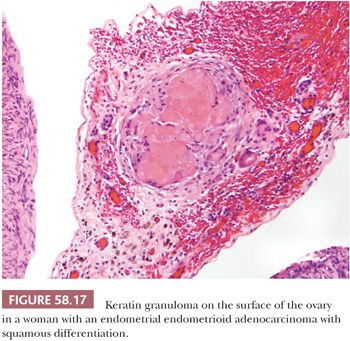
Peritoneal granulomas in response to implants of keratin derived from neoplasms of the female reproductive tract may be confused with metastatic tumor (87). The tumors are most commonly endometrioid carcinomas with squamous differentiation originating in the endometrium or ovary or, rarely, squamous cell carcinomas of the cervix, or atypical polypoid adenomyomas of the uterus. The granulomas consist of laminated deposits of keratin, sometimes with ghost squamous cells, that are surrounded by foreign body giant cells and fibrous tissue (Fig. 58.17). Follow-up data on these patients suggest that the granulomas have no prognostic significance, although they should be thoroughly sampled by the gynecologist and should be carefully examined microscopically by the pathologist to exclude viable tumor. The differential diagnosis includes peritoneal granulomas in response to keratin derived from other sources, as discussed earlier in “Granulomatous Peritonitis.”
INFARCTED APPENDIX EPIPLOICA
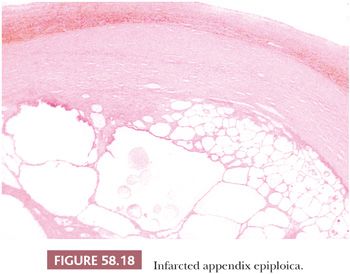
Appendices epiploicae may undergo torsion and infarction (88). Subsequent calcification can result in a hard, tumor-like mass that may be found either attached or loose in the peritoneal cavity. In the late stages, these structures are typically composed of layers of hyalinized connective tissue surrounding a central necrotic and calcified zone in which infarcted adipose tissue is usually recognizable (Fig. 58.18).
CARTILAGINOUS METAPLASIA
Rare cases of cartilaginous metaplasia of the peritoneum have been reported as incidental intraoperative findings in adult women who had undergone previous abdominal or pelvic surgery (89,90). A single lesion was present in two cases (1.6 and 2 cm); in the other case, the lesions were multiple and they ranged from 2 to 7 mm. The nodules consisted of mature hyaline cartilage.
MESOTHELIAL NEOPLASMS
ADENOMATOID TUMOR
This benign tumor of mesothelial origin rarely arises from the extragenital peritoneum, such as the omentum or mesentery (91,92), but it is much more commonly encountered within the male and female genital tracts. It is thus discussed in the chapters dealing with these subjects.
WELL-DIFFERENTIATED PAPILLARY MESOTHELIOMA
Well-differentiated papillary mesotheliomas (WDPMs) of the peritoneum (93–96) usually occur in women who are of reproductive age but occasionally after the menopause. Rare cases have occurred in men. They are usually an incidental finding, but rare cases have been associated with abdominal pain or ascites. Occasional patients have been exposed to asbestos (95), but this may be an incidental association.
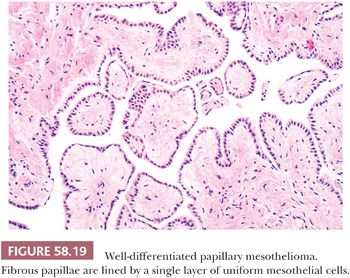
WDPMs are solitary or multiple, gray to white, firm, papillary or nodular lesions measuring less than 2 cm in diameter. The omental and pelvic, including ovarian, peritoneum is typically involved; several examples have also been encountered on the gastric, intestinal, or mesenteric peritoneum (93,94). Multiple lesions should be well sampled in order to exclude a malignant mesothelioma, as some of the latter can focally resemble a WDPM microscopically (see “Malignant Mesothelioma”). Microscopic examination reveals fibrous papillae that vary from slim to broad and that are covered by a single layer of flattened to cuboidal mesothelial cells (Fig. 58.19) with occasional basal vacuoles; the nuclear features are bland, and mitotic figures are rare or absent. Less commonly, the mesothelial cells are arranged in a tubulopapillary pattern, branching cords, or solid sheets (93). The stroma may be extensively fibrotic or rarely myxoid, and psammoma bodies are encountered in occasional cases.
Most WDPMs exhibit benign or indolent behavior; some tumors have persisted for many years (93,95). In a recent study, only 1 of 26 patients experienced a recurrence, in the form of WDPM, 46.5 months after the initial diagnosis (96). In rare cases, the tumors have been aggressive (56,95), or they have “progressed” to malignant mesothelioma, although how thoroughly such tumors had been initially sampled to exclude a malignant mesothelioma ab initio is not clear. Loss of heterozygosity of the neurofibromatosis type 2 gene has been identified as an early molecular alteration in a WDPM associated with malignant transformation (97). In some WDPMs with a fatal outcome, adjuvant therapy might have contributed to the patient’s death (93). In a study of localized and diffuse peritoneal mesotheliomas in women (94), all of the diffuse peritoneal mesotheliomas (i.e., more than one site involved) had at least focally malignant histologic features, whereas localized lesions (i.e., only one site involved) had uniformly benign histologic features. Only tumors in the localized group had a predictably benign clinical course. Accordingly, the diagnosis of WDPM should be reserved for well-sampled tumors that have diffusely bland nuclear features.
MALIGNANT MESOTHELIOMA
Clinical Features
Malignant mesotheliomas of the peritoneal cavity (PMMs) are much less common than their pleural counterparts, accounting for 10% to 20% of all malignant mesotheliomas (94,98–100). They are uncommon in women (98) in whom most malignant papillary neoplasms of the peritoneum are extraovarian papillary serous carcinomas (see “Lesions of the Secondary Müllerian System”). The male-to-female ratio is about 2:1, and the patients are usually middle aged or older; occasional PMMs, however, occur in young adults and children (101–104). The patients typically present with nonspecific manifestations, including abdominal discomfort and distention, digestive disturbances, and weight loss. Some patients have had an elevated CA125 (104). Ascites is present in the majority of cases, and cytologic examination of the ascitic fluid may be diagnostic of PMM in some cases. The diagnosis, however, usually requires laparotomy or laparoscopy and biopsy. PMMs may rarely present as a localized mass, such as within a hernia or hydrocele sac; as a retroperitoneal, hepatic, umbilical, intestinal, or pelvic (including ovarian) tumor (94,105–108); or as cervical or inguinal lymphadenopathy (109). Rare cases have presented as a localized inflammatory lesion that intraoperatively mimicked acute cholecystitis or appendicitis or as an incarcerated umbilical hernia with no grossly apparent tumor (110).
Most male patients have a history of asbestos exposure, but this association is uncommon in females. Other potential etiologic factors include chronic inflammation, organic chemicals, nonasbestos mineral fibers, radiation, and genetic factors (111–113). The majority of reported patients with PMMs, most of whom were male, have survived less than 2 years after diagnosis, although rare long-term survivals have been reported (114,115). Similarly, in some studies of PMMs in women, most of the patients died of disease, or they were alive with disease on follow-up (94). In contrast, a third study of PMMs in women (98) found that a sizable minority (40%) of the tumors had an indolent course; the histologic features of the tumors were not predictive of their behavior. Deciduoid and biphasic subtypes of PMM (see the following section “Pathologic Features”) appear to have a more aggressive behavior than typical PMMs (116). Favorable prognostic factors include an age less than 60 years, low nuclear grade, low mitotic count, minimal residual disease after cytoreduction, and lack of deep invasion (115–117). Rare tumors that are localized may also have a better prognosis. Some IHC markers (see the following discussion) have been shown to be of prognostic use, as low WT1 expression (≤25% positive tumor cells) has been identified as an unfavorable prognostic marker (117). Furthermore, loss of p16 expression has been shown to correlate with homozygous co-deletion of CDKN2A-MTAP and poorer survival (118). Current treatment modalities using cytoreductive surgery and hyperthermic intraperitoneal chemotherapy have been associated with some improvement in long-term survival rates (119,120).
Pathologic Features
At laparotomy, the visceral and parietal peritoneum is usually diffusely thickened or extensively involved by nodules and plaques, although as noted earlier, rare PMMs may be localized (108). The viscera are often encased by tumor and they may be invaded, although local invasion and metastases to the lymph nodes, liver, lungs, and pleura are less frequent than they are in association with carcinomas showing comparable degrees of peritoneal involvement. Significant degrees of invasion or metastatic involvement of abdominal viscera may, however, be encountered at autopsy. In occasional cases, the intraoperative appearance of the process may not be overtly malignant, and it may mimic an inflammatory or reactive process, as noted earlier (110).
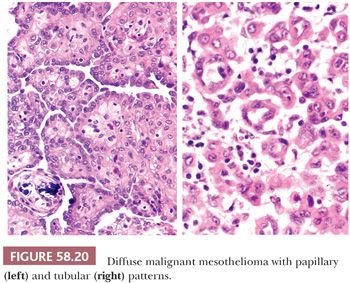
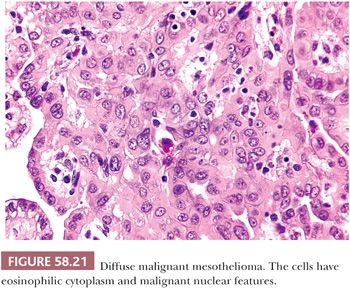
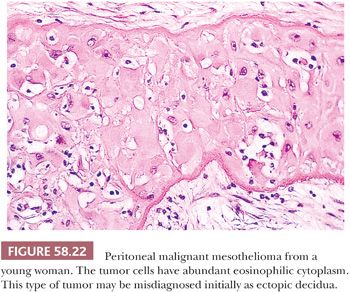
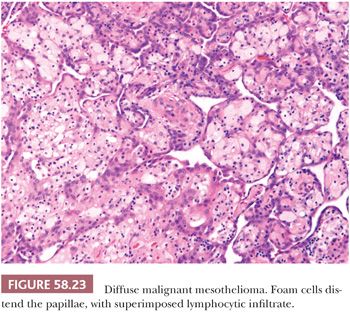
On microscopic examination (Figs. 58.20 and 58.21), most tumors are of epithelial type, with solid, tubular, and papillary proliferations, alone or in combination, typically composed of cuboidal cells with eosinophilic cytoplasm and mild to moderately atypical nuclei. Mitotic figures are usually inconspicuous. Biphasic and purely sarcomatoid tumors occur but are less common than in the pleura; in one biphasic tumor, the sarcomatous component contained rhabdoid cells (105). Rare PMMs are characterized by an exclusively sheetlike pattern composed of polygonal cells with abundant glassy cytoplasm, the so-called deciduoid mesotheliomas (Fig. 58.22) (101,102). These tumors appear to be more common in the peritoneum than in the pleura, and they often affect adolescent or young adult females. It has recently been found that a proportion of deciduoid mesotheliomas are characterized by marked cellular pleomorphism and high mitotic rate, and that these features define a high-grade subgroup which may account for the aggressive clinical behavior associated with this variant (121). A pleomorphic variant of mesothelioma has also been described, which also manifests aggressive clinical behavior (122). Some mesotheliomas may exhibit signet ring cell features and potentially be mistaken for metastatic adenocarcinoma (123). A prominent inflammatory response, which may take the form of a dense lymphocytic infiltrate, granulomas, or numerous foamy, lipid-rich histiocytes, occurs in some PMMs (Fig. 58.23) (99,124). Some tumors have a myxoid stroma that is occasionally striking (99). Occasional PMMs contain numerous psammoma bodies. The IHC (see the following section “Differential Diagnosis”) and ultrastructural features of PMMs are similar to those of their pleural counterparts.
Differential Diagnosis
The differential diagnosis of PMM with atypical mesothelial hyperplasia was already discussed (see “Mesothelial Hyperplasia”). The other lesion in the differential diagnosis is adenocarcinoma with diffuse peritoneal involvement, including metastatic adenocarcinomas, and, in women, adenocarcinomas of primary peritoneal origin (see “Lesions of the Secondary Müllerian System”). Most patterns of PMM are, however, almost diagnostic. Most primary peritoneal adenocarcinomas are papillary serous carcinomas; these are histologically indistinguishable from primary ovarian serous carcinomas, including the frequent presence of cells with high-grade to bizarre nuclear features and numerous mitotic figures, both of which are usually absent in PMMs. Deciduoid PMMs can be distinguished from an ectopic decidual reaction (see “Lesions of the Secondary Müllerian System”) by a variety of features, including prominent nucleoli, mitotic activity, immunoreactivity of the lesional cells for cytokeratin, and ultrastructural findings.
Histochemical and IHC stains can help in the differential diagnosis with adenocarcinoma (125–130). In contrast to adenocarcinomas, PMMs elaborate acid mucin rather than neutral mucin. The former is predominantly hyaluronic acid and is appreciable as Alcian blue–positive, digested periodic acid-Schiff (DPAS)–negative, hyaluronidase-sensitive material, usually within cytoplasmic vacuoles (125). Hyaluronic acid, however, can leach from formalin-fixed tumors, resulting in false-negative staining. Furthermore, the cell borders and stroma of PMMs may stain intensely DPAS positive, and occasional PMMs may contain small, DPAS-positive cytoplasmic granules (125). Immunoreactivity for both cytokeratin (including sarcomatous PMMs) and vimentin, calretinin, tenascin-X, as well as negative staining for a variety of epithelial antigens, including carcinoembryonic antigen (CEA), B72.3, Leu-M1, and Ber-EP4, favors a diagnosis of PMM over carcinoma (56,125,126,131). Similarly, IHC can facilitate the distinction between PMMs from primary or secondary involvement of the peritoneum by serous carcinoma (127–133), although in our experience, this is rarely a diagnostic problem with routinely stained sections. Serous carcinomas are typically positive for PAX8 and PAX2; most PMMs are negative for these markers, but focal or weak staining with PAX8 may occur (132,133). Serous carcinomas but not PMMs are also usually positive for B72.3, Leu-M1, Ber-EP4, MOC-31, CA19-9, and estrogen receptors, whereas calretinin, caldesmon, and thrombomodulin staining is found in most PMMs but only rare serous carcinomas. Accordingly, Comin et al. (129) found that an h-caldesmon+/calretinin+/ER−/Ber-EP4− profile strongly favors PMM over serous carcinoma. Immunoreactivity for podoplanin and D2-40 favors PMM, although these markers are not as reliable because they are present in a minority of serous carcinomas. WT1 and CK5/6 are of no use in this differential diagnosis, as these tend to be positive in both tumors. It should be stressed that no single IHC stain is diagnostic in the separation of PMMs from adenocarcinomas, and the results of a panel of antibodies should be interpreted in conjunction with the hematoxylin and eosin (H&E) and mucin stains.
MISCELLANEOUS PRIMARY TUMORS
SOLITARY FIBROUS TUMOR
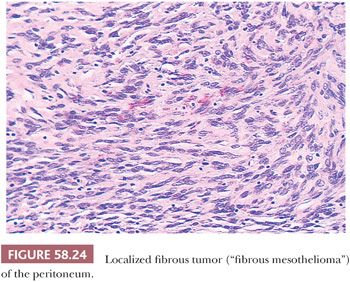
Localized fibrous tumors of the type that involve the pleura are extremely rare in the peritoneal cavity (Fig. 58.24) (134–137). Although these were once referred to as “fibrous mesotheliomas,” they are now generally considered to originate from submesothelial fibroblasts. Their clinical and pathologic features, including their strong immunoreactivity for vimentin and CD34 and negativity for cytokeratin, as well as their usually indolent behavior are similar to those encountered in the pleura and elsewhere, and they facilitate their distinction from desmoplastic mesotheliomas. Rare tumors with focal hypercellularity, marked nuclear pleomorphism, and a high mitotic rate have been interpreted as being histologically malignant (136,137). In one study (137), the worrisome histologic findings were not, however, associated with an adverse outcome.
INTRA-ABDOMINAL DESMOPLASTIC SMALL ROUND CELL TUMOR
Clinical Features
The intra-abdominal desmoplastic small round cell tumor (DSRCT) is of uncertain histogenesis, with a predilection for the peritoneal surfaces of the abdomen and pelvis and, rarely, the pleura (138–145). Solitary examples of DSRCTs arising in the scalp, brain, parotid gland, ethmoid sinus, hand, and liver have been reported. Apparently unique to DSRCTs is the fusion of the Ewing sarcoma (EWS) gene on chromosome 22 and the Wilms tumor gene (WT1) on chromosome 11 resulting from chromosomal translocation t(11;22) (p13;q12)(141). The EWS–WT1 gene fusion transcript, which is present in almost all cases and is detectable by reverse transcriptase-polymerase chain reaction on paraffin-embedded tissue, can be extremely useful in confirming the diagnosis, especially in cases that are not typical histologically.
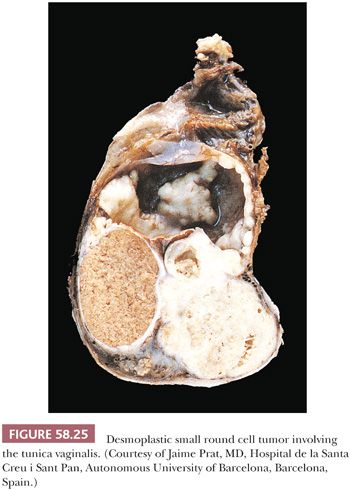
DSRCTs have a strong male predilection (male-to-female ratio of 4:1) and are most common in adolescents and young adults (mean age of 22 years in the largest series [141]). The patients typically present with abdominal distention; pain; and a palpable abdominal, pelvic, or scrotal mass, sometimes in association with ascites. The serum neuron-specific enolase (NSE) or CA-125 has been elevated in some cases. Laparotomy typically discloses a variably sized but usually large intra-abdominal mass associated with smaller peritoneal implants of similar appearance (138). Any portion of the peritoneal cavity or, less commonly, the retroperitoneum may be affected; the tumor is frequently confined to the pelvis. Involvement of the tunica vaginalis can result in a paratesticular mass (Fig. 58.25) (139). Similarly, striking ovarian involvement can mimic a primary ovarian tumor (140).
Pathologic Features
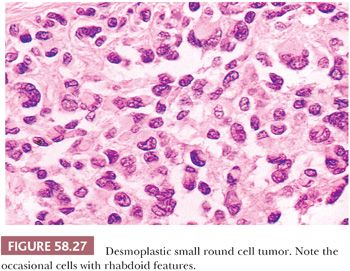
On gross examination, the external aspect of the tumors, which may reach 40 cm in diameter, is smooth or bosselated, and the neoplastic tissue is firm to hard and gray-white, with foci of myxoid change and necrosis (Fig. 58.25). Direct invasion of intra-abdominal or pelvic viscera has been noted in occasional cases. On microscopic examination, sharply circumscribed aggregates of small cells are delimited by a cellular desmoplastic stroma (Fig. 58.26). The aggregates vary in size and shape from tiny clusters or even single cells to rounded or irregularly shaped islands. Other common features include rosette-like or gland-like spaces, peripheral palisading of basaloid cells in some of the nests, and central necrosis within the nests that is sometimes associated with calcification. The tumor cells are typically uniform, with scanty cytoplasm and indistinct cell borders, although cells with eosinophilic cytoplasmic inclusions and an eccentric nucleus, resulting in a rhabdoid appearance (Fig. 58.27), are also frequently present. The small to medium-sized, round, oval or spindle-shaped hyperchromatic nuclei have clumped chromatin and usually inapparent nucleoli. Mitotic figures and single necrotic cells are numerous.
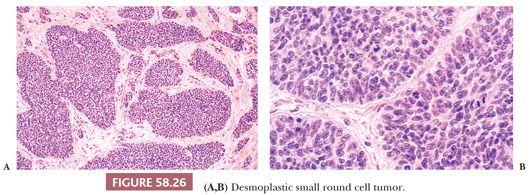
Uncommon architectural features that can occasionally predominate and can lead to diagnostic problems include tubules; glands, occasionally with luminal mucin; cysts; papillae; anastomosing trabeculae; cords of cells that can potentially mimic lobular breast carcinoma; adenoid cystic-like foci; and only a sparse desmoplastic stroma (142). Uncommon cytologic features that can occasionally predominate include spindle cells, cells with abundant eosinophilic or clear cytoplasm, signet ring–like cells, and cells with marked nuclear pleomorphism including bizarre nuclei (142,146). The stroma frequently contains blood vessels with prominent endothelial and perithelial cells (138). Invasion of vascular spaces, especially lymphatics, is a common feature, and lymph nodes are occasionally involved.
The characteristic immunoprofile of DSRCTs suggests a multidirectional phenotype, with immunoreactivity for epithelial (low–molecular-weight cytokeratins, EMA), neural and neuroendocrine (NSE, CD57/Leu-7), and muscle (desmin) markers, as well as vimentin (138,141,143,144). Desmin and vimentin immunoreactivity is typically paranuclear and globular, and it is particularly intense in the rhabdoid cells. Nuclear immunoreactivity for the C-terminal region of the Wilms tumor protein (WT1) is present in most cases (147), a finding that is useful in separating DSRCTs from Ewing sarcoma and primitive neuroectodermal tumors. Atypical staining patterns can rarely occur due to expression of full-length WT1 or variant transcripts (147). Variable staining for a wide variety of other antigens has been reported in a minority of cases (141,143). Ultrastructural features suggest a range of differentiation (138,143). Cell junctions have varied from scant and primitive to more prominent ones, including the intermediate, desmosomal, and tight types. Juxtanuclear intermediate cytoplasmic filaments, which correspond to the cytoplasmic inclusions noted earlier, have been a prominent feature in most of the cases, as has the basal lamina surrounding the nests of tumor.
Differential Diagnosis
DSRCT should be distinguished from other malignant small cell tumors that may involve the peritoneum; this differential diagnosis has been detailed by workers with great experience with the tumor (138,141). The characteristic age of the patient, the distribution of the tumor, and its typical microscopic and IHC features facilitate the diagnosis in most cases. In problematic cases, the diagnosis can be confirmed by identification of the unique reciprocal translocation.
Behavior
The treatment of the tumors has usually consisted of debulking and postoperative chemotherapy, irradiation, or both. Complete cytoreductive surgery and hyperthermic intraperitoneal chemotherapy have been advocated in recent studies in attempt to improve local disease control (148). The tumor is highly aggressive, with more than 90% of patients dying from tumor progression. Some tumors have responded, at least initially, to aggressive chemotherapy. Even in advanced stages, the bulk of the tumor tends to remain within the peritoneal cavity; extra-abdominal metastases have, however, occurred in occasional patients.
MALIGNANT VASCULAR TUMORS
Rare malignant vascular tumors, including epithelioid hemangioendothelioma and epithelioid angiosarcoma, have appeared to arise from the peritoneum (149–151). Some of the tumors have a focal tubulopapillary pattern and/or a biphasic pattern (due to admixed reactive or neoplastic spindle cells) that may suggest the diagnosis of malignant mesothelioma. Variable degrees of vascular differentiation and immunoreactivity of the neoplastic cells for endothelial antigens (and negative or weak cytokeratin staining) exclude the diagnosis of mesothelioma.
INFLAMMATORY MYOFIBROBLASTIC TUMOR
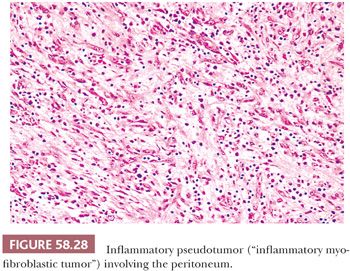
Day et al. (152) reviewed the features of seven cases of abdominal inflammatory pseudotumor, a lesion that has also been referred to as plasma cell granuloma or, more recently, as inflammatory myofibroblastic tumor (153). The abdominal lesions are typically encountered in patients younger than 20 years of age who present with a mass, fever, growth failure or weight loss, hypochromic anemia, thrombocytosis, and polyclonal hypergammaglobulinemia. Laparotomy typically reveals a solid mesenteric mass that on microscopic examination consists of myofibroblastic spindle cells, mature plasma cells, and small lymphocytes (Fig. 58.28). The spindle cells show cytoplasmic immunopositivity for ALK-1, with associated chromosomal translocations, in approximately 50% of cases (154,155). Inflammatory myofibroblastic tumors may be associated with an uneventful postoperative course but are regarded as tumors of intermediate biologic behavior given the tendency for local recurrence and very rare cases which have behaved in a malignant fashion (154,155). It has also been reported that abdominopelvic tumors have a higher recurrence rate than those arising in other anatomic sites and that ALK-negative tumors are more likely to be associated with metastases.
OMENTAL-MESENTERIC MYXOID HAMARTOMA
This designation was applied by Gonzalez-Crussi et al. (156) to a lesion found in infants that was characterized by multiple omental and mesenteric nodules; these were composed of plump mesenchymal cells in a myxoid, vascularized stroma. The diagnosis of the referring pathologists was usually that of some type of sarcoma, but the follow-up was uneventful. The conclusion was that these lesions are probably hamartomatous.
METASTATIC TUMORS
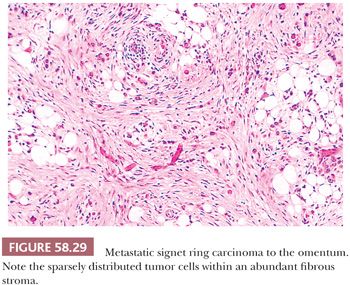
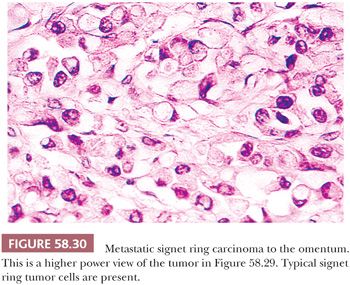
Peritoneal involvement by metastatic tumor is typically a result of seeding from a primary tumor arising within the abdomen or pelvis, most commonly the ovary. Peritoneal serous tumors in which the ovaries are normal or are only minimally involved may arise directly from the peritoneum (see “Lesions of the Secondary Müllerian System”) or represent metastases from a serous papillary carcinoma of the endometrium or fallopian tube. Other tumors that may be associated with peritoneal seeding include carcinomas of the breast (157), gastrointestinal tract (especially colon and stomach), and the pancreas. In such cases, the metastatic tumor may take the form of signet ring cells that are widely scattered in a fibrous stroma (Figs. 58.29 and 58.30). Occasionally, the signet ring cells can have relatively bland nuclear features, further resulting in a deceptively benign appearance. Pseudomyxoma peritonei, which usually is a result of peritoneal spread of a low-grade mucinous tumor of the appendix, is considered in Chapter 56, Miscellaneous Primary Tumors, Secondary Tumors, and Nonneoplastic Lesions of the Ovary.
LESIONS OF THE SECONDARY MÜLLERIAN SYSTEM
These lesions share an origin from the so-called secondary müllerian system, which corresponds to the pelvic and lower abdominal mesothelium and the subjacent mesenchyme of females (158–160). The müllerian potential of this layer is consistent with its close embryonic relation to the müllerian ducts that arise by invagination of the coelomic epithelium. Lesions of the secondary müllerian system include those containing endometrioid, serous, and mucinous epithelium, thus simulating normal or neoplastic endometrial, tubal, and endocervical epithelium. The metaplastic potential of the pelvic peritoneum also includes differentiation toward cells of the transitional (urothelial) type, which is exemplified most commonly by Walthard nests. Proliferation of the subjacent mesenchyme may accompany epithelial differentiation of the mesothelium, giving rise to mixed tumors or a variety of pure mesenchymal lesions composed of endometrial stromal-type cells, decidua, or smooth muscle.
ENDOMETRIOSIS
Endometriosis is defined as the presence of endometrial tissue outside the endometrium or myometrium. Usually, both epithelium and stroma are seen, but, occasionally, the diagnosis of endometriosis can be made when only one component is present (161). Etiologic, pathogenetic, and clinical aspects of endometriosis, as well as endometriosis in extraperitoneal sites, have been reviewed in detail elsewhere (160–162) and are not considered here.
Macroscopic Features
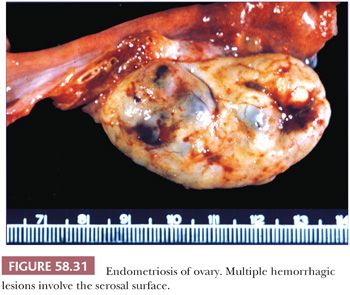
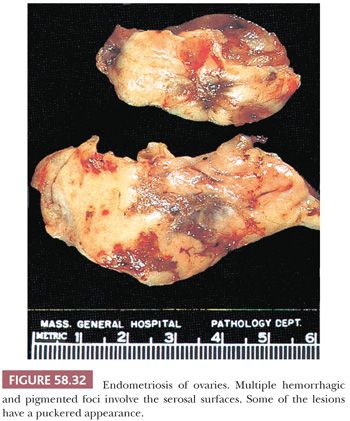
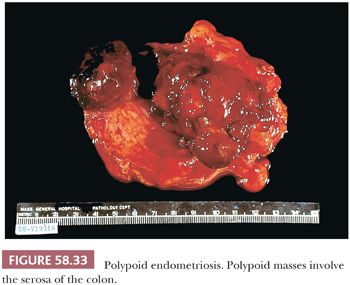
Depending on the age of endometriotic foci and their superficial or deep location in relation to the peritoneal surface, they may appear as punctate, red, blue, brown, or white spots or patches with either a slightly raised or puckered surface (Figs. 58.31 and 58.32) (163,164). Ecchymotic or brown areas have sometimes been described as “powder burns.” The endometriotic foci are frequently associated with dense, fibrous adhesions. The lesions may form nodules, cysts, or both. Rarely, pelvic peritoneal endometriosis may present as multiple, polypoid masses of soft gray tissue (“polypoid endometriosis”) that fill the pelvis, which may clinically or macroscopically mimic a malignant tumor (Fig. 58.33) (165).
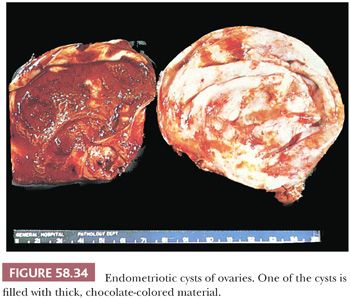
Endometriotic cysts (endometriomas) most commonly involve the ovaries, where they may partially or almost completely replace the normal tissue; bilateral involvement occurs in a third to half of the cases. The cysts rarely exceed 15 cm in diameter; larger examples are more likely to harbor a neoplasm. Endometriotic cysts are commonly covered by fibrous adhesions, which may result in fixation to adjacent structures. The cyst walls are usually thick and fibrotic, with a smooth or shaggy, brown to yellow lining (Fig. 58.34). The cyst contents typically consist of altered, semifluid or inspissated, chocolate-colored material (Fig. 58.34); rarely, the cyst is filled with watery fluid. Any solid areas in the cyst wall or intraluminal polypoid projections should be sampled histologically, as they may be cancers arising from the epithelial or stromal component of the cyst. Rare complications of endometriosis, which typically occur during pregnancy, include rupture (usually ovarian or intestinal) producing acute abdominal symptoms (166,167) or hemorrhage from endometriotic lesions that have undergone decidual transformation (168).
Microscopic Features
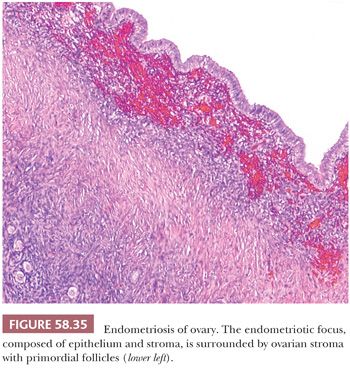
The appearance of endometriotic tissue varies with the extent of its response to the normal hormonal fluctuations of the menstrual cycle and the duration of the process (Figs. 58.35 through 58.39). When the appearances of simultaneously sampled eutopic endometrium and endometriotic foci are compared, the latter exhibit cyclic changes in 44% to 80% of the cases, and considerable variability is observed in glandular morphology (169,170). When more than one endometriotic focus is examined in the same patient, the appearances of the specimens do not differ significantly from one to another. In most postmenopausal patients with endometriosis, the endometriotic tissue is atrophic, with glands that are occasionally cystic and that are lined by flattened epithelial cells and surrounded by a dense, fibrotic stroma (Fig. 58.37); the appearance is similar to that of simple or cystic atrophy of the endometrium (171). In a minority of cases, however, the endometriotic tissue has an active appearance, with or without the metaplastic and hyperplastic changes that are more common in premenopausal women, as considered later (see page 2689).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


