• Mucopurulent cervical discharge. • Pelvic pain; bilateral adnexal tenderness. • Elevated temperature (>101° F). • White blood cell count 20,000/μL, with marked leukocytosis and/or elevated sedimentation rate • Neisseria gonorrhea and Chlamydia trachomatis most common, followed by Ureaplasma urealyticum, Mycoplasma hominis, Streptococcus spp., Escherichia coli, Haemophilus influenzae, Peptostreptococcus, and Peptococcus.* • Transvaginal ultrasound showing thickened, fluid-filled tubes or tubo-ovarian mass. • Acute and chronic endometritis on endometrial biopsy. • Laparoscopy is the gold standard. • New Centers for Disease Control and Prevention (CDC) rec-ommendations are that all sexually active adolescents un-dergo routine screening for Chlamydia during annual pelvic examinations. • CDC also recommends that routine screening of asymptomatic women between ages 20 and 24 years should be considered, particularly if she has a new male sexual partner, has more than one male sexual partner, or does not use barrier contraception. • N. gonorrhea (GC): a delicate and fastidious species with high infectivity, preferring human columnar and transitional epithelia. In less than 1 hour after intercourse GC can establish itself on the urethral mucosa, resisting the flow of urine. Favored sites in the lower female genital tract are Bartholin’s and Skene’s glands, urethra, and endocervical canal. Spreading occurs from the endocervix across endometrium to tubal mucosa or by migration through subendothelial vascular and lymphatic channels. The most common method of spreading is vector; GC attached to spermatozoa are physically carried to the fallopian tubes. Retrograde menstruation or uterine contractions during intercourse are other modes of dissemination. Acute state: GC and polymorphonuclear leukocytes accumulate in subepithelial connective tissue, causing patchy destruction of overlying mucosa. Consequent mucosal thinning may facilitate GC penetration into deeper tissue; GC survive only a short time in the fallopian tubes. Descent of the microbe beyond surfaces being examined makes it difficult to detect. Concomitant infections occur; the primary role of GC is paving the way for secondary invaders from normal vaginal flora. C. trachomatis and anaerobic bacteria superinfections are possible. • C. trachomatis (CT): 20%-30% of PID cases are caused by CT; acute chlamydial PID may be subclinical or silent in 66%-75% of cases. Lab diagnosis is difficult. It is frequently asymptomatic. • Anaerobes: most commonly isolated from fallopian tubes or cul-de-sacs of PID patients. They are probably not the chief causative agents, but opportunists commonly found in immunocompromised hosts. They generally are of endogenous origin; cervixes and vaginas of normal healthy women contain anaerobes and aerobes. Anaerobic infections are more common in older patients and women with a history of prior PID. • Other organisms: facultative aerobic organisms in tuboperitoneal fluids from women with salpingitis include coliforms, Haemophilus influenzae, streptococci, and Mycoplasma hominis. M. hominis is a common agent of polymicrobial milieu of PID, present in 81% of female patients with GC and 64% of those without. • Death from salpingitis is rare and generally from rupture of tubo-ovarian abscess with subsequent peritonitis, with mortality rate of 5.2%-5.9% for tubo-ovarian abscesses. • Fitz-Hugh–Curtis syndrome: perihepatitis complicating primary PID. It has characteristic violin-string adhesions attaching liver to abdominal wall. Adhesions are from local peritonitis involving the anterior liver surface and adjacent abdominal wall. CT is a more frequent cause than GC. • Infertility: PID puts women at risk for recurrence. After fallopian tubes are damaged by infection, normal defense mechanisms are impaired. Reinfection is the most important cause of infertility after PID. • Risk in sexually active 15-year-olds is 1 in 8; in average 24-year-olds, the risk is 1 in 80. Cervical mucus in younger women may be estrogen dominated, creating an environment more accessible to pathogens. • Women with multiple partners have a 4.6-fold greater risk than do monogamous women. • IUDs increase risk. Oral contraceptive (OC) users have less risk of GC but more risk for CT invasion. Barrier methods of birth control decrease PID risk (women with vasectomized partners may have less risk). • Iatrogenic: the following invasive procedures may introduce pathogens or disturb tract flora and induce PID: cervical dilation, abortion, curettage, tubal insufflation, hysterosalpingography, insertion of IUD. PID may not strictly be a sexually transmitted disease (STD). Menstruation, sperm, and trichomonads help transport pathogens into the salpinx. • Infections occurring around menses tend to be GC rather than CT. Menstrual regurgitation may carry sloughed endometrium with attached GC or intracellular CT that proliferate in tubal epithelium or on peritoneal surfaces. • Human sperm: bacteriospermia is a cause of infertility in men; 66%-75% of men who tested positive for GC were asymptomatic. Sperm are vectors. Cervical mucus is an effective mechanical and immunologic barrier between flora of the vagina and upper tract. Yet organisms attached to sperm can easily traverse mucus column. Sperm migrate through menstrual plasma but not during the luteal phase or through cervical mucus of pregnancy. Sperm is intimately associated with cytomegalovirus, Toxoplasma, Ureoplasma urealyticum, and Chlamydia. • Motile trichomonads are another transporter, ascending from the vagina to the fallopian tubes, carrying additional invaders. Key observation: trichomonads are never isolated from human beings when heavy bacterial contamination is absent. Common Signs and Symptoms in Acute PID
Pelvic Inflammatory Disease
DIAGNOSTIC SUMMARY
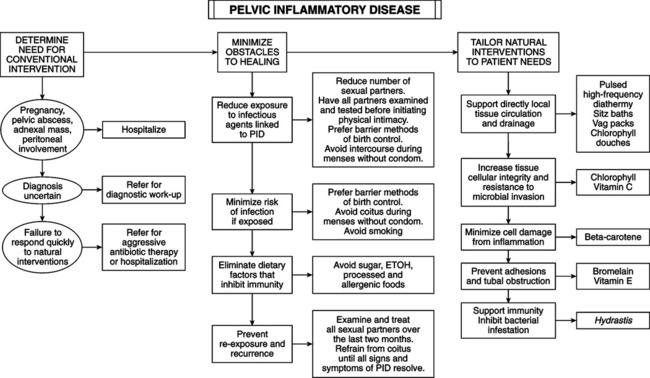
GENERAL CONSIDERATIONS
Etiology
Complications
Risk Factors
Pathogen Access to Upper Female Tract
DIAGNOSIS
Symptom
Incidence (%)
Lower abdominal pain
90
Adnexal tenderness on palpation
90
Pain on movement of the cervix
90
Vaginal discharge
55
Adnexal mass or swelling
50
Fever or chills
40
Irregular vaginal bleeding
35
Anorexia, nausea, and vomiting
25 ![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Basicmedical Key
Fastest Basicmedical Insight Engine
