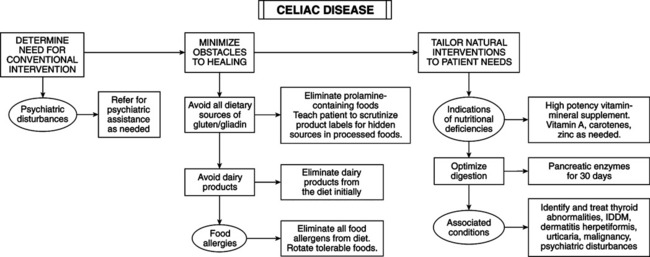
• Chemistry of grain proteins: gluten is a major component of wheat endosperm, composed of gliadins and glutenins. Gliadin triggers CD. In rye, barley, and oats, the proteins triggering CD are secalins, hordeins, and avenins, respectively, and prolamines collectively. The cereal grain family is Gramineae; close taxonomic relation to wheat suggests ability to activate CD. Rice and corn do not activate CD and are further removed taxonomically from wheat. Gliadins are single polypeptide chains (molecular weight 30,000-75,000 mol) with high glutamine and proline. Gliadin’s four electrophoretic fractions are alpha-, beta-, gamma-, and • Opioid activity: pepsin hydrolysates of wheat gluten have opioid activity, the factor linking wheat ingestion to schizophrenia. Hypothesis: gluten is a pathogenic factor in schizophrenia (substantiated by studies). • Pathogenesis: genetic etiology linked to human leukocyte antigen (HLA) DQ2 in 95% of patients and DQ8 in the remainder. These gene loci relate to immunologic recognition of antigens and specific T-cell immune responses. Hypothesis: CD may arise from abnormalities in immune response, rather than gliadin “toxicity.” Gliadin sensitizing is humoral and cell mediated; T-cell dysfunction is a key factor in enteropathy. Circulating antibodies are specific for CD (antiendomysial antibody [AEA]) and are used as serum markers to screen patients and estimate CD prevalence. Tissue transglutaminase (tTG) is the autoantigen for AEA; enzyme-linked assay using recombinant tTG is highly sensitive and specific for CD. Anti-tTG antibody titers fall and can become undetectable during gluten-free diet; tTG-gliadin complexes stimulate gluten-specific T cells to induce anti-tTG antibody production. T cells may react to smaller gliadin peptides, and B cells may respond to the larger tTG enzyme. Breastfeeding has prophylactic effect; breastfed babies have decreased risk, with greater protection when breastfed after gluten is introduced. CD risk is greater when large amounts of gluten are introduced. Early introduction of cow’s milk is a major etiological factor. Breastfeeding plus delayed exposure to cow’s milk and cereal grains are preventive. • Clinical aspects: CD lesions are often histologically indistinguishable from tropical sprue, food allergy, diffuse intestinal lymphoma, and viral gastroenteritis. CD can cause disaccharidase deficiency, leading to lactose intolerance. Increased intestinal permeability causes multiple food allergies. Cow’s milk intolerance may precede CD. • Associated conditions: thyroid abnormalities, insulin-dependent diabetes mellitus, psychiatric disturbances (including schizophrenia), dermatitis herpetiformis, and urticaria are linked to gluten intolerance. Increased risk of malignant neoplasms exists (e.g., non-Hodgkin’s lymphoma) in celiac patients, perhaps because of decreased micronutrient absorption of vitamin A and carotenoids (Textbook, “Beta-carotene and Other Carotenoids” and “Vitamin A”) and gliadin-activated suppressor cell activity. Alpha-gliadin activates suppressor cells of celiac patients but not healthy control subjects or patients with Crohn’s disease. Casein and beta-lactoglobulin do not activate suppressor cells. Depressed immunity increases susceptibility to infection and neoplasm.
Celiac Disease
GENERAL CONSIDERATIONS
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Basicmedical Key
Fastest Basicmedical Insight Engine
