• Affects 2%-13% of population, 80%-90% of which are female. • Chronic widespread pain involving axial pain; pain on left, right, upper, and lower parts of body. • Abnormal tenderness at 11 or more of 18 specific anatomic tender point (TnP) sites. • Associated symptoms include fatigue, stiffness, headache, sleep disturbance, irritable bowel, depression, cognitive dysfunction, anxiety, coldness, paresthesias, sicca symptoms, exercise intolerance, dysmenorrhea. • Two major criteria for fibromyalgia syndrome (FMS) are chronic (>3 months) widespread pain and tenderness. • Hypothyroidism and cellular resistance to thyroid hormone may present with symptoms of FMS. Thyroid testing and a trial of thyroid hormone therapy can determine whether FMS is related to these disorders. • Main disorders for differentiation are arthritis, myopathy, polymyalgia rheumatica, diabetic polyneuropathy, ankylosing spondylitis, discopathy, cardiac or pleural pain, multiple muscle myofascial pain syndromes, and lupus erythematosus. • Distinguish FMS (except for hypothyroidism or cellular resistance to thyroid hormone) by careful pathognomy. Symptoms and signs of most FMS patients are indistinguishable from the subclass of hypothyroid or thyroid hormone–resistant patients for whom pain is the predominant symptom. • Serotonin deficiency hypothesis: central nervous system (CNS) serotonin deficiency reduces efficiency of brainstem-spinal cord descending antinociceptive system, resulting in heightened pain perception in response to normal afferent input. This theory has been refuted. • Hypometabolism hypothesis: contributing factors are hypothyroidism and/or partial cellular resistance to thyroid hormone, pernicious diet, nutritional deficiencies, low physical fitness level, and metabolism-impeding drugs. Inadequate thyroid hormone regulation (ITHR) may be underlying mechanism of two main features of FMS: chronic widespread pain and abnormal tenderness. ITHR of metabolic processes in brainstem-spinal cord descending antinociceptive system can cause the following: Following are two mechanisms of the syndrome: • ITHR increases substance P, which is released from nociceptor neurons, is high in CSF of FMS patients, and facilitates summation of slow nociceptive signals, amplifying nociceptive signals in spinal cord. Thyroid hormone inhibits substance P synthesis and secretion in many CNS cells by repressing transcription of the gene for preprotachykinin-A, a precursor of substance P, and its cognate substance P receptor. Thyroid hormone treatment lowers substance P level in the anterior pituitary, brain nuclei, and dorsal horns of the spinal cord. Excess thyroid hormone reduces substance P to subnormal levels. • ITHR reduces synthesis and secretion of norepinephrine (NE) in the brainstem locus ceruleus cells, which are essential to the normal function of the descending antinociceptive system. • Wholesome diet: vegetarian diets; eliminate excitotoxins monosodium l-glutamate and aspartame; ingest Chlorella pyrenoidosa; uncooked vegan diet of berries, fruits, vegetables and roots, nuts, germinated seeds, sprouts; strict, low-salt, uncooked vegan diet rich in lactobacteria. • Nutritional supplements: vitamins B1, B6, B12, C, E, and beta-carotene have antinociceptive properties. Myers’ cocktail (intravenous B vitamins, vitamin C, calcium, magnesium [Mg]); 5-hydroxy-l-tryptophan (5HT); S-adenosyl-l-methionine (SAM); Mg; malic acid; combined aloe vera extracts, plant saccharides, freeze-dried fruits and vegetables; Dioscorea; vitamin/mineral supplement; collagen hydrolysate; blend of ascorbigen and broccoli powder. • Exercise to tolerance: results of exercise treatment for FMS are mixed. Cardiovascular exercise provides the most improvement, especially low-intensity endurance training. Endurance exercise reduces physical limitations of FMS. Low metabolic efficiency from ITHR renders some patients susceptible to FMS. Vigorous exercise exacerbates symptoms; high density of a2-adrenergic receptors on FMS patients’ platelets indicates receptor density in CNS. Binding catecholamines to high density of membrane receptors inhibits energy metabolism, worsening symptoms. During early phase of treatment, use mild exercise to minimize catecholamine secretion. Thyroid hormone therapy decreases density of a2-adrenergic receptors and increases a2-adrenergic receptors, enabling cells to respond appropriately to high catecholamines. Shifting from a2-adrenergic receptor dominance explains the ability to engage in vigorous activity after thyroid hormone therapy. • Physical medicine: necessary to eliminate FMS pain. Spinal manipulation, soft tissue manipulation, and trigger point therapy relieve pain. Lesions exacerbating FMS: myofascial trigger points and spinal joint fixations. Any nociception-generating neuromusculoskeletal lesion can exacerbate pain because of the impaired antinociceptive system. Neuromusculoskeletal lesions can disturb sleep, increasing symptoms in hypometabolic patients.
Fibromyalgia Syndrome
DIAGNOSTIC SUMMARY
DIFFERENTIAL DIAGNOSIS
GENERAL CONSIDERATIONS
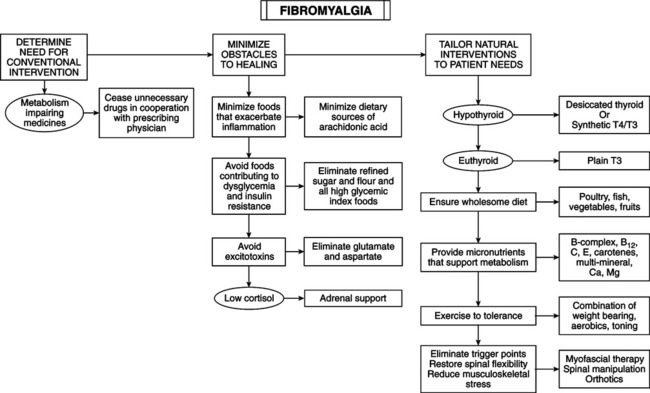
Other metabolism-regulating therapies
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Fibromyalgia Syndrome
