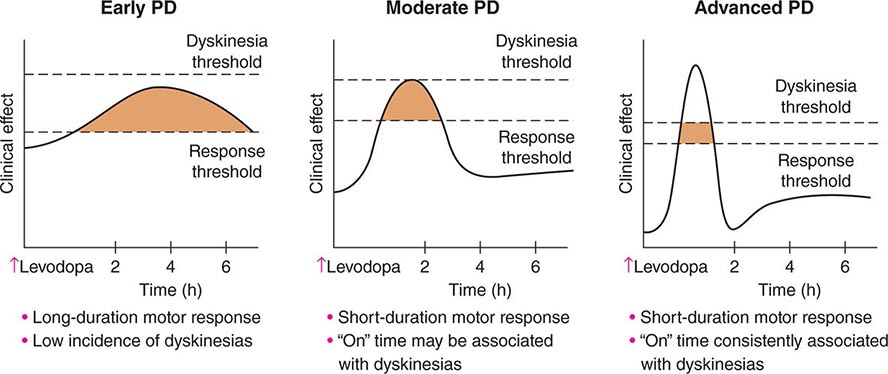
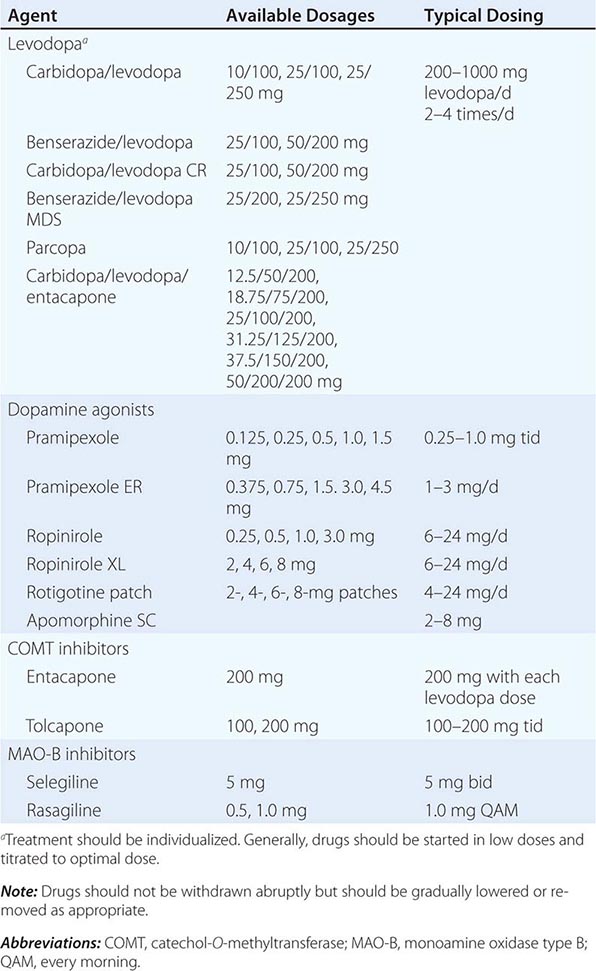
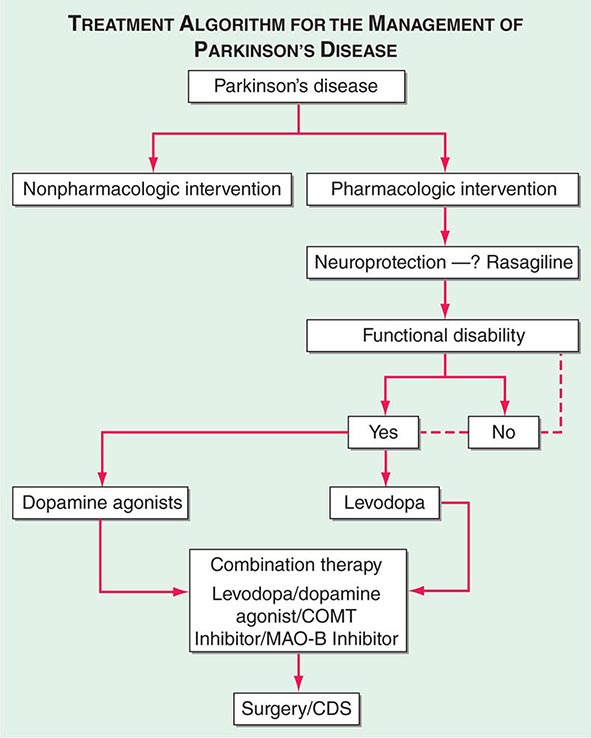
449 Parkinson’s Disease and Other Movement Disorders
PARKINSON’S DISEASE AND RELATED DISORDERS
Parkinson’s disease (PD) is the second commonest neurodegenerative disease, exceeded only by Alzheimer’s disease (AD). Its cardinal clinical features were first described by the English physician James Parkinson in 1817. It is noteworthy that James Parkinson was a general physician who captured the essence of this condition based on a visual inspection of a mere handful of patients. It is estimated that approximately 1 million persons in the United States, 1 million in Western Europe, and 5 million worldwide suffer from this disorder. PD affects men and women of all races, all occupations, and all countries. The mean age of onset is about 60 years. The frequency of PD increases with aging, but cases can be seen in patients in their 20s and even younger. Based on the aging of the population and projected demographics, it is estimated that the prevalence of the disease will dramatically increase in the next several decades.
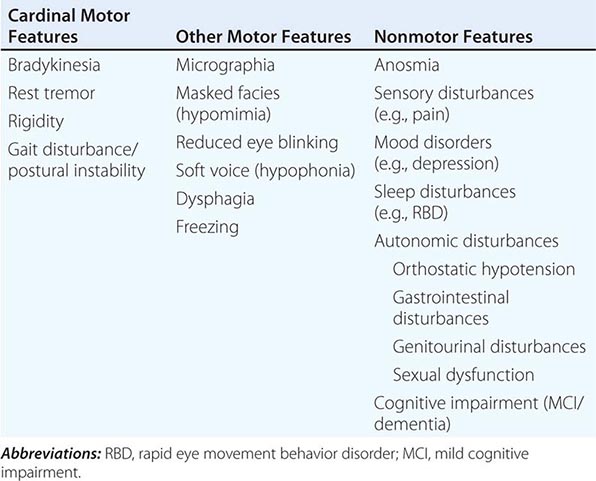
Clinically, PD is characterized by rest tremor, rigidity, bradykinesia (slowing), and gait impairment, known as the “cardinal features” of the disease. Additional features can include freezing of gait, postural instability, speech difficulty, autonomic disturbances, sensory alterations, mood disorders, sleep dysfunction, cognitive impairment, and dementia (Table 449-1).
CLINICAL FEATURES OF PARKINSON’S DISEASE |
Pathologically, the hallmark features of PD are degeneration of dopaminergic neurons in the substantia nigra pars compacta (SNc), reduced striatal dopamine, and intracytoplasmic proteinaceous inclusions known as Lewy bodies that primarily contain the protein alpha synuclein (Fig. 449-1). While interest has primarily focused on the dopamine system, neuronal degeneration with inclusion body formation can also affect cholinergic neurons of the nucleus basalis of Meynert (NBM), norepinephrine neurons of the locus coeruleus (LC), serotonin neurons in the raphe nuclei of the brainstem, and neurons of the olfactory system, cerebral hemispheres, spinal cord, and peripheral autonomic nervous system. This “nondopaminergic” pathology is likely responsible for the development of nondopaminergic clinical features listed in Table 449-1 characterized by their lack of satisfactory response to dopaminergic replacement therapy. There is evidence that Lewy body pathology first begins in the peripheral autonomic nervous system, olfactory system, and dorsal motor nucleus of the vagus nerve in the lower brainstem, and then spreads in a predictable and sequential manner to affect the upper brainstem and cerebral hemispheres (Braak staging). These studies suggest that degeneration of dopamine neurons develops in a mid-stage of the disease. Indeed, epidemiologic studies suggest that clinical symptoms reflecting this nondopaminergic degeneration, such as constipation, anosmia, rapid eye movement (REM) behavior sleep disorder, and cardiac denervation, can precede the onset of the classic motor features of PD.
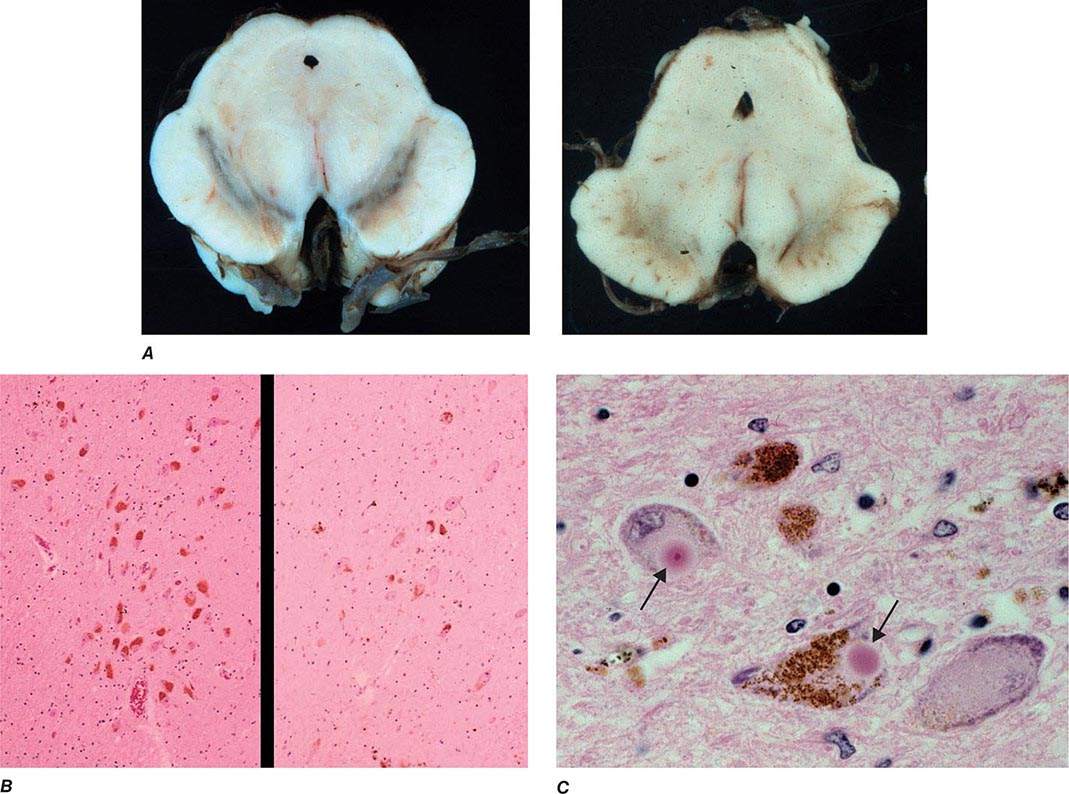
FIGURE 449-1 Pathologic specimens from a patient with Parkinson’s disease (PD) compared to a normal control demonstrating (A) reduction of pigment in SNc in PD (right) versus control (left), (B) reduced numbers of cells in SNc in PD (right) compared to control (left), and (C) Lewy bodies (arrows) within melanized dopamine neurons in PD. SNc, substantia nigra pars compacta.
DIFFERENTIAL DIAGNOSIS
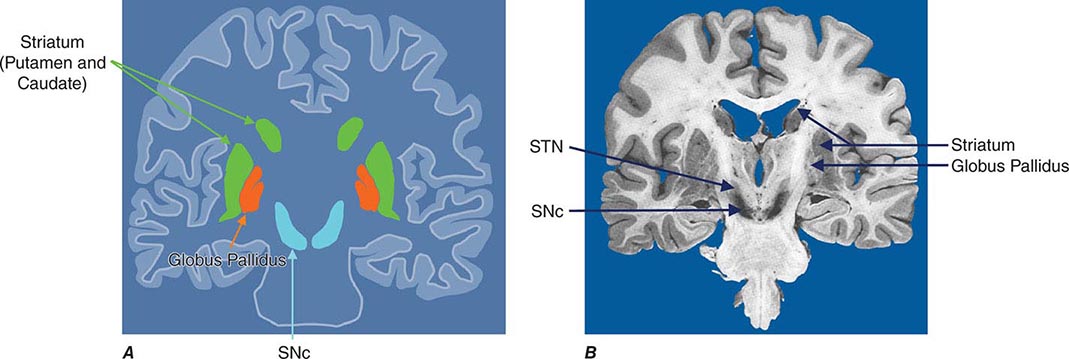
Parkinsonism is a generic term that is used to define a syndrome manifest as bradykinesia with rigidity and/or tremor. It has a differential diagnosis (Table 449-2) that reflects damage to different components of the basal ganglia. The basal ganglia are comprised of a group of subcortical nuclei that include the striatum (putamen and caudate nucleus), subthalamic nucleus (STN), globus pallidus pars externa (GPe), globus pallidus pars interna (GPi), and the SNc (Fig. 449-2). Among the different forms of parkinsonism, PD is the most common (approximately 75% of cases). Historically, PD was diagnosed based on the presence of two of three parkinsonian features (tremor, rigidity, bradykinesia). However, postmortem studies found a 24% error rate when diagnosis was based on these criteria. Clinicopathologic correlation studies subsequently determined that parkinsonism associated with rest tremor, asymmetry, and a good response to levodopa was more likely to predict the correct pathologic diagnosis. With these revised criteria (known as the U.K. Brain Bank Criteria), a clinical diagnosis of PD is confirmed pathologically in as many as 99% of cases. A more complete definition of PD is now needed to incorporate the fact that there is widespread pathology beyond the dopaminergic system, nondopamine and nonmotor clinical features, and a premotor stage of the disease.
DIFFERENTIAL DIAGNOSIS OF PARKINSONISM |

FIGURE 449-2 Basal ganglia nuclei. Schematic (A) and postmortem (B) coronal sections illustrating the various components of the basal ganglia. SNc, substantia nigra pars compacta; STN, subthalamic nucleus.
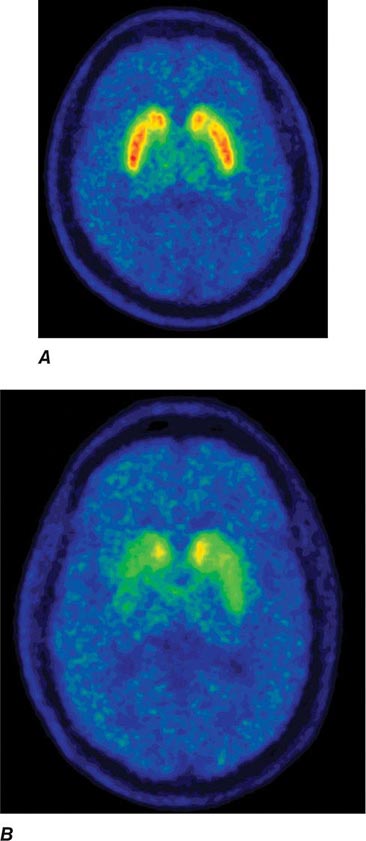
Imaging of the brain dopamine system in PD with positron emission tomography (PET) or single-photon emission computed tomography (SPECT) shows reduced uptake of striatal dopaminergic markers, particularly in the posterior putamen with relative sparing of the caudate nucleus (Fig. 449-3), reflecting the degeneration of nigrostriatal dopamine neurons. Imaging can be useful in patients where there is diagnostic uncertainty (e.g., dystonic tremor, essential tremor) or in research studies, but is rarely necessary in routine practice because the diagnosis can usually be established on clinical criteria alone. This may change in the future when there is a disease-modifying therapy and it is important to make the diagnosis as early as possible. Genetic testing is not routinely used at present, but can be helpful for identifying at-risk individuals in a research setting. Mutations of the LRRK2 gene (see below) have attracted particular interest because they are the commonest cause of familial PD and are responsible for approximately 1% of typical sporadic cases of the disease. Mutations in LRRK2 are a particularly common cause of PD in Ashkenazi Jews and North African Berber Arabs. The penetrance of the most common LRRK2 mutation ranges from 28 to 74% and is strongly correlated to the age of the carrier, with 50% affected by age 60 years. Mutations in the parkin gene should be considered in patients with onset prior to 40 years of age.
FIGURE 449-3 [11C]Dihydrotetrabenazine positron emission tomography (a marker of VMAT2) in healthy control (A) and Parkinson’s disease (B) patient. Note the reduced striatal uptake of tracer, which is most pronounced in the posterior putamen and tends to be asymmetric. (Courtesy of Dr. Jon Stoessl.)
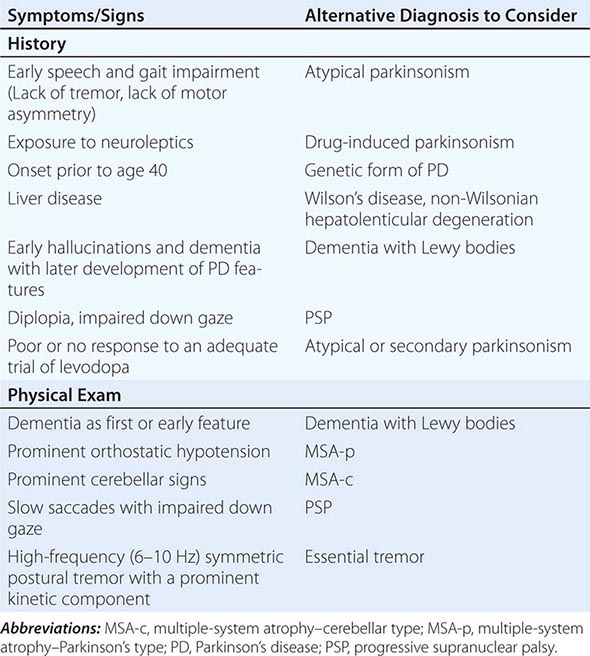
Atypical and Secondary Parkinsonism Atypical parkinsonism refers to a group of neurodegenerative conditions that usually are associated with more widespread neurodegeneration than is found in PD (often involvement of striatum and/or globus pallidus as well as the SNc). As a group, they present with parkinsonism (rigidity and bradykinesia) but have a slightly different clinical picture than PD, reflecting differences in the underlying pathology. In these conditions, parkinsonism is typically characterized by early speech and gait impairment, absence of rest tremor, no motor asymmetry, poor or no response to levodopa, and an aggressive clinical course. In the early stages, they may show some modest benefit from levodopa and be difficult to distinguish from PD. Pathologically, neurodegeneration typically involves degeneration of the SNc but occurs without Lewy bodies (see below for individual conditions). Neuroimaging of the dopamine system is usually not helpful, because dopamine depletion can be seen in both PD and atypical parkinsonism. By contrast, metabolic imaging of the basal ganglia/thalamus network (using 2-F-deoxiglucose PET) may be helpful, showing a pattern of decreased activity in the GPi with increased activity in the thalamus, the reverse of what is seen in PD.
Multiple-system atrophy (MSA) manifests as a combination of parkinsonian, cerebellar, and autonomic features and can be divided into a predominant parkinsonian (MSA-p) or cerebellar (MSA-c) form. Clinically, MSA is suspected when a patient presents with atypical parkinsonism in conjunction with cerebellar signs and/or early and prominent autonomic dysfunction, usually orthostatic hypotension (Chap. 454). Pathologically, MSA is characterized by degeneration of the SNc, striatum, cerebellum, and inferior olivary nuclei coupled with characteristic glial cytoplasmic inclusions (GCIs) that stain for α-synuclein. Magnetic resonance imaging (MRI) can show pathologic iron accumulation in the striatum on T2-weighted scans, high signal change in the region of the external surface of the putamen (putaminal rim) in MSA-p, or cerebellar and brainstem atrophy (the pontine “hot cross buns” sign [Fig. 454-2]) in MSA-c. Mutations in the CoQ2 gene encoding parahydroxybenzoate-polyprenyl transferase, an enzyme involved in the biosynthesis of coenzyme Q10 (CoQ10), a cofactor of the mitochondrial respiratory chain, have been identified in familial and sporadic forms of MSA.
Progressive supranuclear palsy (PSP) is a form of atypical parkinsonism that is characterized by slow ocular saccades, eyelid apraxia, and restricted eye movements with particular impairment of downward gaze. Patients frequently experience hyperextension of the neck with early gait disturbance and falls. In later stages, speech and swallowing difficulty and cognitive impairment become evident. MRI may reveal a characteristic atrophy of the midbrain with relative preservation of the pons, the “hummingbird sign” on midsagittal images. Pathologically, PSP is characterized by degeneration of the SNc, striatum, subthalamic nucleus, midline thalamic nuclei, and pallidum along with neurofibrillary tangles and inclusions that stain for the tau protein.
Corticobasal ganglionic degeneration is less common and is usually manifest by asymmetric dystonic contractions and clumsiness of one hand coupled with cortical sensory disturbances manifest as apraxia, agnosia, focal limb myoclonus, or alien limb phenomenon (where the limb assumes a position in space without the patient being aware of it). Dementia may occur at any stage of the disease. Both cortical and basal ganglia features are required to make this diagnosis. MRI frequently shows asymmetric cortical atrophy. Pathologic findings include achromatic neuronal degeneration with tau deposits. Because other disorders such as PSP can present with a similar clinical picture, the term corticobasal ganglia syndrome should be used until a precise diagnosis can be confirmed pathologically.
Secondary parkinsonism can occur as a result of drugs, stroke, tumor, infection, or exposure to toxins such as carbon monoxide or manganese. Dopamine-blocking agents such as the neuroleptics are the commonest cause of secondary parkinsonism. These drugs are most widely used in psychiatry, but physicians should be aware that drugs such as metoclopramide and chlorpromazine, which are primarily used to treat gastrointestinal problems, are also neuroleptic agents and common causes of secondary parkinsonism (as well as acute and tardive dyskinesias; see below). Other drugs that can cause secondary parkinsonism include tetrabenazine, calcium channel blockers (flunarizine, cinnarizine), amiodarone, and lithium.
Finally, parkinsonism can be seen as a feature of other degenerative disorders such as Wilson’s disease, Huntington’s disease (especially the juvenile form known as the Westphal variant), dopa-responsive dystonia, and neurodegenerative disorders with brain iron accumulation such as pantothenate kinase (PANK)–associated neurodegeneration (formerly known as Hallervorden-Spatz disease).
Some features that suggest parkinsonism might be due to a condition other than PD are shown in Table 449-3.
FEATURES SUGGESTING AN ATYPICAL OR SECONDARY CAUSE OF PARKINSONISM |
ETIOLOGY AND PATHOGENESIS
Most PD cases occur sporadically (~85–90%) and are of unknown cause. Twin studies suggest that environmental factors likely play an important role in patients older than 50 years, with genetic factors being more important in younger patients. Epidemiologic studies also suggest increased risk with exposure to pesticides, rural living, and drinking well water and reduced risk with cigarette smoking and caffeine. However, no environmental factor has yet been proven to cause typical PD. The environmental hypothesis received support with the demonstration in the 1980s that MPTP (1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine), a byproduct of the illicit manufacture of a heroin-like drug, caused a PD-like syndrome in addicts in northern California. MPTP is transported to the central nervous system, where it is oxidized to form MPP+, a mitochondrial toxin that is selectively taken up by, and damages, dopamine neurons. However, MPTP or MPTP-like compounds have not been linked to sporadic PD.
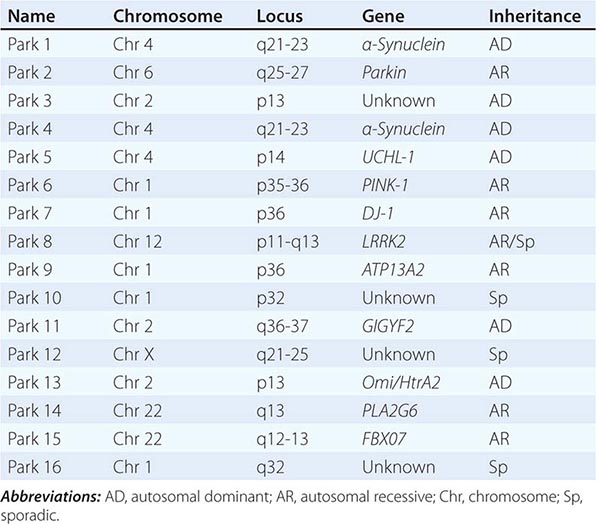
About 10–15% of cases are familial in origin, and multiple specific mutations and gene associations have been identified (Table 449-4). Genetic factors have also been linked to sporadic cases, with several typical PD cases found to carry the LRRK2 mutation, and genome-wide association studies (GWAS) implicating alpha synuclein, tau, and HLA as risk factors. It has been proposed that most cases of PD may be due to a “double hit” involving an interaction between a gene mutation that induces susceptibility coupled with exposure to a toxic environmental factor that may induce epigenetic or somatic DNA alterations. In this scenario, both factors are required for PD to ensue, while the presence of either one alone is not sufficient to cause the disease.
GENETIC CAUSES OF PARKINSON’S DISEASE |
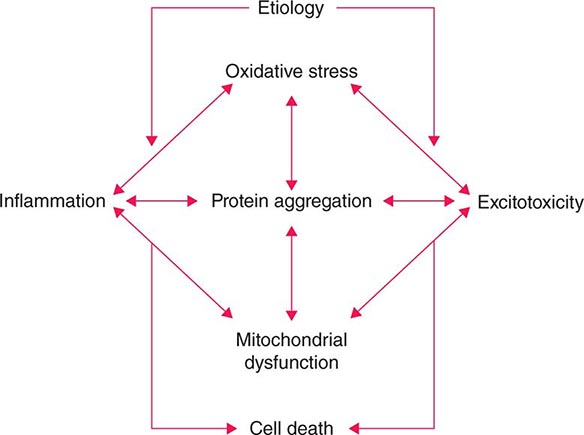
Several factors have been implicated in the pathogenesis of cell death in PD, including oxidative stress, inflammation, mitochondrial dysfunction, and proteolytic stress. Recent studies have demonstrated that with aging dopamine neurons switch from sodium to calcium pacing through calcium channels, potentially making these high-energy neurons vulnerable to calcium-mediated neurotoxicity. Whatever the pathogenic mechanism, cell death appears to occur, at least in part, by way of a signal-mediated apoptotic or “suicidal” process. Each of these mechanisms offers a potential target for neuroprotective drugs. However, it is not clear which of these factors is primary, if the mechanism is the same in each individual case, if they act by way of a network such that a cocktail of agents might be required to provide neuroprotection, or if the findings to date merely represent epiphenomena unrelated to the true cause of cell death that remains undiscovered (Fig. 449-4).
FIGURE 449-4 Schematic representation of how pathogenetic factors implicated in Parkinson’s disease interact in a network manner, ultimately leading to cell death. This figure illustrates how interference with any one of these factors may not necessarily stop the cell death cascade. (Adapted from CW Olanow: Movement Disorders 22:S-335, 2007.)
Gene mutations may not cause all cases of PD, but may be helpful in pointing to specific pathogenic pathways and mechanisms that are central to a neurodegenerative process that might be relevant to all forms of the disease. To date, most interest has focused on pathways implicated by mutations in α-synuclein, LRRK2, and PINK1/Parkin.
Most interest has focused on α-synuclein. Mutations in α-synuclein cause rare familial forms of PD, and α-synuclein constitutes the major component of Lewy bodies in patients with sporadic PD (Fig. 449-1). Furthermore, duplication or triplication of the wild-type α-synuclein can also cause a form of PD, indicating that increased production of the normal protein alone can cause the disease. More recently, Lewy pathology was discovered to have developed in healthy embryonic dopamine neurons that had been implanted into the striatum of PD patients, suggesting that the abnormal protein had transferred from affected cells to healthy unaffected dopamine neurons. Based on these findings, it has been proposed that α-synuclein is a prion and PD is a prion disorder. Here it is proposed that, like the prion protein PrPC, α-synuclein can misfold to form β-rich sheets, generate toxic oligomers and aggregates, polymerize to form amyloid plaques (i.e., Lewy bodies), cause neurodegeneration, and spread to involve unaffected neurons. Indeed, injection of α-synuclein fibrils into the striatum promotes the development of Lewy pathology in host neurons, neurodegeneration, behavioral abnormalities, and the spread of α-synuclein pathology to anatomically connected sites. Further support for this hypothesis comes from the demonstration that inoculation of α-synuclein derived from human Lewy bodies induces widespread Lewy pathology in mice and primates. Collectively, this evidence supports the possibility that neuroprotective therapies for PD might be developed based on inhibiting accumulation or accelerating removal of α-synuclein aggregates.
Mutations in the glucocerebrosidase (GBA) gene associated with Gaucher’s disease numerically represent the most important risk factor for the development of PD. While the responsible mechanism is not precisely known, it is noteworthy that GBA mutations are associated with altered autophagy and lysosomal function and could impair the clearance of α-synuclein.
Six different LRRK2 mutations have been linked to PD, with Gly2019Ser being the most common. The mechanism responsible for cell death with this mutation is not known but is thought to involve changes in kinase activity with altered phosphorylation of target proteins (including autophosphorylation) and possibly lysosomal dysfunction. Kinase inhibitors can block toxicity associated with LRRK2 mutations in laboratory models, and there has been much interest in developing drugs directed at this target. However, kinase inhibitors are likely to be toxic, the physiologic role of LRRK2 is not known, and the large majority of PD patients do not carry a LRRK2 mutation.
Mutations in PINK1 and parkin have implicated mitochondrial dysfunction as a possible cause of PD. Recent studies suggest a role for parkin and PINK1 proteins in the turnover and clearance of damaged mitochondria (mitophagy), and mutations in parkin and PINK1 cause mitochondrial dysfunction in transgenic animals that can be corrected with overexpression of parkin. This is a particularly attractive target because postmortem studies in PD patients show a defect in complex I of the respiratory chain in SNc neurons.
Thus, evidence is accumulating that genetics plays an important role in both familial and “sporadic” forms of PD. It is anticipated that better understanding of the pathways responsible for cell death caused by these mutations will permit the development of more relevant animal models of PD and targets for the development of neuroprotective drugs.
PATHOPHYSIOLOGY OF PD
The classic model of the organization of the basal ganglia in the normal and PD states is provided in Fig. 449-5. With respect to motor function, a series of neuronal circuits or loops link the basal ganglia nuclei with corresponding cortical motor regions in a somatotopic manner. The striatum is the major input region of the basal ganglia, while the GPi and SNr are the major output regions. The input and output regions are connected via direct and indirect pathways that have reciprocal effects on the activity of the output pathway. The output of the basal ganglia provides inhibitory (GABAergic) tone to thalamic and brainstem neurons that in turn connect to motor systems in the cerebral cortex and spinal cord that control motor function. Physiologically, decreased neuronal activity in the GPi/SNr is associated with movement facilitation and vice versa. Dopaminergic projections from SNc neurons serve to modulate neuronal firing and to stabilize the basal ganglia network. The basal ganglia and similar cortical loops are now thought to also play an important role in regulating normal behavioral, emotional, and cognitive functions.
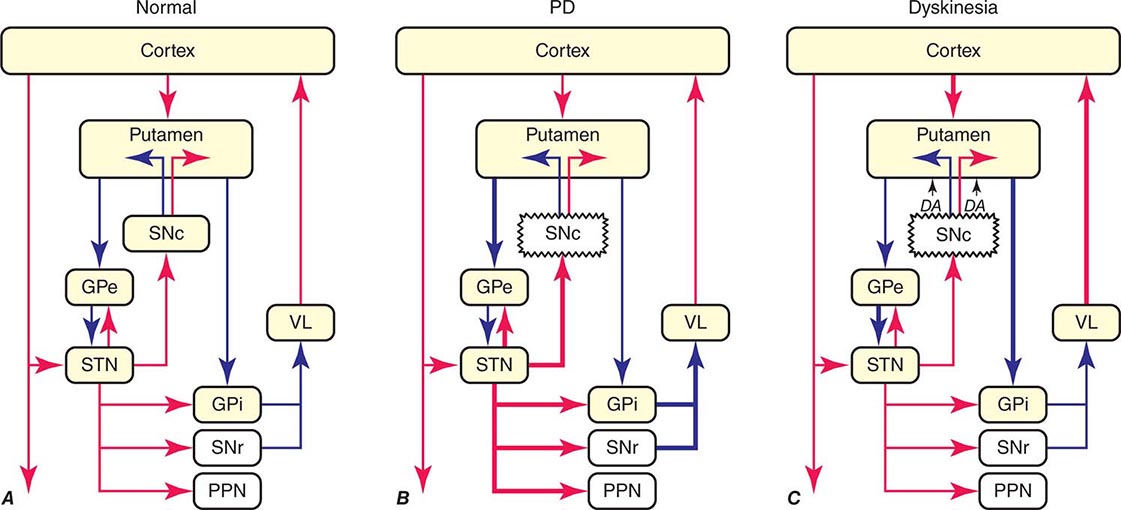
FIGURE 449-5 Basal ganglia organization. Classic model of the organization of the basal ganglia in the normal (A), Parkinson’s disease (PD) (B), and levodopa-induced dyskinesia (C) state. Inhibitory connections are shown as blue arrows and excitatory connections as red arrows. The striatum is the major input region and receives its major input from the cortex. The GPi and SNr are the major output regions, and they project to the thalamocortical and brainstem motor regions. The striatum and GPi/SNr are connected by direct and indirect pathways. This model predicts that parkinsonism results from increased neuronal firing in the STN and GPi and that lesions or DBS of these targets might provide benefit. This concept led to the rationale for surgical therapies for PD. The model also predicts that dyskinesia results from decreased firing of the output regions, resulting in excessive cortical activation by the thalamus. This component of the model is not completely correct because lesions of the GPi ameliorate rather than increase dyskinesia in PD, suggesting that firing frequency is just one of the components that lead to the development of dyskinesia. DBS, deep brain stimulation; GPe, external segment of the globus pallidus; GPi, internal segment of the globus pallidus; PPN, pedunculopontine nucleus; SNc, substantia nigra, pars compacta; SNr, substantia nigra, pars reticulata; STN, subthalamic nucleus; VL, ventrolateral thalamus. (Derived from JA Obeso et al: Trends Neurosci 23:S8, 2000.)
In PD, dopamine denervation with loss of dopaminergic tone leads to increased firing of neurons in the STN and GPi, excessive inhibition of the thalamus, reduced activation of cortical motor systems, and the development of parkinsonian features (Fig. 449-5). The current role of surgery in the treatment of PD is based on this model, which predicted that lesions or high-frequency stimulation of the STN or GPi might reduce this neuronal overactivity and improve PD features.