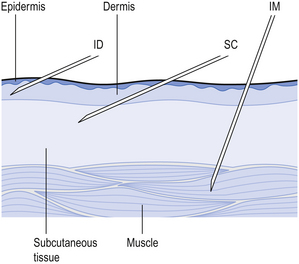
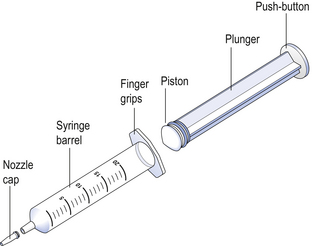
41 In practice, parenteral products are often regarded as dosage forms that are implanted, injected or infused directly into vessels, tissues, tissue spaces or body compartments. Parenteral products are often used for drugs that cannot be given orally. This may be because of patient intolerance, the instability of the drug, or poor absorption of the drug if given by the oral route. From the site of administration the drug is transported to the site of action. With developing technology, parenteral therapy is being used outside the hospital or clinic environment: at a patient’s home or their workplace, allowing self-administration. Parenteral therapy is used to: The vein that is selected for administering the formulation depends on the size of the delivery needle or catheter, the type and volume of fluid to be administered and the rate at which the fluid is to be administered. The fluids are administered into a superficial vein, commonly on the back of the hand or in the internal flexure of the elbow. The intravenous route is widely used to administer parenteral products, but it must not be used to administer water-in-oil emulsions or suspensions. These are injected into the loose connective and adipose tissue immediately beneath the skin in the abdomen, the upper back, the upper arms and the lateral upper hips (Fig. 41.1). Typically, the volume injected does not exceed 1 mL. Following administration, the site of the injection, the body temperature, age of the patient and the degree of massaging of the injection site will all affect the drug distribution. Parenteral products include injection, infusion and implantation. Most small-volume parenteral fluids are currently packaged as either ampoules or vials. Glass ampoules are thin-walled containers made of Type I borosilicate glass (see Fig. 32.2). Injections packaged in glass ampoules are manufactured by filling the product into the ampoules, which are then heat sealed. To achieve the quality required of these products, the packaged solution must be sterile and practically free of particles. These products are prepared in clean room conditions (see Ch. 40). Opening glass ampoules may contaminate the product with glass particles; this is a hazard to the patient. Modern glass ampoules have weakened necks to reduce the number of particles. These are composed of a thick-walled glass container that is sealed with a rubber closure. The closure is kept in position by an aluminium seal (see Fig. 32.4), then covered with a plastic cap. The cap is removed before a needle, attached to a syringe, is inserted through the rubber closure to withdraw a dose of product. The contents of the vial may be removed in several portions. Hypodermic syringes and needles are extensively used for administering small volumes of parenteral formulations to the patient. These syringes have been sterilized by ethylene oxide gas or by gamma irradiation following packaging. Various sizes of hypodermic syringes are available. They are composed of a barrel, having a graduated scale, together with a plunger and a headpiece, known as a piston (Fig. 41.2). These components are often made of polypropylene. Water-miscible co-solvents, such as glycerin and propylene glycol, are used as vehicles in small-volume parenteral fluids. They are used to increase the solubility of drugs and to stabilize drugs degraded by hydrolysis.
Parenteral products
 Reasons for parenteral administration
Reasons for parenteral administration
 Routes available for parenteral administration
Routes available for parenteral administration
 Forms and types of parenteral product
Forms and types of parenteral product
 Design of containers for the administration of parenteral products
Design of containers for the administration of parenteral products
Introduction
 Administer drugs if the oral route cannot be used
Administer drugs if the oral route cannot be used
 Deliver drugs to the unconscious patient
Deliver drugs to the unconscious patient
 Rapidly correct fluid and electrolyte imbalances
Rapidly correct fluid and electrolyte imbalances
Administration procedures
IV injections and infusions
SC injections
Products for parenteral use
Injections
Single-dose ampoules
Multiple-dose vials
Administration of small-volume parenteral products
Formulation of parenteral products
Non-aqueous solvents
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Basicmedical Key
Fastest Basicmedical Insight Engine