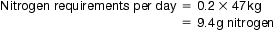44 Today, an increasing number of patients are requesting and being provided with healthcare services at home. Such services include the provision of home parenteral nutrition and home dialysis. This chapter will explore the provision of parenteral nutrition and dialysis for patients in hospital and will explain how these services can be transferred to the home-care setting. Parenteral nutrition formulations are prepared under strict aseptic conditions (see Ch. 40) following guidelines published by the MHRA in Rules and Guidance for Pharmaceutical Manufacturers(2002) and by the DH in Aseptic Dispensing for NHS Patients (Farwell 1995). This chapter concentrates on the provision of adult TPN, although neonatal TPN is available. Some hospitals tailor regimens to individual patients and carry out a number of calculations to determine baseline requirements for each component. In this way they can build up a formulation by matching up the patient’s requirements to commercially available solutions which contain the required components in the correct proportions. Individualized bags tend to be used in patients on long-term TPN. Patients on HPN will always have bags tailored exactly to their nutritional needs. TPN formulations can contain the following components: Lack of nitrogen in the body can result in poor wound healing and interference with the body’s defence mechanisms. To overcome this problem, a utilizable source of nitrogen must be administered to the patient. This is achieved by administering amino acid solutions in a TPN formulation. Nitrogen requirements can be estimated from a 24-hour urine collection. This is done by analysing the total amount of urea excreted and by considering the individual patient’s body weight and clinical ‘type’. The main electrolytes of clinical significance in a TPN formulation include sodium, potassium, magnesium, calcium, phosphate and chloride. The requirement for electrolytes can be met in the form of injectable solutions of varying percentage content. Electrolyte content of each is expressed in terms of mmol/L. The individual role of each electrolyte in a TPN formulation is given in Table 44.1. Vitamins and minerals are normally included in foods taken in orally and must therefore be included in TPN formulations for patients on long-term parenteral nutrition. The NICE guidelines published in 2006 recommend that patients must receive vitamins and trace elements daily in their TPN bags. For patients in hospital, a suitable TPN regimen will be prescribed. On receipt of the prescription, the pharmacist checks the suitability and compatibility of the formulation, the required volume of each component is calculated and details are transferred to a worksheet. Patient details are entered into a computer and labels generated for the worksheet and the final product. In the preparation area, items required for the compounding process are collected together in an appropriate tray ready for transfer to the clean room facility. Batch numbers and expiry dates for each product used are recorded on the worksheet. The pharmacist checks all details, including calculations, before the compounding procedure begins. Compounding of a TPN formulation is carried out under strict aseptic conditions (in a Grade A environment) using a laminar airflow (LAF) cabinet within a clean room facility. Chapter 40 gives details regarding clean room facilities, gowning-up procedures for entry to clean rooms and working procedures for using LAF cabinets. SOPs should be available for all staff carrying out aseptic dispensing procedures.
Parenteral nutrition and dialysis
 Provision of nutritional support for patients
Provision of nutritional support for patients
 Indications for total parenteral nutrition (TPN)
Indications for total parenteral nutrition (TPN)
 Components and compounding of a TPN/home parenteral nutrition (HPN) formulation
Components and compounding of a TPN/home parenteral nutrition (HPN) formulation
 Addition of medicines to a TPN or HPN bag
Addition of medicines to a TPN or HPN bag
 HPN training and potential problems
HPN training and potential problems
 Administration of a TPN/HPN formulation
Administration of a TPN/HPN formulation
 British Parenteral Nutrition Group and British Association of Parenteral and Enteral Nutrition
British Parenteral Nutrition Group and British Association of Parenteral and Enteral Nutrition
 Introduction to home-care for patients on dialysis
Introduction to home-care for patients on dialysis
 Haemodialysis (HD), peritoneal dialysis (PD), including continuous ambulatory peritoneal dialysis (CAPD), intermittent peritoneal dialysis (IPD) and automated peritoneal dialysis (APD)
Haemodialysis (HD), peritoneal dialysis (PD), including continuous ambulatory peritoneal dialysis (CAPD), intermittent peritoneal dialysis (IPD) and automated peritoneal dialysis (APD)
 Provision of services from a hospital renal unit, including home dialysis
Provision of services from a hospital renal unit, including home dialysis
Introduction
Provision of nutritional support
Indications for TPN
 Gastrointestinal disease, including Crohn’s disease, ulcerative colitis, pancreatitis and malabsorption syndrome
Gastrointestinal disease, including Crohn’s disease, ulcerative colitis, pancreatitis and malabsorption syndrome
 Major trauma including severe burns, severe septicaemia
Major trauma including severe burns, severe septicaemia
 Major abdominal surgery; severely malnourished patients may benefit from early peri and postoperative parenteral nutrition if surgery has resulted in a non-functioning gastrointestinal tract
Major abdominal surgery; severely malnourished patients may benefit from early peri and postoperative parenteral nutrition if surgery has resulted in a non-functioning gastrointestinal tract
 Radiation enteritis, when TPN is considered if enteritis is severe after treatment of a primary malignancy
Radiation enteritis, when TPN is considered if enteritis is severe after treatment of a primary malignancy
 High-dose chemotherapy, radiotherapy and bone marrow transplantation. Patients are often ill for a limited time (3–6 weeks) and are unable to eat. TPN can be administered during this period to ensure that the patient’s nutritional requirements are adequately met.
High-dose chemotherapy, radiotherapy and bone marrow transplantation. Patients are often ill for a limited time (3–6 weeks) and are unable to eat. TPN can be administered during this period to ensure that the patient’s nutritional requirements are adequately met.
Assessment of the patient in hospital
The nutrition team
 Training nursing staff in the techniques required for IV administration of TPN fluids
Training nursing staff in the techniques required for IV administration of TPN fluids
 Helping with patient training for HPN
Helping with patient training for HPN
 Monitoring of patients in HPN clinics
Monitoring of patients in HPN clinics
 Liaising with the staff from the home-care company
Liaising with the staff from the home-care company
 Advising on the patient’s drug therapy
Advising on the patient’s drug therapy
Components of a TPN formulation
Protein source
Electrolytes
Vitamins and minerals
Compounding of TPN and HPN formulations
Preparation and training
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Basicmedical Key
Fastest Basicmedical Insight Engine