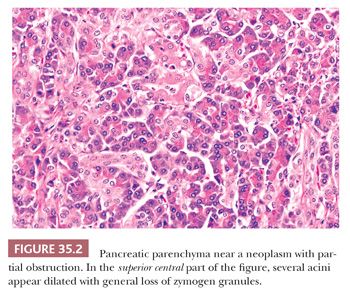
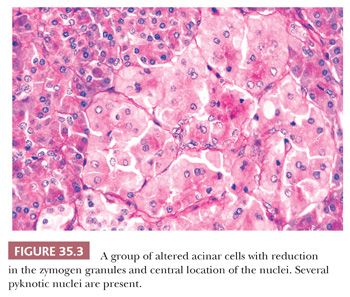
Altered acinar cells are found with moderate frequency when multiple sections of pancreas are evaluated (Fig. 35.2). Acinar dilatation (acinar ectasia) is rare in surgical specimens (28) and has been related to uremia, dehydration, and severe bacterial infections (24). Eosinophilic degeneration of acini, focal acinar cell dysplasia, and localized acinar cytopathology are terms used by various authors to describe a variety of abnormalities apparently having various causes. The most common change consists of small groups of cells having less cytoplasm than normal, with variably reduced basophilia (ribosomes) and zymogen granules. Nuclei are variably placed (often central), often smaller, and appear condensed (even pyknotic in some cells) (Fig. 35.3). Cytoplasmic vacuolation (dilated endoplasmic reticulum) is a fairly common incidental lesion and occasionally may be marked (29). It has been attributed to ischemia including prolonged operations. They seem to be more common in patients with serous cystadenoma. Depending on the cytoplasmic contents, cells are slightly basophilic or slightly eosinophilic. The pallor of the cytoplasm may cause the groups of altered acinar cells to be mistaken for islets of Langerhans (24). These changes are probably retrogressive but may be reversible. In contrast, the basophilic atypical foci that have been also termed as dysplasia often show nuclear enlargement and prominent nucleoli. Their nature and significance is unknown (30,31). Less commonly, there are groups of slightly enlarged acinar cells filled with zymogen granules and with virtually no cytoplasmic basophilia. Rare vacuoles occur in the cytoplasm. Nuclei appear normal and are basal (4). This alteration could represent an abnormal acinar secretory mechanism.
Altered acini have been noted incidentally in a wide variety of disorders, both intrapancreatic and extrapancreatic, including alcoholism and pancreatic endocrine excess. Heavy cigarette smoking and chemotherapy have been associated with the acinar changes just discussed, especially the dysplastic nuclear alterations (31–33).
Dilatation of the major ducts and of tiny peripheral ducts (with inspissated secretions common in the latter) occurs fairly frequently in older individuals (24). Dilated minor ducts tend to be associated with fibrosis.
Multilayered ductal epithelial metaplasia, focal or widespread (including characteristic squamous metaplasia) (1,34), may be present in medium-sized and large ducts in normal pancreata and in chronic pancreatitis. They may appear atypical and mistaken for neoplastic change. There does not appear to be any significant association with carcinoma (1), and in fact, these may have inverse correlation with the incidence of pancreatic intraepithelial neoplasia (PanIN).
Tubular complexes is the name assigned for a form of acinar injury in which the acinar units acquire visible lumen formation and the cells are attenuated. The change is noted most often in chronic pancreatitis and in acute pancreatitis undergoing healing. Some of the “ductulized” acini may contain intraluminal inspissated secretions. In some examples, a transition from acinar units to fully formed ductal units can be observed, some with mucinous cytoplasm. Exaggerated form of this in which the ductal units form a large nodule composed of small mucinous ducts has also been termed adenomatoid ductal hyperplasia is the old literature. Occasionally, such lesions form a grossly visible nodule, in which case it would be more appropriate to classify the process as a variant of intraductal papillary mucinous neoplasm.
Columnar cells with increased amounts of supranuclear mucus-rich cytoplasm (mucinous cell hyperplasia, goblet cell metaplasia) may occur with increased age, in chronic pancreatitis, after large doses of adrenal cortico steroids, and in association with pancreatic ductal carcinomas; they are found most often in ducts of the head and rarely in acini. This change has also been called pyloric gland metaplasia, having mucus that is periodic acid-Schiff (PAS) positive and Alcian blue negative at pH 2.5 (i.e., neutral mucin). A considerable proportion of these cells may contain alterations in the K-ras oncogene (35–38). For this reason, these are now considered part of the PanIN-1A spectrum (see detailed discussion in “Pancreatic Intraepithelial Neoplasia” section) (39).
Pseudopapillary and papillary proliferation (35,38,40) of the ductal epithelium occurs in medium-sized and large ducts and is variably reported with aging, chronic pancreatitis, diabetes mellitus, and ductal carcinoma. These are now regarded as PanINs (39).
Another change occasionally evident is selective congestion and dilatation (peliosis) of the vessels of the islets without marked congestion of the vessels elsewhere in the lobules.
Large islets may occur in the pancreata of middle-aged and elderly persons. These tend to have a greater proportion of non–insulin cells than do normal islets. The pathologist must remember that defining a patchy or localized hyperplasia of neuroendocrine elements (and possible associated ductal structures) versus a small neuroendocrine neoplasm sometimes may be difficult:
1. Patchy hyperplasia has multiple islets, most of which are normal in size or perhaps slightly larger. The islets can be present lying in peripancreatic adipose tissue, individually or in clusters. This phenomenon can be especially striking in the tail of the organ; however, it can be distinguished from a true infiltration of a pancreatic neuroendocrine tumor by the rounded and separated nature of the nests (islets) and their morphology is identical to the other islets in the same pancreas.
2. Occasionally, a fairly large islet, which may or may not have cytology different from other islets, is encountered in a fibrotic pancreas. Although this occurs more commonly in the setting of multiple endocrine neoplasia type 1 (MEN1), occasionally it can be found sporadically. Such lesions may represent precursor (incipient) neoplasia.
Moreover, proliferation and enlargement of islets and abnormal islets may accompany the pancreatic neuroendocrine tumors (41,42). All types of islet cells are represented in these neoislets and enlarged islets, but a shift in the islet cell population may occur; glucagon-producing tumors may be accompanied by islets in which the islet glucagon cells are smaller than usual and sparse. In the pancreas outside an insulin-producing tumor, the insulin cells may be altered in number, and the proportion of glucagon cells and somatostatin cells may be increased (42). Likewise, the presence of abnormal islets, proliferation of islet cells, and various changes in exocrine elements have been noted in instances of the Zollinger-Ellison syndrome (43).
Nesidioblastosis is a term originally employed for the enlarged islets seen in newborns of mothers with hyperglycemia, which leads to in utero suppression and postpartum compensatory hyperactivity of the islets of Langerhans. Boys are affected slightly more frequently than girls, with the disorder usually discovered within the first few weeks of life in large-for-gestational-age babies of nondiabetic mothers (44). The disorder is diagnosed biochemically through a battery of highly specialized tests of glucose, insulin, C-peptide levels, ketones, and glucagon response coupled with arterial calcium stimulation or percutaneous transhepatic pancreatic venous sampling. There may be deviations from the normal histologic pattern of the pancreas expected for the age of the child, but often, morphologic alterations are absent or minimal (45). The disorder is found in diffuse (functional abnormality of islets in the whole pancreas) or focal forms (focal islet cell hyperplasia), with different treatment implications. When histologic changes are present, they consist of the following:
1. Relatively large, localized, hypertrophic collections of islet cells displacing acinar tissue and containing a neoproliferation of islet cells from ducts (focal adenomatosis, ductuloinsular complexes)
2. The very rare, but similar, diffuse proliferation of islet cells (generalized adenomatosis)
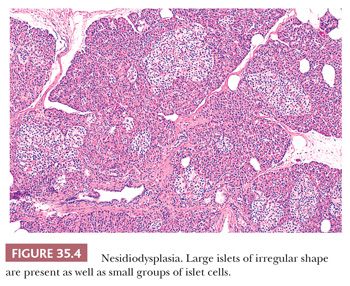
3. Neuroendocrine cell “dysplasia” or nesidiodysplasia (loss of the usual centrilobular concentration of larger islets, increased numbers of small aggregates of islet cells distributed irregularly in the lobules, irregularity of the contour of the islets) (Fig. 35.4)
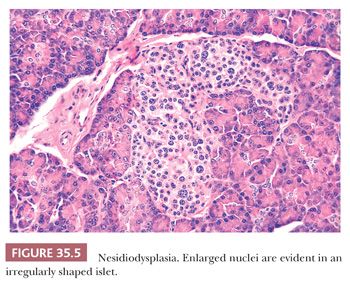
4. The presence of scattered islet cells (mostly insulin cells) with hypertrophic nuclei (Fig. 35.5) (46–49)
For many of these changes, there are no reliable numeric or morphologic criteria. In fact, changes similar to these can be observed in normal population. Specific genetic alterations (SUR1 [sulfonylurea receptor gene at chromosome 11p15.1], Kir6.2 [inwardly rectifying potassium channel], or GCK/GLUT1, ABCC8, and KCNJ11 mutations) suggest the severity of the disease, the type of β-cell (insulin-producing cell) abnormality, and which therapy would be most appropriate (45,49,50). Partial to near-total pancreatectomy is the treatment of choice in cases refractory to aggressive medical management, although enucleation of focal islet cell adenomatous hyperplasia may be of value in controlling the symptoms. Careful examination of different parts of the pancreas allows for such a determination based on the extent of the disease. Although axiomatic, diabetes mellitus and pancreatic insufficiency (malabsorption) are complications (44,50,51).
Of note, hyperinsulinemic hypoglycemia in adults is usually the result of a neuroendocrine neoplasm releasing insulin in abnormal amounts or at inappropriate times. However, in a small number of adult patients with hyperinsulinemic hypoglycemia or other evidence of endocrine hyperfunction, no neoplasm has been detected (47,52–54). Instead, other features have been described, such as (a) numerous islet cells (singly or in clusters, with or without cytologic abnormalities), apparently arising from ducts (Fig. 35.6); (b) increase in the size and number of otherwise normal islets; (c) irregularly shaped large islets unevenly distributed through the lobules; (d) numerous septal islets; (e) localized extraordinary increases in the amount of islet tissue (focal adenomatosis); and (f) abnormal characteristics of hyperplastic islets in cell cultures in vitro (54). The condition is called endocrine cell nesidiodysplasia or adult nesidioblastosis (47,52–54), which may be encountered after gastric bypass surgery (55).
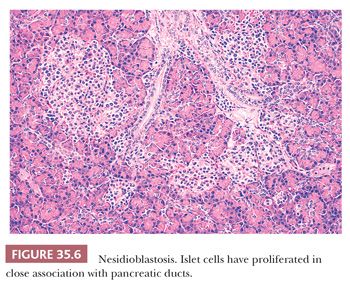
It is important to note here, again, that the findings described in these conditions are often subtle and nonspecific but, in the right context, would be confirmatory of the clinical impression. However, in any of these conditions, before the diagnosis can be rendered, a diligent search for a neuroendocrine tumor is essential. In fact, the patient ought to be considered to have a neuroendocrine tumor unless definitively proven otherwise with all possible methods.
DIAGNOSTIC TECHNIQUES
Fine-needle aspiration (FNA) has become a primary diagnostic method for pancreatic lesions. In the differential diagnosis of chronic pancreatitis and pancreatic ductal adenocarcinoma, an FNA specimen may in fact be more informative than a needle core biopsy specimen because the FNA can sample a wider area and also avoids the dilution by the desmoplastic stroma because typically most of the aspirate is the epithelial component (adenocarcinoma) (56–63). Complication rates are also lower. For this reason, percutaneous biopsies are obtained only in select patients nowadays.
Inadequate samples result from failure to master these deceptively simple techniques. Common problems in wedge biopsies and in core needle biopsies are crushing or otherwise distorting the tissues, failure to obtain enough tissue for adequate interpretation, and the frequent presence of chronic pancreatitis obscuring the ductal carcinoma. Difficulties with FNA result from missing the lesion because of improper localization and/or incorrect placement of the needle, applying too much suction (which causes bleeding, dilutes the sample, and interferes with further sampling), using too long a needle (which bends, misses the target, and makes the procedure awkward), and using a needle larger than 22 gauge (59,64).
The interpretation of aspirates requires that the pathologist be acquainted with the appearance of the following normal cells:
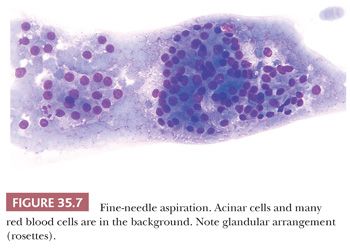
1. Acinar cells (Fig. 35.7) are arranged in small groups or clusters resembling rosettes and sometimes may show a central lumen but otherwise indistinct cellular borders. They have a moderate amount of cytoplasm (finely granular and with few vacuoles) and small, round, regular nuclei with visible nucleoli.
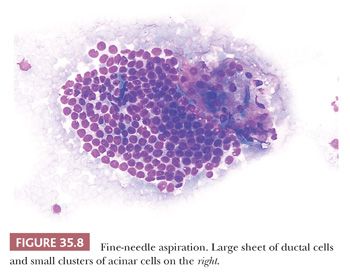
2. Ductal cells (Fig. 35.8) are arranged in monolayers of more than 50 nuclei. These nuclei are smaller and have denser chromatin than those of acinar cells; nucleoli are not evident, and the cytoplasm is scanty. The cells appear tightly packed and in a honeycomb arrangement.
3. Neuroendocrine (islet) cells have features intermediary between acinar and ductal. They typically form monolayers. The distinctive salt-and-pepper chromatin pattern is characteristic. They may also form rosette-like structures and show granules.
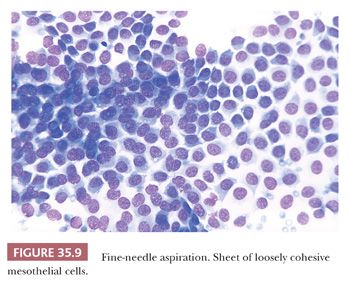
4. Mesothelial cells (Fig. 35.9) may be a source of false-positive diagnosis. They have a distinctive pattern at low magnification, appearing as large sheets or monolayers of cells with moderate amounts of bluish cytoplasm and well-demarcated cell borders. Frequently, windows or separations between adjacent cells are seen. Cytoplasmic vacuoles of variable sizes are present. Large nuclei (two to three times the size of ductal cell nuclei) and prominent nucleoli are common.
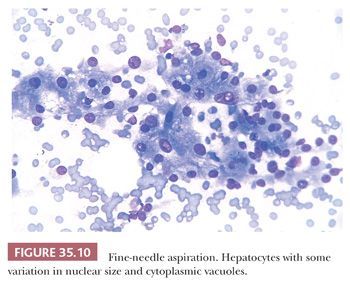
5. Hepatocytes (Fig. 35.10) are arranged in groups and cords of variable sizes and also are seen as single cells or naked nuclei. There is more variation in nuclear size than in acinar, ductal, or mesothelial cells. Nucleoli are larger than those of mesothelial cells. The abundant cytoplasm usually has pigment granules (either bile or iron) and/or vacuoles of different sizes.
It is important not to overdiagnose the minor alterations of epithelial cells that may be identified when chronic pancreatitis is present. In addition to its considerable success in separating ductal carcinomas from benign lesions, aspiration cytology has allowed the recognition of other pancreatic neoplasms and has provided cells for histochemical, ultrastructural, biomarker, and DNA studies (65–67).
PANCREATIC TRANSPLANTATION
Transplantation of the pancreas (often combined with kidney transplantation) has become increasingly accepted as a treatment for young to middle-aged adults afflicted with insulin-dependent diabetes mellitus (type 1) (68–75). Allograft rejection can be assessed by needle biopsies of the graft (76–78) and/or cytologic studies of pancreatic juice drained from the graft (74,79).
In allogeneic grafts undergoing acute T-cell–mediated allograft rejection, depending on the grade of rejection, the lesions include acinar inflammation with or without acinar cell injury/necrosis, ductitis (infiltration of ductal epithelium by mononuclear and/or eosinophilic inflammatory infiltrates and ductal epithelial cell damage), septal inflammation (predominantly mononuclear lymphocytes and variable numbers of eosinophils), neural and perineural inflammation, venulitis (subendothelial inflammatory infiltration and endothelial damage/lifting), as well as arteritis ranging from minimal intimal arteritis (rare subendothelial/intimal mononuclear inflammatory infiltration with no evidence of endothelial damage) to necrotizing arteritis (transmural inflammation) (78). In general, diffuse acinar inflammation with multicellular/confluent acinar cell necrosis and/or intimal arteritis and/or necrotizing arteritis is associated with decreased allograft survival.
Antibody-mediated allograft rejection is best identified by a combination of serologic (identification of circulating donor-specific antibodies) and morphologic findings including acinar/interacinar inflammation, acinar cell injury (cytoplasmic swelling and vacuolization as well as apoptotic or necrotic cell dropout), interacinar capillaritis, as well as C4d immunohistochemical/immunofluorescence staining in interacinar capillaries (77,80). Of note, acute T-cell–mediated allograft rejection and antibody-mediated allograft rejection may coexist and should be recognized and graded independently (I–mild, II–moderate, and III–severe; for details, refer to Banff Schemas for grading pancreas allograft rejection [77,78]).
Chronic allograft arteriopathy (fibrointimal arterial thickening with narrowing of the lumen) was initially considered to be an expression of T-cell–mediated allograft rejection (81), but recent studies have shown that acute and chronic arterial lesions can be also associated with antibody-mediated allograft rejection (82). Accordingly, this lesion is now listed as a separate morphologic category (77). Recognition of chronic allograft arteriopathy in biopsy samples is clinically important because it indicates ongoing (chronic) alloimmune injury and for its association with late graft thrombosis (71).
In chronic allograft rejection/graft fibrosis, there is fibrosis, depending on the grade, ranging from only expansion of fibrous septa to extensive fibrosis with isolated areas of residual acinar tissue and/or islets present (77,78). A trichrome stain is particularly useful to demonstrate interacinar fibrosis in the earlier stages and to assist in the identification of specific structures or pathologic changes (i.e., denuded ducts, fibrinoid necrosis in arterial walls, etc.)
Forms of rejection (acute T-cell–mediated allograft rejection vs. antibody-mediated rejection [77] vs. chronic allograft arteriopathy vs. chronic allograft rejection/graft sclerosis) need to be separated from nonimmunologic causes of allograft dysfunction (e.g., donor disease, ischemic/preservation injuries, thrombosis, bacterial or fungal infection, cytomegalovirus pancreatitis, posttransplantation lymphoproliferative disease, etc.). Immediately after surgery, grafts are altered by interstitial edema and blood, fat necrosis, mild acinar cell injury, and a slight infiltrate of neutrophils and lymphocytes with histiocytes (posttransplantation ischemic pancreatitis). Later, even in syngeneic grafts, there are frequent sparse inflammatory cell infiltrates (mostly lymphocytes) of the acinar tissue, loss of acinar cells, fibrosis, and slight ductal alterations (inflammation, dilatation, and some cellular atypia). Acute pancreatitis, which may occur early or late in the graft, has neutrophilic infiltrates, necrosis of acinar cells, and fat necrosis (69). It is important to note that biopsy techniques are not without their complications, including hemorrhage, fistula, severe pain, and pancreatitis (68,83). As time passes, a successful graft may be affected by recurrent autoimmune diabetes mellitus: The islets are infiltrated by mononuclear cells (predominantly T lymphocytes), insulin cells decrease in number, and there is a substantial relative increase in glucagon cells. Posttransplantation lymphoproliferative disorder is often Epstein-Barr virus related and may overlap histologically with allograft rejection. However, the atypical, plasmacytoid B-cell proliferation affecting the parenchyma in a random fashion (rather than predilection for the acinar tissue), with expansive nodules extending into the peripancreatic soft tissue, will help to make the separation; treatment is diametrically opposite, making accurate diagnosis critical (69,84).
INFLAMMATORY CONDITIONS
ACUTE PANCREATITIS
Acute pancreatitis is fundamentally a clinical syndrome characterized by abdominal pain suggestive of pancreatitis (epigastric pain often radiating to the back), elevated serum amylase and lipase levels (at least three times the normal), and characteristic findings of acute pancreatitis on imaging (85). Its overall incidence varies in different parts of the world, ranging from 5 to 80 people per 100,000 of the population (86). The major etiologic factors, although geographic region dependent, include high alcohol consumption (especially in men) and biliary tract disease (especially calculi in older women), whereas ischemia, systemic shock, various drugs (e.g., estrogens, corticosteroids, immunosuppressives, azathioprine), instrumentation, pancreatic trauma (often a severe disease course), hypercalcemia, hyperlipidemia, infections (viruses, especially in human immunodeficiency virus [HIV]–infected patients), pregnancy, and genetic factors (mutations in cystic fibrosis gene [CFTR], trypsinogen gene [PRSS1], and pancreatic secretory trypsin inhibitor gene [SPINK1] recurrent acute pancreatitis only) round out the top conditions (86–89) (Table 35.1). No matter what the trigger, there is cell injury with acute inflammatory mediators and vascular compromise, leading to necrosis and possible multiorgan dysfunction (90,91). Radiographic assessment of the extent of inflammation and necrosis within 48 hours allows for a more standardized diagnosis, severity assessment, treatment plan, and complication identification (92–97). Nevertheless, the process may be difficult to separate from chronic pancreatitis. The surgical pathologist rarely receives tissue, but occasionally, a pancreatic biopsy or partial resection of damaged tissue is performed. The gross appearance ranges from a slightly swollen, wet, firm organ to hemorrhagic and/or necrotic tissue. Fat necrosis is manifested as grayish-yellow plaques and nodules in extrapancreatic and intrapancreatic adipose tissue.
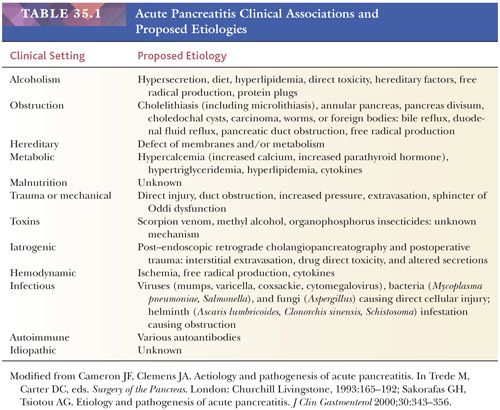
Histologic features vary with the severity of the process and yield a different clinical course (85,86,98–100). The revised Atlanta Classification (101) separates acute pancreatitis into acute edematous pancreatitis and acute necrotizing pancreatitis, although both show a variable degree of autodigestive tissue necrosis (85,101,102). In edematous (or interstitial) pancreatitis, the interstitial fibrous tissue, adipose tissue, and pancreatic parenchyma are edematous and may contain scattered acute inflammatory cells (86). It should be kept in mind that neutrophils may be limited in acute pancreatitis. Limited fat necrosis may be present in and around the gland (86) (Fig. 35.11). The pancreatic acini adjacent to the fat necrosis often are dilated, and some acinar cells may be necrotic (autodigested). However, zonal necrosis of acinar tissue is very uncommon in conventional acute pancreatitis other than rare locally ischemic or infected cases. Because changes in edematous (or interstitial) pancreatitis are most marked in the fibrous and adipose tissues and much of the pancreatic parenchyma is preserved (although altered), a substantial reconstitution of normal structure and function is possible, provided the acute attack is only minimally destructive. It is important to note that edematous (or interstitial) pancreatitis has been detected without any known symptoms or signs of pancreatic inflammation during the patient’s life.
When acute necrotizing pancreatitis is present, marked hemorrhage, panlobular coagulative necrosis, and extensive fat necrosis of peripancreatic tissues occur (86,89,98,99,103). A layer of neutrophilic leukocytes lies in the region between the intact tissues and the necrosis; thrombi are common in capillaries and venules in this same region. Some of the intact pancreatic acini adjacent to the necrotic tissues are dilated, contain inspissated secretions, and have flattened cells with smaller and fewer zymogen granules. Intraductal and periductal inflammation, ductal dilatation, and ductal disruption have been described, but many ducts are intact. It is possible that these changes occur more often in cases of acute exacerbation of chronic pancreatitis than in acute pancreatitis. Viral or bacterial pancreatitis may be manifest by necrosis of individual acinar cells (99).
In addition to the revised Atlanta Classification, there are other systems used to classify the severity of acute pancreatitis, including the Acute Physiology and Chronic Health Evaluation (APACHE II) Scale, Ranson Criteria, Glasgow, and the computed tomography severity index (CTSI) (86,95–97,104–106). Each of these systems has utility, although there are also individual drawbacks (107,108).
Major anatomic complications of acute pancreatitis seem to develop more commonly in acute necrotizing pancreatitis and include peripancreatic fluid collections, pancreatic and peripancreatic necrosis (sterile or infected), pseudocyst (occur after 3 to 5 weeks; see discussion later in this chapter), walled-off necrosis (sterile or infected), splenic and portal vein thrombosis, and fat necrosis in distant sites (100,109–111). These result in possible organ failure. These changes, in particular, pseudocyst, appears to be much more commonly associated with alcoholic rather than other types of acute pancreatitis. Although uncommon, there is a definite mortality associated with acute pancreatitis (overall, up to 8%), often related to inflammatory mediators that are released, which lead to multiorgan dysfunction or failure and septic complications (Escherichia coli derived from the intestines). Nearly half of all deaths occur within the first weeks after onset of pancreatitis. Significant long-term morbidity in exocrine (fatty stools or chronic pancreatitis) and endocrine insufficiency (diabetes mellitus) is seen in up to 50% of patients, whereas chronic pancreatitis develops in approximately 10% of patients. Recurrences are common if chronic alcoholism and cholelithiasis are not treated (88,109–111). Enteral nutrition, supportive care, and possible antibiotic therapy if there is infected necrosis help improve patient outcome (92).
CHRONIC PANCREATITIS
Chronic pancreatitis is an ill-defined disorder with many etiologic factors, many classification systems, significant morbidity, as well as a variety of genetic factors, which influence the type of therapy that will yield the best long-term patient outcome. Needless to say, the significant architectural and cytologic alterations of the pancreas, whether predominantly focal, segmental, or diffuse, are of interest to the surgical pathologist because they simulate a pancreatic neoplasm on gross examination or because a neoplasm often is accompanied by “chronic pancreatitis” (peritumoral pancreatitis) (112,113). Because such chronic changes are so common in resected pancreata, we believe it is better not to render the diagnosis of chronic pancreatitis unless there were clinical manifestations of chronic pancreatitis.
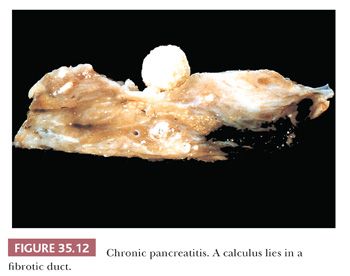
The pathogenesis includes a host of factors that, through a variety of pathways, result in acinar cell injury and upregulation of trypsin activation, producing an inflammatory response with cytokines. The cytokines (produced from leukocytes, macrophages, platelets, and acinar cells) cause activated pancreatic stellate cells to secrete collagen, and fibrosis begins, starting the pathway to chronic pancreatitis (113–115). Ethanol consumption and ductal obstruction by calculi are the most common etiologies in much of the world (Fig. 35.12), similar to the causes of acute pancreatitis (89,99,113,116) (Table 35.1). However, alcohol alone appears to confer only marginal risk for chronic pancreatitis (117), with risk increasing with tobacco smoking (118). Other etiologic factors include toxic or metabolic factors (e.g., tobacco smoking, drugs, toxins, chronic renal failure, hypercalcemia, hyperlipidemia), recurrent and severe acute pancreatitis, autoimmune factors (including Sjögren syndrome, primary biliary cirrhosis, Crohn disease, ulcerative colitis), obstructive factors (e.g., pancreatic divisum, sphincter of Oddi disorders, duct obstruction [i.e., stones, tumor], primary sclerosing cholangitis), and idiopathic factors (113–115,119–129). Continued exposure to these etiologic agents (especially ethanol) probably leads from multiple relapses of recurrent acute pancreatitis to progressive chronic pancreatitis, which is clinically expressed through steatorrhea, diabetes mellitus, and pancreatic calcifications (113–115,130,131), although this process does not develop in all patients.
Genetic variants in cationic trypsinogen gene (PRSS1), anionic trypsinogen gene (PRSS2), pancreatic secretory trypsin inhibitor gene (SPINK1), cystic fibrosis transmembrane conductance regulator gene (CFTR), and to a lesser extent, chymotrypsinogen gene (CTRC) and calcium sensing receptor gene (CASR) have been identified as being associated with susceptibility to chronic pancreatitis. Although all of the current major genetic susceptibility factors center on the control of trypsin activity within the pancreas, there are additional genetic variants being evaluated, which will likely be added to this list in the future (87,117,132–135).
A variety of radiographic studies are helpful in diagnosing chronic pancreatitis; however, they are usually performed when it is already well developed. Endoscopic ultrasound (EUS) and 2-(18F)-fluoro-2-deoxy-D-glucose positron emission tomography (FDG-PET) are the studies frequently used, the latter assisting with differentiation from carcinoma (136). More recently, magnetic resonance imaging (MRI) has become the method of choice.
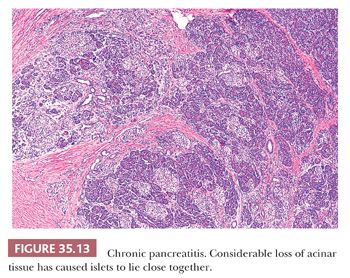
In chronic nonobstructive pancreatitis, inflammation, fibrosis, and acinar atrophy are unevenly distributed, with some lobules almost untouched and others markedly involved (Fig. 35.13) (99,100). Even in advanced disease, the lobular pattern of the organ is preserved. The extent of the chronic inflammatory infiltrates is variable, and such infiltrates can be minor. The inflammation can be centered around the ducts. Intrapancreatic nerves are increased in number and diameter. Fibrosis (periductal, intralobular, and interlobular) is a major feature but is variable in amount and distribution (Figs. 35.14 and 35.15) (100,114,137). Part or the entire organ is enlarged and hard. Progression leads to a shrunken and distorted gland in the late stages of the disease.
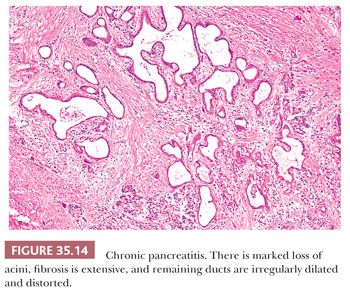
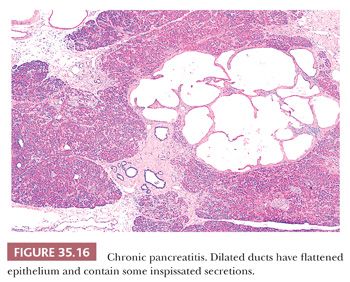
The normal ductal pattern is altered variably by deletion, stricture, and dilatation (Fig. 35.16) (99,100). In alcoholic and idiopathic pancreatitis, protein-rich secretions (often calcified) plug many small ducts and ductules; well-developed calculi of various sizes frequently occur; and the adjacent ductal epithelium undergoes metaplasia, atrophy, or disappears altogether. Many small ducts are dilated, with marked ectasia of even the smallest ducts evident in some lobules. Dilated periductal vessels are engorged with erythrocytes. Groups of tiny ducts may be present and probably are the result of several factors, including the loss of zymogen granules and reduction in the height of acinar cells (formation of tubular complexes); possible loss of acinar cells; the persistence of ductular-ductular and ductular-acinar anastomoses; and possible proliferation of cells of the smallest ducts, redifferentiating into tubular complexes through morphologic plasticity. Saccular dilatations of larger ducts are common and are visible on radiologic examination or gross inspection. Pseudocysts (see discussion later in this chapter) are identified in and adjacent to the pancreas due to the resorption of peripancreatic adipose tissue. Autodigestive (fat) necrosis is seen concomitantly with pseudocyst formation.
Alterations in the ductal epithelium are frequent and include local ulcerations, reactive atypia, squamous metaplasia, atrophy, as well as mucinous changes (PanIN-1A) and proliferative changes of more advanced PanINs (116,137). K–ras mutation, p53 alterations, and increased Ki-67 labeling index have been identified in epithelial alterations of chronic pancreatitis. Aspirates from pancreatitis (acute and/or chronic) show many acinar cells, a mixture of inflammatory cells (neutrophilic leukocytes, lymphocytes, plasma cells, and histiocytes), and a few sheets of ductal cells (138,139). Granular debris, precipitated enzymatic secretions, and hematoidin pigment are characteristic findings.
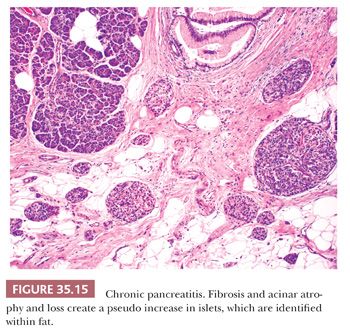
The islets of Langerhans are relatively resistant to chronic pancreatitis in comparison with the pancreatic acini (140). As acini disappear and fibrosis and fatty infiltration occur, the lobules collapse and the islets tend to be concentrated together, resulting in an apparent increase in islet cell mass. Although this phenomenon has been labeled islet cell hyperplasia in the past, there is no evidence of true proliferation. In fact, as the process progresses, the insulin cells are reduced in number, and glucagon cells are proportionately increased (140). Thus, the better term is islet aggregation (Fig. 35.15). Eventually, many patients with chronic pancreatitis develop diabetes mellitus.
When chronic pancreatitis results from obstruction of a major pancreatic duct, the affected tissue may be more evenly involved, the process typically is less severe, and the ductal epithelium is less altered than in chronic calcifying pancreatitis. Intraductal proteinaceous plugs and small calculi are rare. The process may be more marked in the head than elsewhere in the gland or may involve only one group of lobules, depending on the location of the obstruction.
Chronic pancreatitis involving the tail of the pancreas often afflicts the peripancreatic soft tissues including the splenic vessels. Splenic complications such as focal necrosis, foci of inflammation, and granulomas are not uncommon in patients with chronic pancreatitis.
Any pancreatic neoplasm can compress or obstruct the ducts, thereby favoring the development of pancreatitis in part of the gland, and this inflammation and fibrosis in turn make recognition of the neoplasm more difficult. The fibrosis and calculi that occur in many cases of chronic pancreatitis not only obstruct the ducts of the pancreas but also may block the biliary tree. If the resultant jaundice is accompanied by marked fibrosis of the pancreatic head, both the surgeon and the pathologist find it difficult to exclude the presence of carcinoma. In fact, chronic pancreatitis is considered to be a risk factor for the development of pancreatic carcinoma. Therefore, when major pancreatic resection for complicated chronic pancreatitis is performed to relieve the patient’s symptoms, careful examination is suggested to exclude concurrent carcinoma.
Endotherapy and surgery are used for the treatment of painful chronic pancreatitis to try to improve the patient’s quality of life. Surgery and/or drainage versus sphincterotomy and stenting can yield different long-term results depending on the factors managed (pain and/or weight gain). Pain is separated into short and relapsing (type A) versus continuous and requiring constant analgesics (type B); although pain may be modulated, the specific type of pain treated will skew results (116,141–146). Complications and mortality are related to pancreatic carcinoma, bleeding, anastomotic leaks, diabetes mellitus, continued smoking, and constant pain. Cessation of alcohol consumption is one of the most important factors in long-term survival. Some forms of chronic pancreatitis appear to benefit more from surgery than others.
AUTOIMMUNE PANCREATITIS
Autoimmune pancreatitis (AIP), a relatively recently defined distinct form of pancreatitis, has been divided into two types—type 1 and type 2—which share certain clinical similarities but are vastly different in terms of pathology and extrapancreatic features (147–153).
Type 1
Type 1 AIP (previously known as lymphoplasmacytic sclerosing [LPSP]) is regarded as a prototypical organ manifestation of IgG4-related disease (154,155), which can occur alone or either simultaneously or metachronously with other organ complications. The pancreas was the first organ in which IgG4-related disease was identified, but the disease has now been described in virtually every organ system: the biliary tree, meninges, orbital tissues (e.g., lacrimal gland, extraocular muscles, and retrobulbar space), salivary glands, lymph nodes, thyroid gland, lungs, pericardium, aorta, breast, kidneys, prostate, retroperitoneum, and skin (154,156–161).
AIP type 1 specifically lacks conventional risk factors for pancreatitis, including alcohol use and cholelithiasis. The disease predominantly affects elderly males, a demographic profile that does not overlap with other forms of chronic pancreatitis (162). The patients exhibit symptoms of chronic pancreatitis and painless obstructive jaundice (158–161). The serum IgG4 concentration is elevated (>135 mg/dL) in many patients but it may be normal in up to 40% of patients with biopsy-proven AIP type 1 (163). To date, there have been studies of AIP and antibodies to lactoferrin, carbonic anhydrase isoforms II and IV, pancreatic secretory trypsin inhibitor (PSTI; product of the SPINK1 gene), as well as to less sensitive or specific markers of autoimmunity, such as antinuclear antibody and rheumatoid factor association. Although there are some strengths of association with PSTI antibodies, none of these biomarkers appears to be sensitive or specific enough to serve as distinctive evidence of AIP (164–166).
There is a localized to diffuse swelling of the pancreas, centered in the head, with irregular narrowing of the pancreatic ductal system (159–162,167–172). The characteristic appearance on CT imaging for diffuse pancreatic involvement is a sausage-shaped enlargement with homogeneous attenuation, moderate enhancement, and a peripheral halo at the rim of hypoattenuation. Although these findings may mimic a pancreatic head carcinoma (in focal disease), pancreatic ductal narrowing in the pancreas is highly suggestive of AIP. ERCP shows focal, diffuse, or segmental attenuation of the main pancreatic duct with loss of right angle branches (162,168). EUS is also used, especially to guide FNA or biopsy of the hypoechoic parenchyma (168,169,172–174).
The three major histopathologic features associated with AIP type 1 (and IgG4-related disease in general) are the following:
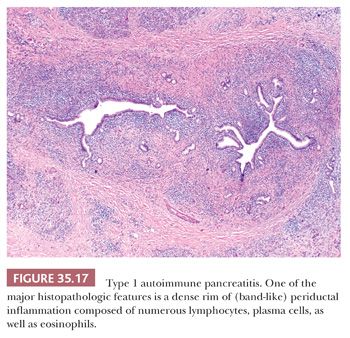
1. Dense lymphoplasmacytic infiltrate (Fig. 35.17)
2. Fibrosis
3. Obliterative phlebitis (Fig. 35.18) (147,149–151,154,155,157,162,167,171,175–177)
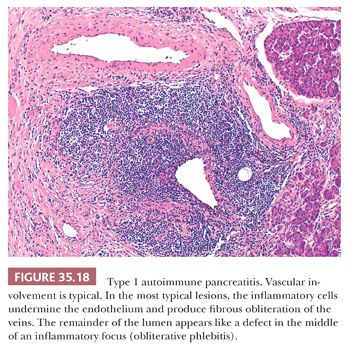
The inflammatory infiltrate is composed of lymphocytes (predominantly of T cells, with scattered aggregates of B cells), plasma cells, as well as eosinophils, which may be prominent enough to raise the possibility of “eosinophilic pancreatitis.” Although the inflammatory cells tend to aggregate around ducts (159–161,168,171,178), this infiltrate invariably extends into the lobules, the peripancreatic adipose tissue, as well as the intrapancreatic portion of the bile duct. The fibrosis, characterized by robust fibroblasts/myofibroblasts buried within the inflammatory infiltrate, is invariably organized in a storiform pattern (155). In rare cases, the amount of fibroblastic activity resembles inflammatory myofibroblastic tumor (159,179). The venous channels are obliterated by a dense lymphoplasmacytic infiltrate (obliterative phlebitis) (180). Fully obliterated veins may require elastin stains for identification. Obliterative thrombophlebitis, especially involving larger venules, appears to be significantly more common in AIP type 1 than other inflammatory injury of the pancreas, including tumors (180). Calcification, fat necrosis, and cyst formation are not seen.
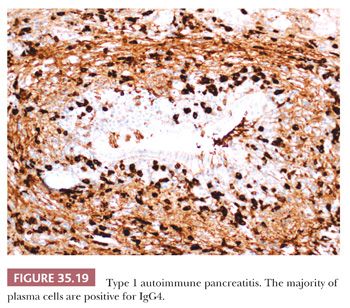
On immunohistochemistry, the majority of plasma cells are positive for IgG4 (Fig. 35.19). The finding of more than 50 IgG4+ plasma cells/hpf is considered highly specific for AIP type 1 (147,155,162,181–183). On biopsy specimens, the presence of more than 10 IgG4+ plasma cells/hpf has been proposed as one component of a comprehensive diagnostic panel (168). However, an elevated IgG4+-to-IgG+ plasma cell ratio of more than 40% is more meaningful than IgG4+ plasma cell counts alone in establishing the diagnosis (155,157). Of note, there is no gold standard approach for counting IgG4+ plasma cells. Because the IgG4+ cell distribution may be patchy, counting only areas of intense IgG4 focus (“hot spots”) might be more representative. It should be kept in mind, though, that neither an increase in serum IgG4 nor the finding of elevated numbers of IgG4+ plasma cells in tissue is specific for AIP type 1 (or IgG4-related disease in general). Thus, the diagnosis of AIP type 1 requires both characteristic histologic features (described earlier) and increased numbers of IgG4+ plasma cells (or an elevated IgG4+-to-IgG+ ratio) in tissue (155).
To identify the full spectrum of changes occurring in AIP, one must recognize its five cardinal features (the Mayo Clinic’s HISORt criteria): suggestive Histology showing lymphoplasmacytic infiltrate with storiform fibrosis, Imaging showing a diffusely enlarged pancreas, Serology showing elevated IgG4 levels, or evidence of Other organ involvement and Response to steroid therapy (168,184). AIP should be suspected in patients with obstructive jaundice, pancreatic mass/enlargement, or pancreatitis who have one or more HISORt criteria (168,184).
Type 2
Clinical data from histologically confirmed AIP type 2 (previously known as idiopathic duct-centric pancreatitis) cases show that they have distinctly different profile compared with AIP type 1 cases. AIP type 2 seems to be a pancreas-specific disorder. It is not associated with either other organ involvement or with serum IgG4 elevation typically seen in AIP type 1. However, lack of other organ involvement or absence of serologic abnormalities in patients with AIP does not necessarily imply the diagnosis of type 2, as type 1 also can be without other organ involvement and seronegative. Although inflammatory bowel disease seems to be associated with both forms, these are more common in type 2. Approximately 30% of reported cases of AIP type 2 have associated inflammatory bowel disease, such as ulcerative colitis. Patients with AIP type 2 are, on average, a decade younger than AIP type 1 patients and do not show a sex predilection. Currently, AIP type 2 lacks a serologic biomarker (149,151).
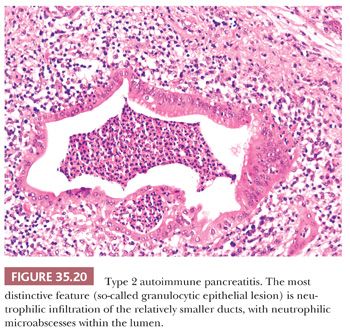
The most distinctive feature of the AIP type 2 is a dense periductal lymphoplasmacytic inflammation accompanied by neutrophilic microabscesses within the lumen (Fig. 35.20), the so-called granulocytic epithelial lesion (GEL), involving medium-sized and small ducts as well as in acini (147,149–153,162,185). The inflammation is generally not as dense as that seen in type 1, although it often leads to the destruction and obliteration of the duct lumen. The interlobular fibrosis lacks fibroblastic/myofibroblastic cell infiltrate, and storiform-type fibrosis is rarely prominent. Although some veins are focally involved by the lymphoplasmacytic infiltrate, overt obliterative phlebitis is uncommon. AIP type 2 cases has none or very few (<10 cells/hpf) IgG4+ plasma cells (147,162). As mentioned earlier, type 1 can be diagnosed without histology, but type 2 requires an adequate histologic specimen to make a definitive diagnosis (149,151).
As AIP does not uniformly involve the pancreas, core needle biopsy and FNA seldom yields sufficient proof for the diagnosis of AIP. If specific features such as obliterative phlebitis or GELs are identified, they are almost pathognomonic for AIP, with obliterative phlebitis favoring type 1 and GEL favoring type 2 (186). In most cases, however, such changes are either partially present or difficult to recognize. Under these circumstances, careful correlation with the clinical scenario and imaging characteristics is often required to arrive at a definitive diagnosis (150,152,155,186,187).
Recently, a distinct subset characterized by arteritis with fibrinoid necrosis in medium-sized and large pancreatic arteries and lacking the stigmata of well-known two subtypes has been described (188). This arteritic-type AIP appears to be associated with collagen vascular diseases such as systemic lupus erythematosus (188).
Regardless of subtype, it is important to recognize AIP because it is considered a reversible pancreatitis (189). The pancreatic (and extrapancreatic) manifestations respond to steroid therapy within an interval of a few months (159–161,169,190). Although relapses are common, especially in AIP type 1 (191), retreatment with steroids remained effective at inducing remission (149,189). If endocrine function is also compromised, it may be a reversible form of diabetes mellitus.
Recent reports have described the development of synchronous and metachronous pancreatic adenocarcinomas in patients with AIP type 1, raising the possibility that patients with AIP may be associated with an elevated risk of malignancy (162,189,192,193). It should be reiterated that ordinary peritumoral pancreatitis can show many features of an AIP type 1, including periductal lymphoplasmacytic inflammation and even occasional periphlebitis.
EOSINOPHILIC PANCREATITIS
Eosinophilic pancreatitis is an exceedingly uncommon pancreatitis that usually occurs either in the setting of eosinophilic gastroenteritis or hypereosinophilic syndrome with systemic manifestations such as peripheral eosinophilia, elevated serum IgE levels, and/or eosinophilic infiltrates in other organs. Isolated eosinophilic infiltration of the pancreas is less common (194,195). Patients present clinically with either symptoms of acute pancreatitis or pancreatic obstructive lesion suspicious for carcinoma. Radiographic findings reveal obstruction, suggestive of tumor. The histologic features include a dense eosinophilic infiltration of the pancreas (and/or bowel wall) along with a history of atopy. Rare pseudocyst formation has been reported (196). Surgery is used to yield a diagnosis. The differential diagnosis includes allograft rejection, AIP type 1, and inflammatory myofibroblastic tumor and histiocytosis X. Some cases appear to be eosinophil-rich variant of paraduodenal pancreatitis described in the following section.
PARADUODENAL (GROOVE) PANCREATITIS
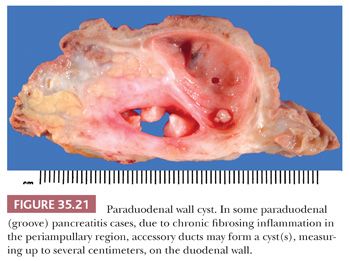
Paraduodenal pancreatitis, also known as groove pancreatitis or cystic dystrophy of heterotopic pancreas, is a distinctive variant of pancreatitis that occurs in the “groove” area, the tissue between the duodenal wall and the pancreatic head. It is often centered around the accessory duct and accessory (minor) ampulla (197–199). The vast majority of patients are young males in their 40s, often with a history of alcohol abuse. A significant proportion of paraduodenal pancreatitis cases present with a clinical diagnosis of “periampullary cancer” or “pancreatic cancer” (137,200,201). Focal thickening and abnormal enhancement of the second portion of the duodenum and “tubulocystic” change in the vicinity of the accessory duct and duodenal wall are highly specific features of this entity by MRI (202–204).
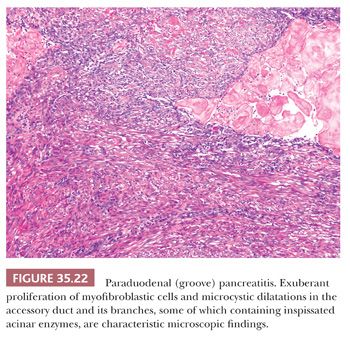
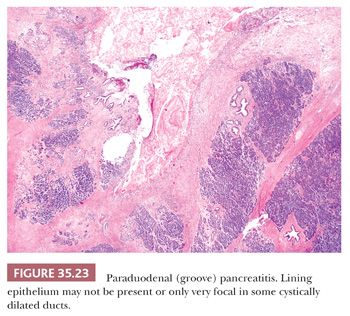
The reasons for this process to develop especially around the accessory ampulla or accessory duct are not known. In some cases, pancreas divisum (persistence of embryologic-type, dorsal-ventral separated drainage systems) is suspected. One possibility is the occlusion of a functionally overactive accessory duct by yet unknown mechanisms in some cases precipitated by alcohol abuse. However, the macroscopic and microscopic findings are quite distinctive: The process leads to narrowing of the duodenal lumen and the duodenal mucosa often acquires a nodular or cobblestone appearance (205). Upon sectioning, the duodenal wall, especially in the vicinity of the minor ampulla, shows a trabeculated appearance to the duodenal musculature, which is often accompanied by cystic change (Fig. 35.21). Some cases designated clinically as duodenal duplication prove to be paraduodenal pancreatitis. In some cases, cyst formation may be prominent, measuring up to several centimeters in size (“paraduodenal wall cyst”). Microscopically, the duodenal mucosa often reveals Brunner gland hyperplasia and there is an exuberant myofibroblastic proliferation (Fig. 35.22), often arranged in fascicles accompanied by small, well-circumscribed lobules of pancreatic tissue (“myoadenomatosis” pattern) or variably sized ducts (“cystic dystrophy of heterotopic pancreas”). These ducts may contain inspissated acinar enzymes (Fig. 35.23). The cyst contents may extravasate and lead to the development of a foreign body giant cell reaction and stromal eosinophilia. Some cysts are devoid of epithelium. Instead, they are lined by more cellular fibroblastic tissue. On occasion, the lining fibroblasts may appear epithelioid and raise concern for a sarcomatoid carcinoma (199,204).
OTHER INFLAMMATORY DISEASES
Infections
Specific infectious and parasitic diseases sometimes involve the pancreas, and these have the pathologic features expected for the particular organism (206–210). In the immunosuppressed patient, opportunistic infections can involve the pancreas (211).
Idiopathic Granulomatous Inflammation
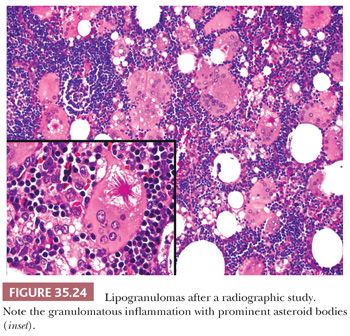
Nonspecific granulomatous inflammation of the pancreas should not be mistaken for sarcoidosis (212), mycobacterial disease (209), or fungal disease (213) (Fig. 35.24). Possible causes of granulomas in chronic pancreatitis, different from granulomatous pancreatitis, include Crohn disease, rheumatoid, and foreign body granulomas (postsurgical, iatrogenic, inspissated secretions) (214).
Tropical (Calcific) Pancreatitis
Tropical pancreatitis is a disorder that seems to present in patients from developing countries of the tropics. Patients present at a young age, mostly as teenagers, with malnutrition, abdominal pain, steatorrhea, and diabetes and lack of alcoholism or biliary tract disease. There is marked pancreatic damage with multiple large duct calculi (215–217). An anomalous pancreaticobiliary ductal union, which may be related to the etiology, was reported to be more common in tropical pancreatitis (218). Mutations in pancreatic secretory trypsin inhibitor (SPINK1) and cathepsin B (CTSB) genes have been found to be associated with tropical pancreatitis (219,220). Recently, novel variants in chymotrypsin C gene (CTRC) in tropical pancreatitis have also been reported. Interestingly, the mutation spectrum of CTRC in Indians appears to be different from Europeans, suggesting allelic heterogeneity (221). There is an established increase in the incidence of carcinoma associated with this disorder.
Hereditary Pancreatitis
Hereditary pancreatitis is an unusual form of recurrent acute pancreatitis and chronic pancreatitis that begins in childhood (median age of onset, 10 years) or young adulthood (115,121,122,127,134,135,222–230). It is a rare, heterogeneous familial disease with a prevalence of 0.3 per 100,000 in Western countries (226). Hereditary pancreatitis should be suspected in any patient who has suffered at least two attacks of acute pancreatitis for which there is no underlying cause and unexplained chronic pancreatitis with a family history in a first- or second-degree relative (231). The majority of hereditary pancreatitis has been shown to be due to gain-of-function mutations of the cationic trypsinogen gene (PRSS1) (134). Recent whole exome sequencing identified deleterious genetic changes in two other major pancreatitis-associated genes (pancreatic secretory trypsin inhibitor gene [SPINK1] and cystic fibrosis transmembrane conductance regulator gene [CFTR]) (222). PRSS1 mutations are inherited in an autosomal dominant pattern with variable penetrance, whereas mutations in SPINK1 and CFTR can be inherited in multiple modalities (134). These patients have a high incidence of pancreas cancer (226,227,231). The relative risk of cancer appears to be increased in smokers (223).
PSEUDOCYSTS
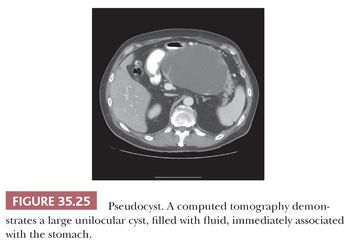
A pseudocyst is a cystic cavity intimately associated with the pancreatic tissues by an inflammatory reaction without an epithelial lining (214,232,233). A pseudocyst may or may not be connected with a pancreatic duct. Acute fluid collections (in early acute pancreatitis) and pseudocysts (acute or chronic) account for approximately 80% of all cystic lesions of the pancreas, although pseudocysts are uncommon (~1 in 100,000 population/year) (106,234,235). Pancreatitis (acute, chronic, or hereditary), trauma (including iatrogenic), ductal calculi, and obstructing neoplasms are the common causes (Fig. 35.25) (100,234,236), although a significant majority of the cases prove to be alcoholism related. The lesions result from resorption of necrotic peripancreatic adipose tissue and are usually unilocular (90%). Pseudocysts range from 2 to 35 cm. The usual contents are watery to thick fluid containing necrotic debris, fibrin thrombi, serum, and blood. The fluid may be rich in amylase, lipase, and trypsin (234,237) and have a low carcinoembryonic antigen (CEA) level. A variety of classification systems exist, but terminology is confusing because “acute” and “chronic” are not used consistently and apply to both timing and histologic composition. The acute fluid collection lacks epithelium and a well-defined wall (85,234). In early stages, a pseudocyst has a wall composed of shaggy and friable granulation tissue, granular debris, outlines of necrotic or healing adipose tissue, and part of the pancreas; it contains an inflammatory infiltrate (including eosinophils) and lacks an epithelial lining (Fig. 35.26) (194,233,234). In later stages, the wall often becomes more fibrotic and paucicellular. Hematoidin pigment is often present. These findings, however, are not uncommon in true cystic neoplasia of the pancreas, which often undergo secondary inflammatory and degenerative changes. Therefore, specimens should be carefully examined for epithelial cells or other characteristic components of cystic pancreatic tumors. Solid pseudopapillary tumors are particularly prone to be mistaken, both clinically and microscopically, as pseudocysts. Mucinous cystic neoplasms also become infected and/or inflamed quite often (232). The pseudocyst often lies outside the pancreas (usually between the stomach and transverse colon), may become infected (an abscess is a dangerous complication), and may erode into blood vessels, resulting in massive intra-abdominal bleeding. Perforation into a hollow viscus and compression of adjacent organs are other complications. The management generally entails excluding a cystic neoplasm using radiographic techniques, FNA of the fluid, and fluid evaluation. Surgical removal of the cyst is much less commonly employed than previously. Most cases are managed conservatively or by marsupialization (238). Occasionally, surgical resection of the cyst is used for symptomatic patients, after complications (e.g., infection, bleeding, obstruction), or when malignancy cannot be excluded. In fact, many cases that undergo surgical resection prove to be a neoplasm mimicking pseudocyst (see the following discussion). Percutaneous drainage, endoscopic drainage, or internal drainage can complement one another depending on location and anatomic variables (235,239). Pseudocysts located in the head and measuring less than 4 cm in greatest dimension are more likely to undergo spontaneous regression or persistence without symptoms (235). Factors associated with morbidity include older age, alcohol, clinical severity of pancreatitis, residual necrosis, and type of treatment.
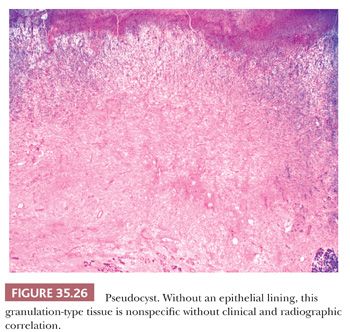
It should be noted here that the cysts encountered in paraduodenal (groove) pancreatitis are also inflammation-associated but may have an epithelial lining in some areas because they are typically connected to the inspissated ducts with rupture (“cystic dystrophy of heterotopic pancreas”).
DUCTAL NEOPLASMS
Most pancreatic neoplasms are of ductal lineage (Table 35.2). Ductal differentiation in this organ is characterized by tubular configuration, mucin production (except serous neoplasms), papilla formation, and/or intraductal growth. There is a spectrum of ductal neoplasms in the pancreas (Table 35.3).

The most important ductal neoplasm is the invasive ductal adenocarcinoma, also known as pancreatobiliary-type adenocarcinoma, characterized by infiltrating tubular units composed of low cuboidal cells with variable amounts of intracytoplasmic mucin. There are morphologic variants of pancreatobiliary-type adenocarcinoma with more foamy or clear cells, larger tubules, papillary or cribriform patterns, and others, and these are all regarded within the realms of pancreatobiliary-type adenocarcinoma. There are also carcinomas of ductal origin that are regarded separately from pancreatobiliary-type adenocarcinoma, although they are closely related to and may be even admixed with the latter in some cases. These are colloid, adenosquamous, medullary, hepatoid, undifferentiated, and undifferentiated with osteoclast-like giant cell–type carcinomas.
Preinvasive ductal neoplasms can fundamentally be regarded in two categories: (a) microscopic forms of dysplastic changes, namely PanINs; and (b) intraductal neoplasms (tumoral intraepithelial neoplasms), namely intraductal papillary mucinous neoplasms (IPMNs) and intraductal tubulopapillary neoplasms (ITPNs), which are fundamentally mass-forming (often cystic) forms of dysplastic process (i.e., adenoma-carcinoma sequence). Closely related to this group are mucinous cystic neoplasms (MCNs), which are in many ways similar to intraductal neoplasms but are characterized by ovarian-type stroma and are believed to be de novo ductal neoplasms.
Serous cystadenomas are the only ductal neoplasm that is characterized by nonmucinous cells and thus believed to be centroacinar/intercalated duct origin, and it is also the only ductal neoplasm without any overt potential for malignant transformation into invasive carcinoma, presumably related to the nonmucinous nature of the cells.
DUCTAL ADENOCARCINOMA
Demographics and Clinical Features
Pancreatic ductal adenocarcinoma (PDAC) is the most frequent solid neoplasm of the pancreas, demonstrating a phenotype similar to ductal epithelium. The incidence varies from 1 to 12 per 100,000 population in developed countries. It is the fourth leading cause of cancer deaths in the United States, with more than 39,000 annual deaths, as compared to 29,000 by prostate (240). The trajectory in the incidence of pancreas cancer–related deaths is such that it is estimated that it is most likely going to be higher than that of breast cancer (40,000 annual deaths in the United States) within a few years (241). PDAC is one of the deadliest of all cancers, with a 5-year survival still below 5%. The patients are usually between 60 and 80 years of age; men are affected slightly more commonly than women, with African Americans having a distinctly higher rate than whites (1,242–244). Rarely, patients are less than 40 years old (245). Symptoms are nonspecific, including abdominal pain, weight loss, jaundice, and pruritus. Many patients present with ongoing back pain before the diagnosis is established. Carcinoma in the head of the pancreas commonly causes painless obstructive jaundice. Carcinoma of the body and tail tends to extend to the peritoneum, the spleen, the stomach, and the left adrenal, resulting in higher stage disease as a result of delayed detection in this anatomic site (242,243). A few patients with pancreatic cancer have a recent onset of diabetes mellitus. Ductal carcinomas usually grow rapidly and are discovered after they have already spread beyond the pancreas. Only about a quarter are deemed resectable at the time of diagnosis. Metastases to lymph nodes, peritoneal surfaces, and the liver are common, regardless of the location of the primary focus or the size of the neoplasm (242). Cigarette smoking, chronic pancreatitis, gastric surgery, chemical exposure, radiation, and long-standing diabetes mellitus are suggested possible etiologic factors or associations (242–244). Some families have high incidence (246–251). A variety of radiographic studies can assist in the diagnostic workup and include MRI, ERCP, and EUS. Among these, CT is still one of the most widely used, although in expert hands, MRI has clear superiority in the diagnosis and differential diagnosis. These studies can also assess lymph node disease and extent of involvement. Nowadays, tissue diagnosis is established by EUS-guided biopsy in most cases. Tumor markers (CA19-9, DuPan-2, CEA, and CA125) and mucin profile analysis on fluid or cytology specimens may also help with diagnosis (57,59,64,65,138,252–254), but the sensitivity and specificity of these markers are suboptimal (255).
Clinical Differential Diagnosis; Pseudotumors
The diagnosis of PDAC is a challenge at the clinical as much as it is at microscopic level. Chronic pancreatitis of any type and a variety of pseudotumors can closely mimic PDAC. In fact, about 3% to 14% of pancreatic resections performed with the clinical diagnosis of PDAC prove to be a benign condition on microscopic examination (256–259). This figure is 5% in our experience. Among chronic pancreatitides especially, two entities are notorious for their mimicry of cancer (what we call pseudotumoral pancreatitis) and are typically indistinguishable from carcinomas even in expert hands utilizing best imaging modalities and expertise: (a) AIP and (b) paraduodenal (groove) pancreatitis. As discussed previously, AIP may show the distinctive sausage-shaped enlargement and a peripheral halo by imaging; however, this is identifiable only in a limited percentage of cases (162,168). Serum IgG4 levels above 135 mg/dL can be helpful but not entirely specific (163). For paraduodenal pancreatitis, the challenge is even more dramatic at times as paraduodenal pancreatitis can have a pattern of “infiltration” to the mesenteric/portal vessels (199–201). Recognizing the duodenal wall alterations including the tubulocystic change in the vicinity of accessory duct and accessory ampulla are the main clues to the diagnosis at MRI (202–204). There are other lesions that are prone to be mistaken as cancer including adenomyomatous hyperplasia of the ampulla, hamartomas (260), etc. (see further discussions in pseudotumors).
Macroscopic Features
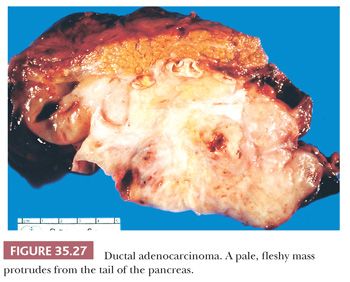
On gross examination, ductal carcinomas usually appear as a poorly defined, scirrhous, pale, hard mass (Fig. 35.27), with a mean size of about 2.5 to 3.5 cm, which may be accompanied by enlarged lymph nodes (1). Approximately two-thirds of pancreatic adenocarcinomas involve the head with consequent duct dilatation; the remainder involves the body and tail. In a few instances, the entire gland appears to be involved, but these are mostly arising from intraductal neoplasms. Usually, there is only a single focus, but in a few patients, there are multiple foci (either multicentric origin or intraglandular spread by cancerization of the ducts and/or acini). Cystic presentation is uncommon (236,261).
Microscopic Features
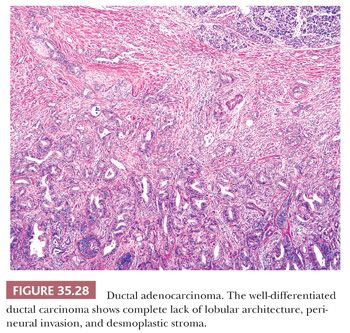
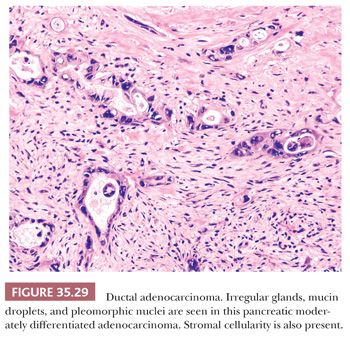
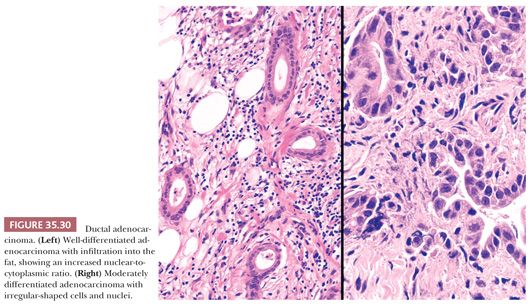
Ductal carcinoma is characterized by atypical cells forming irregular, often incomplete, often complex, tubular or glandular structures, usually accompanied by dense stroma (Figs. 35.28 and 35.29) (1,242). Typically, the tubular structures are usually relatively small and widely scattered and are lined by one to two cell layers of low cuboidal cells. Many cells and their nuclei are much larger than those of normal ducts, and the nuclear-to-cytoplasmic ratio is high. Variation in the shape and size of the nuclei is common (Fig. 35.30). Conspicuous nucleoli are frequently present, and mitotic figures are usually easy to find. The cellular arrangement is often haphazard (the cells’ polarity is disturbed), no definite basement membrane is present around the epithelial structures, and isolated atypical neoplastic cells are noted in the stroma (262). Incomplete duct formation (abortive gland formation), often accompanied by free mucin, is characteristic of carcinoma. Most ductal adenocarcinomas are well to moderately differentiated and tubule formation is identifiable, at least in some foci. In well-differentiated carcinoma, the architecture may be the only hint that a carcinoma is present (1,242).
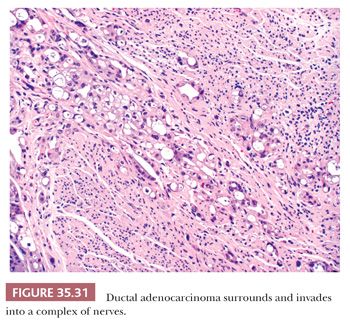
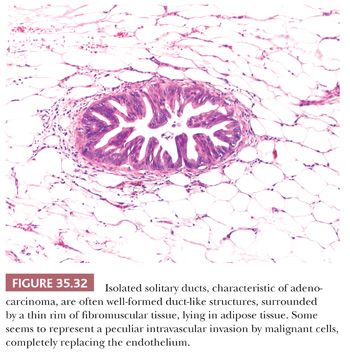
Necrotic cellular debris, some admixed with neutrophils, can be seen in the neoplastic ductal lumens. Invasion into tiny vessels, the islets, and around nerves can almost always be found at the time of diagnosis (Fig. 35.31). Invasion of intrapancreatic nerves is frequent, and spread into the nerves outside the pancreas is correlated with shorter postoperative survival (263,264). Lymphovascular invasion results in distal nodal and liver, lung, or adrenal gland metastases (265). Inappropriately large droplets of mucus may be seen in the cytoplasm of individual carcinoma cells, and pools of mucus may occur in the stroma. Cancer can extend through the ducts beyond the grossly evident tumor (cancerization of the ducts) (266). Similarly, sampling of soft tissues covering the pancreas often yields subtle microfoci of carcinoma composed of isolated solitary ducts lying individually within the adipose tissue (Fig. 35.32), away from the main tumor, most representing intraparenchymal vascular spread (267–269). Thromboemboli are moderately common and may be more likely when the tumor involves the tail. Lymph nodes around the pancreas nearly always contain metastatic foci, but the groups of nodes involved vary depending on the location of the cancer in the organ. The adjacent uninvolved pancreatic parenchyma frequently shows intraductal neoplasia (precursor lesions), atrophy (as a result of duct obstruction or direct compression by the tumor), a “relative” increase in the number of islets or neuroendocrine cells (islet aggregation), and a lack of calcifications (usually seen in chronic pancreatitis of alcoholism).
Immunohistochemical and Molecular Features
Seldom used for diagnostic purposes, immunohistochemistry studies may be of value in separating primary from metastatic disease (either in the pancreas or in other sites). The neoplastic cells show reactivity for CK7, CK8, CK17, CK18, CK19, Cam5.2, MUC1, MUC3, MUC4, MUC5AC, MUC6, CA19-9, CA125, CEA(m), DuPAN-2, TAG72, HER-2/neu, and p16 immunohistochemistry studies, whereas they are negative with vimentin, MUC2, and β-catenin (66,242,270–273). CK20, if present, is usually focal. Sialylated MUC1 glycoprotein expression (membrane or cytoplasmic) reportedly is associated with a more aggressive patient outcome, whereas MUC2, a marker for more indolent behavior, is seen in IPMNs and colloid carcinoma. MUC4 expression increases progressively with increasing architectural disturbances and cytologic atypia (66,270,274,275).
Chromosomal abnormalities are common (276). Cytometry usually has shown that a majority of ductal carcinomas are nondiploid; these are less likely to be resectable, and patients with such tumors have considerably shorter survivals. Molecular analyses have demonstrated multiple different abnormalities associated with PanIN and invasive ductal adenocarcinoma. New evidence suggests that different mutations are associated with variable tumor progression and patient outcome. K-ras and HER-2/neu are seen early, with p16 detected in precursor lesions and p53 and SMAD4 (DPC4) alterations later in the carcinogenic pathway. K-ras mutations, specifically at codon 12, are the most commonly identified mutations (>90%) (244,277–285). The tumor suppressor gene SMAD4 (DPC4) in the transforming growth factor (TGF)-β signaling pathway found on chromosome 18q21.1 (a nuclear transcription factor) is inactivated in many infiltrating adenocarcinomas, possibly associated with a worse clinical outcome (279,286–289). It has been advocated that loss of SMAD4 (DPC4) immunohistochemical expression, which is seen in almost half of PDACs, can be used as strong evidence for pancreatic carcinoma, both in the differential diagnosis from noninvasive lesions as well as with other carcinoma types. Analysis of gene expression has revealed a number of other overexpressed molecules within ductal adenocarcinomas (e.g., fascin, mesothelin, claudin-4, S100AP, S100A6, and S100P), some of which have been proposed as potential immunohistochemical markers to help distinguish reactive (nonneoplastic) glands from carcinoma (290–294). A list of prognostic factors for exocrine neoplasms of the pancreas is illustrated in Table 35.4, although it is by no means exhaustive.
Up to 10% of patients with pancreatic cancer have a family history of this disease (272,295,296). Also, some pancreatic cancers arise in patients with recognized genetic syndromes, including hereditary pancreatitis, familial atypical multiple mole melanoma, BRCA2 kindred, Peutz-Jeghers syndrome, and in hereditary nonpolyposis colon cancer families (242,293). p16 appears to be the molecular link in the patients with familial atypical multiple mole melanoma (FAMMM) syndrome (297,298).
Morphologic Variants of Pancreatic Ductal Adenocarcinomas
In addition to the fundamental pattern described earlier, PDACs can exhibit a variety of morphologic variants that ought to be recognized because they can be helpful in their accurate diagnosis and differentiation from other tumor types.
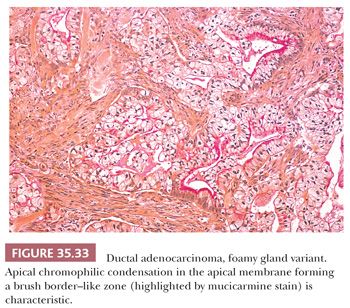
Foamy Gland Variant. Some examples are characterized by abundant very pale foamy/microvesicular cytoplasm, in which the vesicles are very fine and even. Typically, the glands are very well formed and the nuclei are well polarized at the periphery of the cells, and thus the overall pattern is deceptively benign-appearing. In addition to its distinctive cytoplasmic characteristics, this variant can be distinguished from benign noninvasive ducts by the common presence of an apical chromophilic condensation in the apical membrane, forming a brush border–like zone (Fig. 35.33). The cytoplasmic borders are also distinct. Moreover, the nuclei, if pushed at the periphery, are often hyperchromatic and raisinoid, with frequent irregularities caused by the vesicles indenting the nucleus. If the nuclei are more central and preserved, then the contour irregularities are less prominent but nucleoli may be visible. Occasionally, foamy cells form stromal clusters mimicking collections of macrophages. Foamy cell change is also fairly common in cases treated with neoadjuvant treatment (299).
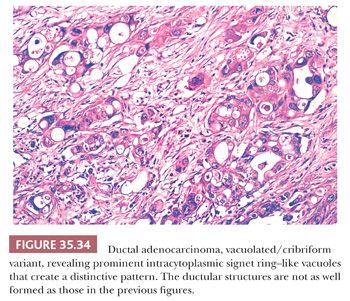
Vacuolated/Cribriform Variant. PDAC is associated with prominent intracytoplasmic signet ring–like vacuoles that create a distinctive microcystic or cribriform pattern (300). Typically, the vacuoles are large, multicell size (Fig. 35.34). When prominent, they can resemble lipocytes and can be mistaken in frozen section specimens as degenerating adipose tissue, or as lipogranulomas in lymph nodes, which are common in this region. Typically, some of the vacuoles contain mucinous material, which may form a targetoid appearance. This pattern is misdiagnosed under the heading of “signet ring” carcinoma; however, typically, the signet ring–shaped cells are seen in clusters forming a cribriform architecture and not infiltrating the stroma as individual cells or cords that are required to define signet ring (“poorly cohesive”) carcinomas. Often, the mucin in the vacuoles is accompanied by nuclear debris and granular material. Invariably, by close inspection, the nuclei are large, hyperchromatic, and pleomorphic. Thus, this variant is rather easy to recognize as malignant by high-power examination. However, the vacuoles can be helpful in the diagnosis in cytologic specimens. Additionally, this distinctive pattern is fairly specific for PDACs and can be very helpful in recognizing it in metastatic locations, particularly in the liver (301).
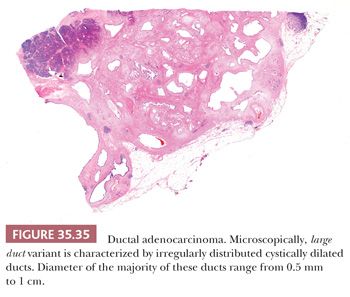
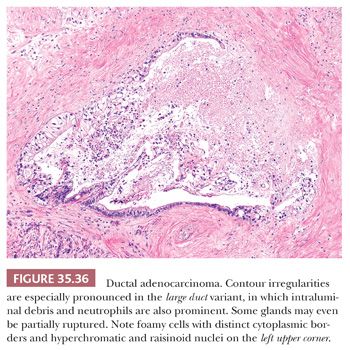
Large Duct Variant. Although most PDACs are characterized by small tubular pattern, in some cases, the infiltrating glands may be fairly large and well-defined and thus mimic preinvasive neoplasia (302,303), such as PanINs or intraductal neoplasms. In fact, the tubular elements can be so large and cystic that these have been also mistaken as MCNs. However, the large duct variant of PDAC often has very angulated contours and wide, open lumen formation (Fig. 35.35) in contrast with preinvasive neoplasia, in which duct contours are typically smooth and undulating, and the lumen is compressed or filled with epithelial elements. In addition, in large duct variant of PDAC, although some ducts may have well-organized papillary elements within, in most units, the epithelium will be flat or irregular. Often, the cytologic features are foamy variant or show other subtle features described for adenocarcinomas earlier. Partial duct rupture akin to the microcystic, elongated, and fragmented (MELF) pattern described in endometrial cancers is not uncommon, and often, the lumen has neutrophils or necrotic granular debris (Fig. 35.36).
Although most PDACs are solid scirrhous lesions, some cases present as a cystic lesion. There are several mechanisms for this to develop. Some examples appear to be a markedly cystic version of the large duct cases described earlier. In some, there appears to be marked secondary duct ectasia of the upstream ductal system that leads to the cystic mass. Often, the cyst lining shows colonization (cancerization) by invasive carcinoma glands. In some cases, the cystic component is attributable to a residual IPMN or MCN component that is now mostly replaced by the invasive adenocarcinoma. Yet some other examples appear to be carcinomas arising in otherwise innocuous-appearing cystic duct ectasia, a process that we refer to as cystic mucinous duct lesion (304).
Poorly Cohesive (with/without Signet Ring Cells) Variant. Focally, a PDAC can exhibit cordlike and even individual cell infiltration pattern, but this is invariably in the context of and closely admixed with an ordinary tubular pattern of PDAC. Usually, even in the same area, abortive glandular formations are evident. Furthermore, these areas typically have significant cytologic atypia and pleomorphism that is unusual for ordinary poorly cohesive–type carcinomas of the gastrointestinal (GI) tract. The monotony and insidious pattern that characterize gastric poorly cohesive carcinoma, mammary lobular carcinoma, or plasmacytoid urothelial carcinoma is typically nonexistent in PDACs. In fact, if a diffuse infiltrative carcinoma with the usual pattern is identified in the pancreas, this typically proves to be of ampullary origin rather than pancreatic or a metastasis from another site.
So-Called Papillary Adenocarcinoma Variant. Papilla formation is one of the common and defining features of ductal differentiation in the pancreas. As such, any ductal tumor in the pancreas may show some papilla formation. For tumors like IPMN and ITPN, the papilla formation is such an integral part of the tumor that it has been incorporated into their name. Florid papillary nodules, detectable grossly, are also seen in about 15% of MCNs. Moreover, low level of papilla formation is also seen in PanINs and PDACs, and in fact, in large duct variant of PDAC, this can be quite prominent. The cases reported in the literature under the heading of “papillary adenocarcinoma” are mostly cases that would be classified nowadays as either pancreatobiliary-type IPMNs or large duct adenocarcinomas. Additionally, ampullary and common bile duct (CBD) carcinomas are also often rich in papilla formation and ought to be considered in the differential diagnosis of papilla-forming tumors.
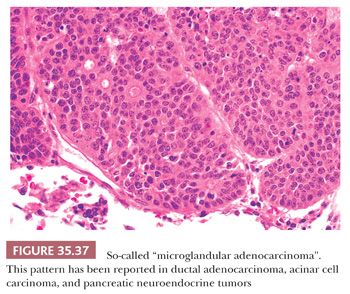
So-Called Microglandular Adenocarcinoma Variant. The nature of the cases (Fig. 35.37) that had been reported under the heading of the so-called “microglandular adenocarcinoma” is debated (305–308). There are different tumor types that can form a pattern qualified as “microglandular.” It has been suggested that microglandular adenocarcinoma is just a pattern of growth seen in ductal, acinar, neuroendocrine, and mixed tumors (308).
Micropapillary Variant. True micropapillary pattern, with solid clusters of cells suspended in lacunar spaces, usually with intraepithelial and stromal tumor infiltrating neutrophils, are very rare in the pancreas. In fact, if there is a poorly differentiated carcinoma with extensive micropapillary pattern in the pancreas, it typically proves to be of ampullary origin (309).
Cytologic Diagnosis
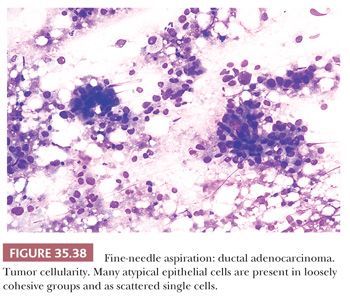
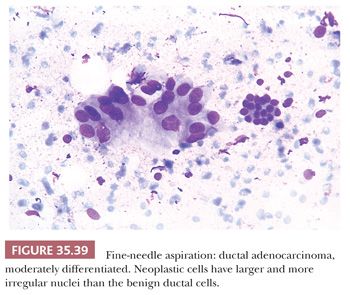
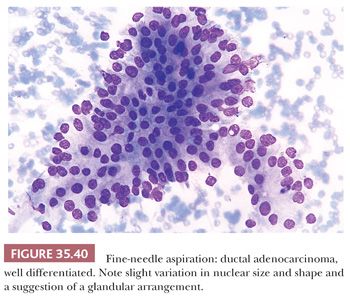
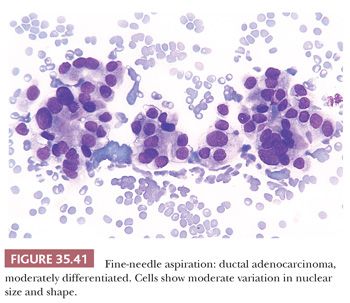
Aspirates from ductal adenocarcinoma yield specimens of variable cellularity (Fig. 35.38). EUS-guided FNA biopsy is the most common modality used in current practice. Consistent cytologic features include a paucity of acinar cells and increased numbers of atypical ductal cells arranged in sheets, three-dimensional clusters, or single cells. Contaminants from the GI tract may be problematic; however, these can be distinguished from ductal adenocarcinoma by their honeycomb arrangement, the presence of goblet cells, and a distinct brush border. The latter two features are seen in duodenal epithelial contaminants but not in gastric epithelium. The neoplastic cells of PDAC vary in size, shape, and degree of cohesiveness. Cell borders are usually easily identified and tumor cells have scant to abundant cytoplasm. When tumor cells form disorganized “drunken” honeycomb sheets, their nuclei are often only minimally enlarged and cells may show palisading, slight crowding, or overlapping. Nuclear enlargement is a very helpful feature in the diagnosis of PDAC, especially when well-differentiated and if benign clusters are available for comparison (Fig. 35.39). The degree of nuclear atypia and cellular cohesion varies according to the degree of differentiation of the carcinoma (Figs. 35.38 to 35.41). For less differentiated tumors, more bizarre nuclei and single cells are seen. A greater than 4:1 variability in nuclear size (so-called fourfold anisonucleosis) is considered a very helpful diagnostic clue, although this refers only to a given cell cluster and not the entire specimen (310,311). When present, foamy gland features (which include foamy/microvesicular cytoplasm; distinct cytoplasmic borders; mild cellular disorganization; and hyperchromatic, irregular, raisinoid nuclei) are also helpful clues to the diagnosis (310). Additionally, the presence of large intracytoplasmic vacuoles containing targetoid mucin or debris is also diagnostic of PDAC. Marked nuclear contour irregularity is seldom a prominent feature in benign conditions (like chronic pancreatitis) as it is in adenocarcinoma. In some examples of well-differentiated adenocarcinoma, the nuclei appear more hypochromatic than hyperchromatic and may be easily overlooked as benign ductal cells if one is not careful (311).
Differential Diagnosis in Surgical Specimens
For the pathologist, the principal problems relate to distinguishing ductal adenocarcinoma from chronic pancreatitis and intraductal and other pancreatic neoplasms that have a less grave prognosis. Chronic inflammation and fibrosis accompany ductal adenocarcinoma (242,276), and any pancreatic neoplasm that obstructs a principal duct may cause ductal dilatation, parenchymal atrophy, and fibrosis, thereby confusing the gross anatomic findings and, possibly, the microscopic features (Table 35.5) (242).
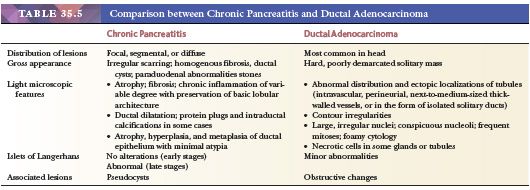
Fundamentally, the most important differential is with benign noninvasive ducts (137,312). Because there are no myoepithelial or basal layers, unlike in breast and prostate, there are no immunohistochemical markers to determine noninvasive ducts. Therefore, cytoarchitectural changes, associations, and the distribution are to be relied upon for this distinction. However, PDAC cells have a very peculiar ability to form very well-differentiated units not only in the stroma but also when they infiltrate into other structures including nerves and vessels. In fact, even in poorly differentiated cases, the cells that infiltrate into the nerves and vessels may form well-defined ductal units that can mimic native ducts (137).
In low-power examination, the lobularity of the process, its organoid nature, and formation of well-demarcated nodules are helpful to distinguish adenosis-type lesions from invasive carcinomas. Invasive carcinomas often show haphazard distribution and contour irregularities (137). Invasive ducts are often localized in places they do not belong. For example, the presence of small- to medium-sized ducts in the interlobar region, the presence of ducts next to medium-sized/thick-walled blood vessels, perineurial invasion, ducts surrounded by a thin cuff of smooth muscle cells (which is indicative of vascular invasion), isolated solitary ducts in adipose tissue (without any accompanying acini or islets), and the presence of ducts on duodenal musculature (away from ampulla major or ampulla minor region) are all in keeping with adenocarcinoma (137,200,242,267,313,314).
In addition to angulated contours, invasive adenocarcinoma glands often show open, round lumina formation, in contrast with the compressed lumen and undulating contours of noninvasive glands (Fig. 35.42). Intraluminal debris with necrotic material or neutrophils, partial rupture of the duct in a MELF pattern, and vacuolations within the ductal structure are also features of adenocarcinoma. In contrast, the presence of enzymatic (corpora amylacea–like) secretions is more in keeping with a benign process (137,200).
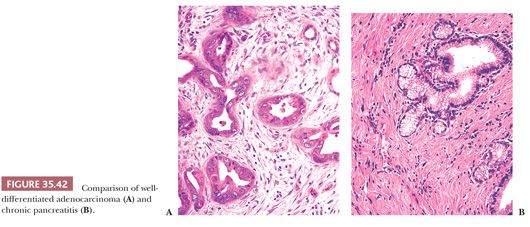
In terms of cytology, many of the features described previously in “Cytologic Diagnosis” section are also applicable to histologic preparations. Typically, the nuclei are enlarged, hyperchromatic, and pleomorphic and there is loss of polarity in adenocarcinomas. In contrast with atrophic acini with ductulization, which typically show a basophilic appearance due to the attenuation of acinar cytoplasm and paradoxical high nuclear-to-cytoplasmic ratio, carcinoma cells typically acquire fair amount of acidophilic cytoplasm. If the cytoplasm is abundant, the presence of foamy gland features including pale microvesicular cytoplasm, raisinoid nuclei, and apical chromophilic condensation at the apex are characteristic (299). In a variant of PDAC, the cytoplasm may appear attenuated, which, along with the extremely well-differentiated appearance of the glands, may create a picture similar to tubular carcinomas of the breast. The presence of nuclear grooves or focal mega-nucleoli or intracytoplasmic vacuoles may be the only clues to the diagnosis in this variant.
In terms of stromal changes, there are significant overlaps between benign conditions and PDAC. Having said that, the presence of myxoid basophilic stroma, especially with vacuolation, are significantly more common in adenocarcinomas. Individual hyperchromatic boxcar-shaped nuclei in the background of a paucicellular stroma without granulation tissue–type changes are also mostly indicative of an adenocarcinoma (137,200).
Differential Diagnosis from Other Similar Tubular Adenocarcinomas
PDACs are morphologically very similar, if not identical, to biliary tract and gallbladder carcinomas, and for this reason, they are regarded under one category of pancreatobiliary-type adenocarcinomas. In fact, in a biopsy from this region, it is preferable to designate the carcinoma as “pancreatobiliary-type” because its precise origin as to cholangiocarcinoma (intra- or extrahepatic) or gallbladder, or pancreas, cannot be determined definitively.
Pancreatobiliary-type adenocarcinomas also have substantial overlapping features with other foregut carcinomas, in particular, gastroesophageal, not only at the morphologic level but immunohistochemically as well (255). Subtle findings may have to be relied upon. Whereas intestinal-like features, poorly cohesive cell pattern, and clear cell pattern (with the nuclei located suprabasally) are much more common in gastric carcinomas, in contrast, foamy gland, vacuolated, and large duct patterns are more common in pancreatobiliary.
Ovarian carcinomas can also have overlapping features with pancreatobiliary-type adenocarcinomas. This extends to the clinical findings, in particular, abdominal carcinomatosis and increased serum CA19-9 or CA125 levels. The findings that favor ovarian origin are overall basophilia, formation of large interconnecting and anastomosing glandular elements with projection and papillary configuration, and, if present, the monotony of nuclei with prominent nucleoli. Although their specificity has not been tested extensively, a panel composed of WT1, PAX8, SMAD4, MUC5AC, and CK20 may be helpful in distinguishing ovarian serous carcinomas from pancreatobiliary-type adenocarcinomas in omental or peritoneal biopsies, which often proves to be a challenging differential diagnosis. WT1−/PAX8−/SMAD4−/MUC5AC+ phenotype would point toward pancreatobiliary-type adenocarcinomas, whereas WT1+/PAX8+/SMAD4+/MUC5AC− is highly in favor of ovarian primary (255,315). If present, CK20, and, less reliably, CDX2 would also be more compatible with a diagnosis of pancreatobiliary-type adenocarcinomas (316).
In the liver, metastatic intestinal adenocarcinomas may mimic pancreatobiliary-type adenocarcinomas. The findings that favor colonic origin are overall basophilia, more columnar appearance of nuclei with pseudostratification, larger glandular units with branching, and intraluminal necrotic material (dirty necrosis). If present, the vacuolated pattern of pancreatobiliary-type adenocarcinomas, which is quite different than the cribriform pattern of intestinal carcinomas, can be helpful in this distinction. Immunohistochemistry may be helpful, with a CK7+/MUC1+/MUC5AC+/MUC6+/CK20−/CDX2−/MUC2− profile being in favor of pancreatobiliary-type adenocarcinomas, with intestinal tumors showing the opposite pattern (255).
One of the peculiar aspects of pancreatobiliary-type adenocarcinoma is that it has a predilection to undergo a multilocular cystic transformation when it metastasizes to the ovary and is often misdiagnosed as primary “mucinous cystadenoma,” not even borderline, because the epithelial lining of the cysts is usually single layer. This mimicry can be truly astonishing. For the distinction, the bilaterality of ovarian involvement, the relatively smaller size (<8 cm), and the degree of cytologic atypia despite the lack of architectural complexity (absence of tufts or papilla formation in the epithelium) are more typical of pancreatobiliary metastasis. In addition, in close inspection, the glands in the stroma between the cysts often show characteristics of pancreatobiliary adenocarcinomas.
Similarly, pancreatobiliary metastases in the lungs also often mimic primary mucinous bronchioloalveolar carcinomas due to their striking lepidic growth and mucinous cytology. If present, foamy gland cytology and the degree of cytologic atypia especially in the glands between the alveolar spaces are helpful to the diagnosis.
Surgical Pathology Reporting and Prognostic Evaluation
Although tubule formation is identifiable in virtually all PDACs, at the same time, a significant proportion of the cases have nonglandular patterns in a mixture. There are various grading systems proposed. The one endorsed by the World Health Organization (WHO) is based on the Kloppel proposal (317) and comprises separate evaluation of four parameters—tubular pattern, mucin production, mitotic activity, and nuclear atypia—each in three tiers, which is then summed and divided and translated into a table to formulate a final grade. It appears that most pathologists find this approach cumbersome, and thus it is seldom employed in daily practice. In contrast, the grading scheme advocated by the American Joint Committee on Cancer/tumor-node-metastases (AJCC/TNM) staging system utilizes only the amount of tubule formation, with 95% for well, 50% to 95% moderate, and less than 50% for poor differentiation. Recently, a grading scheme similar to Gleason for prostate, also incorporating the presence of ill-defined glandular patterns (similar to Gleason pattern 4), was proposed (262). In daily practice, however, most appear to apply a gestalt approach rather than any of these specific grading schemes.
For staging of PDAC, in daily practice, oncologists fundamentally regard PDACs in three categories: resectable, locally advanced, and metastatic. The synoptic reporting of College of American Pathologists (CAP), however, utilizes the AJCC/Union for International Cancer Control (UICC) TNM stage (265), which presents several challenges (318). T1 and T2 are defined based on the size of the tumor. Accurate measurement of the size of PDAC requires close correlation of subtle gross findings with microscopic confirmation, which can be difficult if the sampling is not done according to a protocol. T3 is defined as spread of tumor “beyond the pancreas,” which is interpreted by CAP as the involvement of “peripancreatic soft tissue” (319). This may be difficult to determine, considering that the pancreas does not have a capsule and fat can be found throughout the organ, and furthermore, peritumoral changes further obscure the interphase of pancreatic lobules with adipose tissue. In a recent study (320), by “orange peeling method” sampling and examining the peripancreatic adipose tissue, clusters of carcinoma cells were found in 90% of the cases and, as such, leaving only a handful of cases to qualify as T1/T2.
For involvement of duodenum, the extension of carcinoma cells into the duodenal musculature is regarded as adequate. For CBD involvement, there are different interpretations. In the Armed Forces Institute of Pathology (AFIP) fascicle (1), only involvement of extrapancreatic CBD is considered as T3, whereas in other texts, invasion into any component of CBD is accepted.
As for margins of pancreatoduodenectomy specimen, there are three specific structures on which all authors agree to be reported as margins: CBD, pancreatic neck (duct), and uncinate (superior mesenteric artery; retroperitoneal) margins. There are other surfaces of the pancreatoduodenectomy specimen on which different authors express different views. The vascular bed (groove), where the portal and mesenteric veins lie originally, is regarded as a part of the uncinate margin by some (319). We report this as a separate surface (vascular bed) (321). The posterolateral surface of the pancreas covered by serosa where it adjoins to the duodenum is regarded as part of the “retroperitoneal margin” by some authors. We report this region as posterior free surface (321). Anterior free surface is disregarded by most, although we report it (322–324).
Other findings to be examined and documented microscopically include vascular and perineural invasion. Vascular invasion can manifest in different formats: carcinoma cells in thrombotic nodules; detached micropapillary clusters; and the most common and most challenging, tubule formation with the carcinoma cells lining the endothelium. The latter often forms a very well-defined ductal structure and can be mistaken for normal ducts or PanIN (267,269).
Evaluation of Postneoadjuvant Cases
Neoadjuvant treatment is increasingly employed in the management of PDAC, presumably selecting patients who would benefit from surgery. The tumors that have received prior treatment often show a distinctive sclerosis and cytologic changes including vacuolization of cells, foamy cell changes, and individual hyperchromatic cells lying in the stroma. Blood vessels also often show reactive changes. Extensive sampling may be necessary to identify residual carcinoma in some cases. Complete remission with neoadjuvant therapy is extremely uncommon in PDAC, requiring examination of the entire specimen microscopically and reevaluation of the original biopsy material before making such a determination.
In assessment of the response, a few schemes for the histologic grading of the extent of residual carcinoma in posttreatment pancreatectomy specimens have been proposed: Ishikawa et al. proposed to group the tumor response into three categories based on the percentage of severely degenerative cancer cells, specifically into thirds (324a). Evans et al. (325,326) proposed a four-tiered grading system based on the percentage of tumor cell destruction (residual viable tumor cells): grade I, less than 10% tumor cell destruction; grade II, 10% to 90% tumor cell destruction; grade III, less than 10% viable-appearing tumor cells; and grade IV, no viable tumor cells. The CAP suggests using a four-tiered grading system for the extent of residual carcinoma: grade 0, no viable residual tumor (pathologic complete response); grade 1, marked response (minimal residual cancer with single cells or small groups of cancer cells); grade 2, moderate response (residual cancer outgrown by fibrosis); and grade 3, poor or no response (extensive residual cancer) (319). However, we believe the proposal by Wang et al. (327) from MD Anderson Cancer Center is the most applicable. Fundamentally, this approach regards the response in two groups: Response Group 1 includes the cases with pathologic complete response (CAP grade 0) and those with minimal residual tumor (<5% viable residual tumor, CAP grade 1); Response Group 2 is composed of patients who had 5% or more viable residual tumor (327). Response Group 1 appears to correlate with better survival (327). Complete remission is exceedingly uncommon, and in such cases, the primary diagnosis of carcinoma must be verified.
Clinical Outcome
PDAC is one of the deadliest of all cancers (240). Median survival of all comers is about 9 months. Patients with metastatic disease have a median survival of 4 to 6 months if untreated, and this number has increased to 6 to 9 months with recent gemcitabine and FOLFIRINOX (Folinic Acid, Fluorouracil, Irinotecan Hydrochloride, Oxaliplatin) protocols (328–331). For resected cases, the survival is about 15 months (332–334). If neoadjuvant therapy is employed and the patient remains/becomes resectable after the therapy, the median survival is about 2 years. It is becoming clear that pancreatoduodenectomy for PDAC is essentially a debulking process (268), but most patients benefit significantly from this debulking, and considering that the mortality of this operation has now dropped below 2% in experienced hands, this operation is fully justifiable in most instances (335,336). There are groups who now do surgery for recurrences/persistent disease (337), although the merit of this approach has yet to be proven.
Overall 5-year survival is still less than 5% (240). Most cases recorded as “long-term survivors” in the literature or in surgical databases prove to be other tumor types (acinar, neuroendocrine, colloid, secondary tumors, etc.). Having said that, there are rare bona fide long-term survivors of PDAC. Those are generally small localized tumors, often centered in the vicinity of distal CBD, occurring in younger patients (334,338).
OTHER INVASIVE CARCINOMA TYPES OF DUCTAL ORIGIN
There are other types of invasive carcinoma that occur in the pancreas and are regarded separately from PDACs, although they are also presumed to be of ductal origin and thus closely related to PDAC, and some may also have mixed PDAC component.
Colloid (Mucinous Noncystic) Carcinoma
Recognition of IPMNs as an entity (see the following discussion) has brought to light another distinct invasive carcinoma type in the pancreas—colloid carcinoma (339). The term colloid was coined due to its striking resemblance to those occurring in other exocrine organs, namely breast and skin, where colloid carcinoma has been shown to be associated with a very good prognosis, significantly better than the ordinary carcinomas of those sites, even when they are fairly large.
Colloid carcinoma is seen in patients in their 60s. The patients present with nonspecific symptoms. The tumors can be fairly large (up to 12 cm), with a median of 5 cm, and are often relatively demarcated, in contrast with ordinary PDAC. Colloid carcinoma is characterized by distinct nodular pools of mucin that contain scanty clusters of bland carcinoma cells in strips, stellate clusters, or individual signet ring–like cells, all floating within the mucin (Fig. 35.43). It is believed that mucin acts as a containing factor preventing the spread of the carcinoma cells; thus, it has become a requirement for the diagnosis of this tumor type that it lacks (or shows only minimal) cellular nonmucinous infiltrates into the stroma (340–342). Calcifications are also present, not uncommonly, in colloid carcinomas. Perineurial invasion and lymph node metastasis are seen and often display the same pattern as the primary tumor. Invaded nerves may appear as small structures in large pools of mucin.
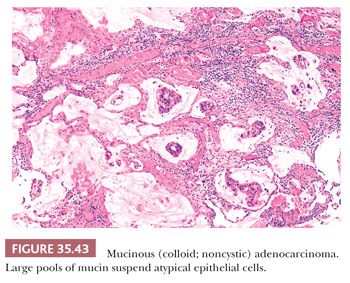
There is an alteration in CEA expression (stroma-facing surfaces rather than luminal surface), a lack of basement membrane, and high expression of MUC5AC and MUC2 (gel-forming mucin), the latter secreted toward the stroma. MUC2 mucin forms strong bonds with the stroma, possibly leading to slower growth or spread (339,340,343,344). Consequently, there seems to be an improved patient survival compared to ordinary ductal adenocarcinoma, with 5-year survival of resected cases being 57% versus 12% in PDAC (342). The expression of SMAD4 (DPC4) protein is intact in all cases (287). K-ras oncogene mutations are infrequently identified (342).
Aspirates of mucinous adenocarcinomas are difficult to smear thinly on the slides because of abundant mucus (Figs. 35.44 and 35.45). On microscopic examination, the mucus pools are large and may be acellular in some smears. However, other smears show the groups of malignant epithelial cells. Nodular nature of the mucin lakes, with cells floating within this mucin, may also be recognizable in aspirates in some cases (1).
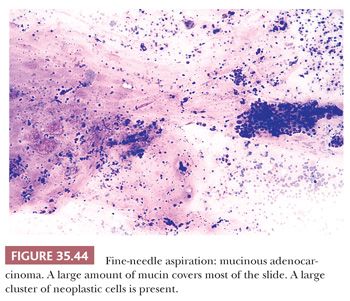
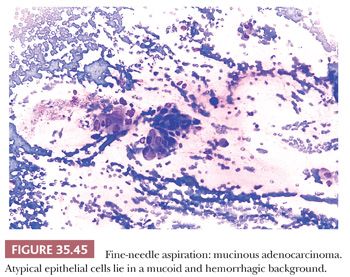
Many colloid carcinomas arise in the background of IPMNs. Virtually all of these IPMNs are intestinal-type, characterized by diffuse MUC2 and CDX2 expression, as are colloid carcinomas (39,274,345,346). Because these two markers are essentially absent in the normal pancreas or other pancreatic tumors, it is believed that this intestinal lineage is an important characteristic of this group and may contribute to the protracted clinical behavior of these tumors, possibly along with the delimiting effect of the secreted mucin, which is produced by the carcinoma cells toward the stroma instead of the lumen of the glands (346). Also, intuitively, it may be more appropriate to treat these cases with intestinal protocols rather than pancreatic. Interestingly, colloid carcinoma is seldom, if ever, seen in association with a MCN (347). In the original description of the entity, one example was reported with MCN, but that case did not have ovarian-type stroma and would now be classified as IPMN instead. In one study, the incidence of thromboembolism was high and seen in patients who underwent open biopsy (342). More importantly, all the patients with open biopsy later developed disseminated disease, whereas those without open biopsy often had a protracted clinical course.
It should be noted here that, unfortunately, the term mucinous has been applied to a variety of tumors in the pancreas, from ordinary PDACs with foamy, vacuolated (signet ring–like), or clear cell features to those PDACs that have focal stromal mucin deposition, which are rare, to true colloid carcinomas. More importantly, carcinomas of ampullary duodenum often have mucinous components, and being large, they often infiltrate into the pancreas (348). These have been included in studies on “mucinous carcinomas” of the pancreas, leading to contradictory impressions on the behavior of mucinous tumors in this region. For this reason, in a given case, it is crucial to specify what is meant by mucinous. In fact, this term should be avoided outside the setting of the entities that are recognized with the name mucinous (IPMNs, MCNs, mucinous adenocarcinomas of the ampulla or duodenum). Also, it should be remembered that, in colloid carcinomas, even a small ordinary (nonmucinous) infiltration pattern may confer a more aggressive behavior as has been amply documented for the mammary examples. In the original description in the pancreas, colloid pattern of more than 80% was required (342), although nowadays, more than 90% is the more commonly used one, akin to the criteria in the breast. The studies that have defined this less stringently, such as those using 50%, have failed to find the survival advantage. Many of these mixed mucinous carcinomas originate in the ampulla and have a more aggressive behavior than pancreatic colloid carcinomas (348).
Adenosquamous and Squamous Carcinomas
Adenosquamous carcinoma affects older age men more frequently than women. Patients present clinically with a picture similar to PDAC including weight loss and/or jaundice, among other symptoms. The pancreatic body and tail is affected more often than the head (349). Dual differentiation toward adenocarcinoma and squamous cell carcinoma is found in adenosquamous carcinoma (Fig. 35.46) (297,350–353). Because small foci of squamous differentiation are not uncommon in ductal adenocarcinomas, an arbitrary squamous component of 30% has been proposed for a carcinoma to be regarded as adenosquamous (242). In general, the two major cellular types are so intermingled that this pattern is unlikely to be overlooked if several sections are taken from different parts of the neoplasm (Fig. 35.47). Although the squamous elements may be extensive in the primary neoplasm, the adenocarcinomatous element may predominate or may be the only pattern in the metastatic foci (282). Keratin immunoreactivity for AE1/AE3 is identified, with other keratins variably expressed; CA19-9, CEA, and p63 are noted in the majority of cases (p63 specifically in the areas of squamous differentiation) (34,354). Loss of SMAD4 (DPC4) protein expression and K-ras oncogene mutations are seen in the majority of cases (282,354). Adenosquamous carcinoma appears to be even more aggressive than ordinary PDAC. Despite appropriate surgery, the outcome is fatal within a few months (282,349,350,355–360).
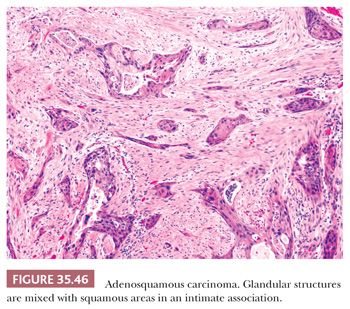
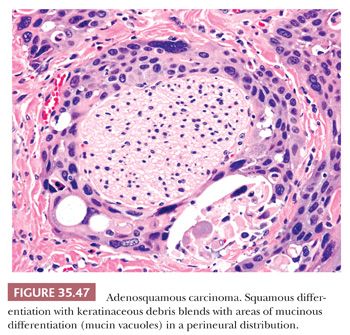
Aspirates from adenosquamous carcinoma show a distinctive necrotic background (351). In the granular amorphous debris are dense blue globules, ghosts of squamous cells with angular borders, and anucleate squames. Atypical single cells with dense cytoplasm and enlarged pyknotic nuclei are present. Also seen are sheets and clusters of atypical cells with moderate to abundant cytoplasm (that varies from dense to vacuolated) and nuclei of variable sizes and shapes in a disorderly arrangement (Fig. 35.48).
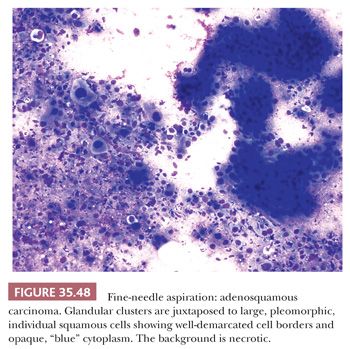
Pure squamous cell carcinoma of the pancreas is very rare. Most cases contain a glandular component (e.g., adenosquamous carcinoma) on careful histologic examination, and any amount of glandular differentiation is sufficient for a predominantly squamous carcinoma to be regarded as adenosquamous. Exposure of the pancreas to ionizing radiation might predispose to the occurrence of adenosquamous carcinoma (282).
Medullary Carcinoma
Medullary carcinoma, as defined in the lower GI tract or the breast, by a nodular pattern with a pushing border, nonglandular syncytial growth, and variable amounts of inflammatory infiltrates, may also be seen in the pancreas (361–363). Many cases involving the pancreatic head prove to be originating from the ampulla or duodenum; however, bona fide examples arising in the pancreas including the tail of the organ are also reported. Some are mixed with colloid carcinoma. The patients seem to be somewhat younger than ordinary PDAC. The association with microsatellite instability appears to be even stronger than it is with their colonic counterparts (363–366). Medullary carcinomas may arise sporadically or in patients with Lynch sydrome (364,367). Most medullary carcinomas do not harbor K-ras mutations (362). Although the data are limited, their prognosis seems to be better than that of ordinary PDAC (361–365,367).
Undifferentiated Carcinoma
In some PDACs, the hallmarks of ductal differentiation may be minimal. Such cases are classified as “undifferentiated carcinoma” (368–371). In some, epithelial-to-mesenchymal transition can be so extensive that the tumor may resemble sarcomas (370) and only after adjunct studies is the ductal nature of the tumor revealed (370). However, in most examples, additional sampling reveals the ductal/tubular patterns characteristic of PDAC. There are several histologic variants of undifferentiated carcinoma on record with overlapping microscopic features: spindle cell (sarcomatoid) carcinoma (Fig. 35.49), anaplastic giant cell carcinoma, and carcinosarcoma (283,370,372), all of which are characterized with either spindle cells or anaplastic giant cells (very pleomorphic and very large cells with multilobated nuclei). The latter is not to be confused with osteoclastic giant cells (see the following section) or with the symplastic giant cells of neuroendocrine tumors. It is an exceedingly uncommon occurrence for anaplastic giant cells to predominate the picture, or be the sole pattern. In such cases, it may be imperative to look for the more conventional PDAC pattern in other areas, and if this is not found, consideration must be given for a secondary tumor, such as the ampulla. The term carcinosarcoma is used when a separate glandular component is also present (373). Immunohistochemically, some of the undifferentiated carcinomas express both epithelial (such as keratin or epithelial membrane antigen [EMA]) and mesenchymal (vimentin) markers (283,368,374,375). In others, evidence of epithelial differentiation may be absent, even at the immunohistochemical level. It has been demonstrated that noncohesive pancreatic cancers, including undifferentiated pancreatic carcinomas, are characterized by the loss of E-cadherin protein expression, which might explain the poor cohesion of many undifferentiated carcinomas (376). The prognosis is very poor, and average survival is just 5 months (283,368,372).
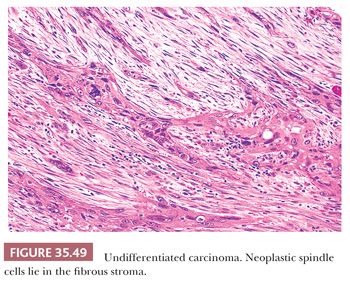
Undifferentiated Carcinoma with Osteoclast-Like Giant Cells
Occasionally, a PDAC may exhibit a focal sarcomatoid growth pattern and even a storiform spindle cell arrangement. This is typically very limited in amount and is usually seen in the context of a densely hypercellular stroma with individual cell infiltration of the carcinoma cells that acquire spindle cell morphology and thus create a sarcomatoid pattern. In virtually every case, there is a conventional tubular PDAC pattern in the background.
In contrast, undifferentiated carcinomas with osteoclast-like giant cells (Fig. 35.50) are characterized by a sea of ovoid to spindle-shaped, highly pleomorphic mononuclear cells, in which a variable number of osteoclast-like giant cells are suspended (374). Recent studies confirmed that the malignant cells are actually the smaller, ovoid to spindle-shaped, highly pleomorphic mononuclear cells, whereas the osteoclast-like giant cells are benign histiocytic giant cells that are attracted to the undifferentiated carcinoma, presumably by factors secreted by the tumor or factors within the tumor environment. Such osteoclastic cells are recorded in sarcomatoid tumors of other types and locations, including sarcomatoid mesotheliomas or sarcomatoid melanomas, but seldom, if ever, as abundant as they are in the undifferentiated carcinomas with osteoclast-like giant cells of the pancreas. A component of benign histiocytic mononuclear cells is also present within these tumors (377–380). Some cases may contain anaplastic giant tumor cells in addition to osteoclast-like giant cells (377).
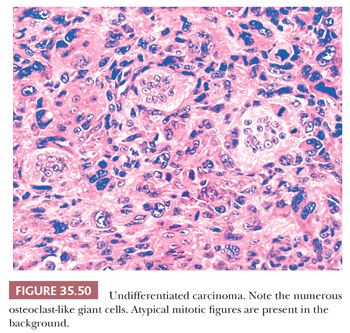
Undifferentiated carcinomas with osteoclast-like giant cells seem to be less than 1% to 2% of malignant pancreatic tumors, and men in the seventh decade of life are affected most frequently, although the age range is broad (242). Patients present with weight loss and pain, along with a variety of other nonspecific abdominal symptoms identical to ductal carcinomas. The tumors are usually large lesions (mean size, 9 to 10 cm) and present slightly more frequently in the head, although any site in the pancreas can be involved. There is often a nodular and even polypoid growth pattern with well-demarcated nodules. Examples are reported with prominent intraductal growth and a protracted clinical course (381). Hemorrhage, necrosis, and cystic degeneration often are conspicuous, and the tumor may be soft. Direct extension into surrounding organs and nodal metastases may be present. Liver and lung are frequent sites of distant metastasis.
Heterologous elements such as bone and cartilage can be seen in undifferentiated carcinomas with osteoclast-like giant cells. In some cases, the bone can form a rim at the periphery of the nodules. Usual PDAC is found if aggressively sought, no doubt the source of the undifferentiated carcinoma (Fig. 35.51), whereas MCNs or IPMNs are rarely associated findings (380,382–384).
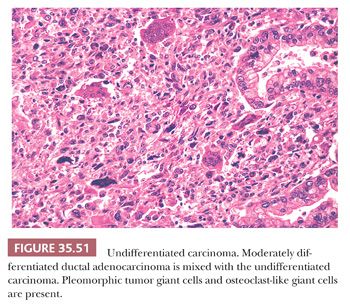
Histochemically and immunohistochemically, a few malignant cells may contain intracytoplasmic mucin droplets. Cytokeratin immunoreactivity within both the smaller ovoid to spindle-shaped and the anaplastic giant malignant cells (Fig. 35.52) is seen in approximately 75% of cases (368). It should be kept in mind, however, that undifferentiated carcinomas with osteoclast-like giant cells can be negative for epithelial markers. The malignant cells also show a high Ki-67 proliferation index, p53 labeling, and K-ras gene mutations (368,379,380,383). The osteoclast-like giant cells are positive for vimentin, leukocyte common antigen (LCA, CD45), and histiocytic markers (CD68, KP1) while being nonreactive for CKs, EMA, or CEA (374,378,385). However, “tumor cannibalism” (i.e., presence of malignant cells in the benign osteoclast-like giant cells) is fairly common in this entity and ought to be considered in evaluating these markers (255). Of note, the osteoclast-like giant cells do not harbor K-ras mutations (368,379,380,383).
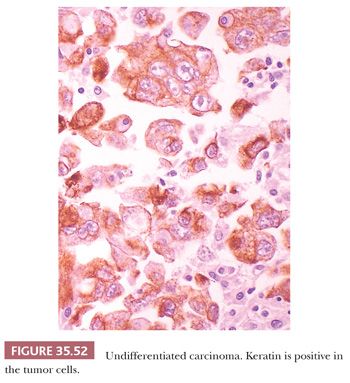
The clinical behavior of undifferentiated carcinomas with osteoclast-like giant cells has been reported to be very aggressive (1); however, examples with prominent nodular and intraductal growth pattern appears to have more protracted clinical course (381). Of note, several cases were reported under the diagnosis of benign osteoclastic giant cell tumor, interpreted to be pancreatic counterparts of similar bone lesions (386). The nature of these cases is unclear. The positivity of CD163 in the background neoplastic cells was given as the evidence of their histiocytic origin. However, undifferentiated carcinomas with osteoclast-like giant cells also show CD163 expression in the background cells (377). Therefore, these cases may also represent undifferentiated carcinoma, although possibly a more indolent example of the entity.
Other Invasive Carcinoma Types of Ductal Origin
In rare pancreatic tumors, the tumor cells display abundant acidophilic cytoplasm and single prominent nucleoli, reminiscent of a hepatocellular carcinoma (hepatoid differentiation) (387–392). Bile production and intracytoplasmic hyaline globules are described (387). Hepatoid differentiation can occur in association with an ordinary PDAC or in a pure form. However, pure forms are exceedingly uncommon. Pancreatic metastases from an occult hepatocellular carcinoma are probably much more common, and a metastasis needs to be excluded before establishing the diagnosis of a primary hepatoid carcinoma of the pancreas.
Oncocytic carcinomas are unlikely. Most solid oncocytic tumors in the pancreas are oncocytic variants of either pancreatic neuroendocrine tumors (PanNETs) (393), solid pseudopapillary neoplasms (394), or solid variants of oncocytic IPMNs (394,395). Some poorly differentiated examples of PDACs can exhibit oncocytoid or hepatoid cytology, but this is usually a very focal finding, typically accompanied by the ordinary PDAC pattern elsewhere.
Along the same lines, cases reported as “clear cell carcinoma” (396–399) are likely a highly heterogenous group of tumors, with most examples representing foamy gland adenocarcinoma or clear cell variants of PDAC and possibly metastatic renal cell carcinoma. Lipid-rich and clear cell variants of PanNETs (400,401) and clear cell variant of solid pseudopapillary neoplasm (402) also can form solid tumors. PEComas occasionally also form solid clear cell tumors in the pancreas (403–407).
PREINVASIVE DUCTAL NEOPLASMS
Preinvasive ductal neoplasms of the pancreas are regarded in two types (Table 35.3):
1. Incidental, microscopic forms of dysplasia, which are now termed pancreatic intraepithelial neoplasia.
2. Tumoral intraepithelial neoplasms that present as clinically and grossly detectable intraductal lesions that are by WHO definition larger than 1 cm. This category comprises two distinct entities, IPMNs (1,39,345,408–410) and ITPNs (411–413). MCNs may also be regarded as mass-forming preinvasive neoplasms, and as such closely related to IPMNs and ITPNs, but are discussed separately because they are believed to be de novo preinvasive neoplasms rather than intraductal tumors (414).
PANCREATIC INTRAEPITHELIAL NEOPLASIA
PanIN is used to describe a morphologic spectrum of intraductal precursor lesions (39,415–421) of microscopic incidental nature. The classification was proposed by a working group consensus meeting to narrow the terms used by pathologists to include a wide variety of epithelial abnormalities of the pancreatic ductal epithelium to provide for a more uniform documentation of changes (420). This classification was put forth to facilitate consistent reporting to accommodate further research in the field of pancreatic duct lesions and their biologic significance. It is intended to be used for incidentally discovered lesions in predominantly smaller caliber ducts, whereas “IPMN” should be used for lesions identified radiographically or macroscopically in large ducts.
There is a four-part classification of PanIN, presented here with the terminology that has been used in the past as a point of comparison and clarification (Table 35.6). These lesions are believed to represent precursor intraductal lesions, often segmental, that progress through a series of architectural and cytologic changes that ultimately may develop into or are strongly associated with invasive ductal adenocarcinoma (36,37,416,417,419,420,422–424).

• PanIN-1A: flat epithelium composed of tall columnar mucin-producing cells with basally located small nuclei (Figs. 35.53 and 35.54). The cells show virtually no cytologic atypia but show molecular alterations of neoplastic changes and are thus regarded as the earliest precursor lesions.
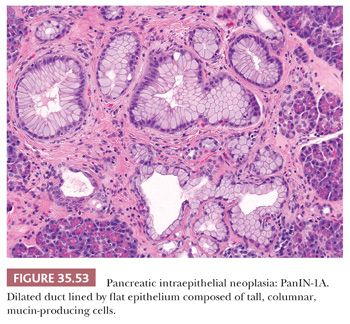
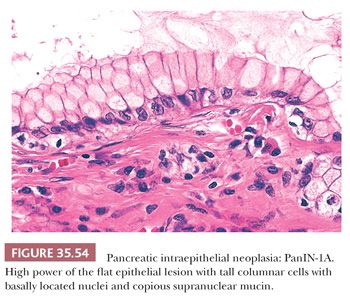
• PanIN-1B: identical to PanIN-1A except for subtle papillary, micropapillary, or basally pseudostratified architecture (Figs. 35.55 and 35.56).
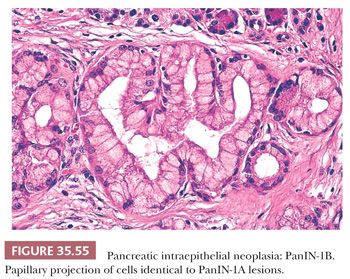
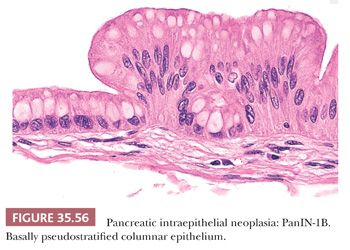
• PanIN-2: flat to papillary proliferations with focal or mild nuclear abnormalities, such as nuclear enlargement, nuclear crowding, hyperchromatism, and pseudostratification (Figs. 35.57 and 35.58).
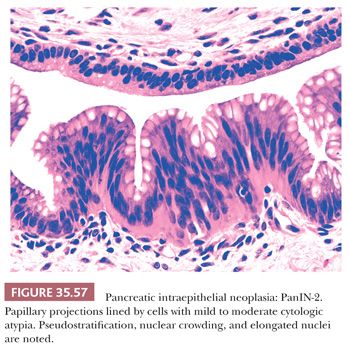
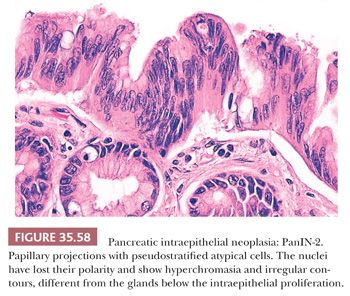
• PanIN-3: predominantly papillary or micropapillary architecture, although cribriforming and luminal necrosis can be seen. Cytologically, these lesions have loss of polarity; dystrophic goblet cells; large cells; enlarged, irregular nuclei and prominent nucleoli; and increased mitotic figures, including atypical forms (Figs. 35.59 and 35.60). Tufting is a common characteristic feature.
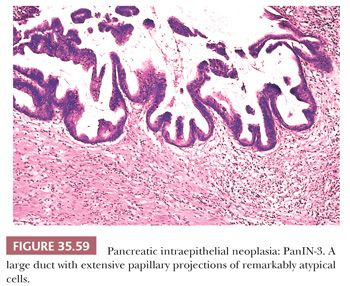
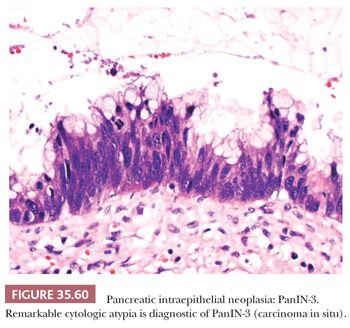
The transition from one grade to the next may be present in the same duct and elsewhere in the pancreas; in this circumstance, the “highest” grade component is reported. These lesions are very frequently associated with invasive ductal adenocarcinoma, which suggests that some of the “lesions” might represent intraductal (pagetoid) spread of the cancer (425), whereas in others, there is a strong association with IPMNs (424). Further refinement of the lesions, where “preneoplasia” begins, and their role in tumor development versus part of normal aging require further evaluation for lower grades of PanIN (426–428). Many genetic abnormalities are seen within these PanINs, similar to those identified in invasive ductal adenocarcinoma, supporting the significance of recognizing these small duct lesions. Too numerous to list in detail here, a selection of these abnormalities includes the following: early molecular changes include telomere shortening and activation of the K-ras oncogene, p21 upregulation, and expression of MUC5AC, MUC6, HER-2/neu, and fascin; intermediate steps include cyclin D1 and MUC1 overexpression, inactivation of tumor suppressor gene p16CDKN2A, and proliferation antigen expression; and late stages include BRCA2, SMAD4 (DPC4), and TP53 tumor suppressor gene (chromosome 17p) inactivation, and overexpression of Ki-67 and mesothelin (35,36,274,289,416,422–424,429–435). Recently, GNAS or BRAF gene mutations have been reported as early changes especially in K-ras wild-type PanINs (436). Increasing Ki-67 labeling indices has been shown with increasing grades of “dysplasia” in PanINs (423). Further understanding of these molecular mechanisms will help with prevention, detection, and therapy.
PanIN-1 and PanIN-2 are very common incidental findings in the general population (428,437,438). If searched thoroughly, they can be found in more than half of the population over the age of 50 years (415). In fact, the incidence may be even higher. For this reason, these low-grade PanINs are believed to be clinically inconsequential by themselves. Accordingly, it is not necessary to document PanIN-1 or PanIN-2 in the surgical pathology report. In contrast, PanIN-3 is seldom seen in pancreata without invasive ductal adenocarcinoma (428). Therefore, if PanIN-3 is identified in the seeming absence of invasive adenocarcinoma, a thorough investigation of the pancreas is warranted because there is a very high likelihood of a missed invasive carcinoma.
INTRADUCTAL PAPILLARY MUCINOUS NEOPLASMS
Definition, Demographics, and Clinical Features
IPMNs (39,408,410) is a category created to unify tumors that had been previously reported in the literature under the heading of ductectatic MCNs, mucinous duct ectasia, mucin-producing tumor, adenoma(tosis) of the ducts, and intraductal papillary neoplasms. Clinically, they are regarded in two groups (439):
1. Branch duct–type, which typically present as cystic tumors in the head of the pancreas, mostly in the uncinate (440,441), and by definition showing minimal, if any, dilatation of the main pancreatic duct.
2. Main duct–type is characterized by dilatation of the main duct, usually at least 7 mm, accompanied by nodularity (442–446). Main duct IPMNs may be extensive and may also involve the entire pancreas in some cases (447,448).
Multifocality has been well established in IPMNs and thus the patients ought to be carefully investigated for skip lesions (449–452).
Branch duct–type IPMNs have become one of the most common “incidentalomas” due to the recent improvements in and widespread use of imaging modalities (453). Often, a small cystic lesion is detected in the head of the pancreas during a workup for other conditions (454). IPMNs are found at a broad age range; however, they are significantly more common in the elderly. Median age at diagnosis is about 66 years (455,456). There seems to be a slight male predominance (446,455,457). Those that are symptomatic often present with vague abdominal complaints related to ductal obstruction and low-grade pancreatitis (446,457). Main duct cases seem to be more commonly associated with pancreatitis (458). Whether this is due to ductal obstruction leading to pancreatitis, or pancreatitis being a risk for development of main duct–type IPMN, is not known. But it is well documented that some patients present with episodes of acute pancreatitis but lack cholelithiasis or a history of alcohol abuse (39,232,236,266,441,444–446,456,459–467). Bulging of the papilla into the duodenal lumen, seen radiographically, is nearly pathognomonic of IPMN (Fig. 35.61). Endoscopically, a patulous orifice of the ampulla from which mucin extrudes is also diagnostic, especially for main duct–type cases; however, this finding is present in only a small percentage of the cases nowadays.
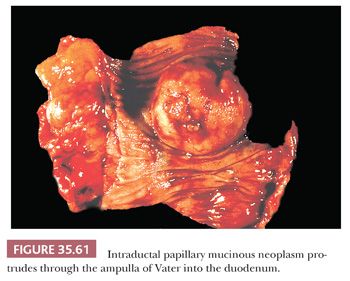
There appears to be an increased incidence of other malignancies, synchronous or metachronous, suggesting careful preoperative evaluation (colon and stomach most commonly) (468–472). It is not clear whether this reflects a specific propensity in these patients to develop neoplasia, or coincidence due to old age, or both.
A previous history of diabetes, especially with insulin use; chronic pancreatitis; other tumors especially of the GI tract; and family history of PDAC have all been documented with higher frequency in patients with IPMN (252,473,474). Whether these are true biologic associations or epiphenomena of old age or organ injury or representation of a common propensity to develop these disorders have yet to be determined.
There are different management protocols devised for branch versus main duct–type IPMNs (408,444,475). Most incidental cysts of the pancreas prove to be branch duct IPMNs that are typically gastric foveolar–type and with low-grade dysplasia. For this reason, watchful waiting is commonly employed for those that are noncomplex (not showing mural nodules) and relatively small (<3 cm) without any changes in close follow-up (408,410,441,476–483). However, the imaging follow-up needs to be performed by experts who can appreciate the subtle changes in these lesions (484,485). The incidence of invasive carcinoma is predicted to be about 15% in these cases (443,455,456,486–489). Some authors advocate surgery in younger patients even if the suspicion of malignancy is very low, citing the difficulty of intense long-term follow-up, a lifelong cumulative risk, and better outcome of pancreatic surgery in younger ages. For the management of main duct–type IPMN, there is wide consensus that resection is indicated if clinically feasible because the incidence of in situ or invasive carcinoma is fairly high (441,446,485,490,491).
Cytologic Diagnosis
The role of FNA in the management of IPMNs is debated. It is seldom employed in Europe and Japan, citing the limited benefits and the concern for complications including tumor spread. However, it is increasingly becoming the norm in the United States. Experience has shown that the concern for tumor spread is probably not an issue, and the complication rate is fairly low. There is no question that FNA can be quite helpful in select patients if evaluated in the context of the clinical findings and with close imaging correlation (492–494); however, whether it needs to be performed in every suspected case is in question.
Cytologic findings include abundant, thick, viscid mucus in the background in nearly all cases. The smears range from hypocellular to hypercellular, with isolated single cells or loosely cohesive groups of neoplastic cells (495–500). Papillary structures or sheets containing mucin-producing columnar cells with mild to moderate pleomorphism may be seen. Goblet cells are frequently present. The cytologic features are usually limited, but when combined with the clinical and radiographic features, they are diagnostic of an IPMN (497,499,501–504).
In the evaluation of an EUS-guided FNA specimen from a potential IPMN case, contamination by GI mucosa is a challenge. The lining epithelium of branch duct IPMNs, which are mostly gastric foveolar–type, is virtually indistinguishable from gastric mucosa (409). For such cases, a diagnosis of “gastric-type epithelium may represent branch duct IPMN if from a cystic lesion” may be preferable. Conversely, the presence of dense, sticky mucin may be meaningful even if the specimen is acellular. It should also be noted that IPMNs cannot be distinguished from MCNs by cytology because ovarian stroma that characterizes the latter is hardly ever present in the aspirates. For such cases, the diagnosis of neoplastic mucinous cyst is more than adequate, followed by the differential and assessment of the degree of cytologic atypia.
It is often difficult to determine the degree of dysplasia in IPMNs. Additionally, it can be impossible to distinguish an invasive carcinoma from an in situ carcinoma (high-grade dysplasia), unless there are overt malignant features. In such uncertain cases, the diagnosis of high-grade atypia and a discussion of the possibility of in situ versus invasive carcinoma ought to be provided.
Cyst Fluid Analysis
Cyst fluid analysis is often performed in the diagnosis of IPMNs (and MCNs) and its differential diagnosis from nonmucinous cystic lesions. Very high levels of pancreatic enzymes, amylase and lipase, is more in keeping with a pseudocyst, but low to moderate increase in the enzymes can be encountered in IPMNs. CEA levels of more than 192 ng/mL, although not entirely specific, is often strongly in favor of a mucinous lesion (505–507). Preoperative cyst fluid analysis with MUC stains is also reported to be reliable for identifying the histologic subtype of IPMN (486,508).
Recently, molecular analysis of cyst fluid is also being advocated (509,510). There are commercial laboratories that offer studies based on the DNA content, loss of heterozygosity, and fluorescence in situ hybridization (FISH) for several cancer-associated chromosomal abnormalities. In the right context, these tests may serve as adjunct to other findings in determining malignant potential; however, there is not enough evidence that they are by themselves conclusive for diagnosis (511–513).
Macroscopic Features
Systematic macroscopic examination and extensive sampling are crucial for the correct diagnosis (321) because the tumor is in contiguity with the pancreatic duct(s), frequently showing invasion. Branch duct IPMNs typically present as multilocular cystic lesions embedded in otherwise unremarkable pancreatic parenchyma. The cyst contents are commonly easily expelled and serosanguineous, and a mucinous nature may not be evident. The cyst wall is typically thin and the lining may be smooth and glistening. Areas with papilla formation, if present, are represented as feathery granulations or polypoid granular lobulated projections. In branch duct IPMN cases, it may be helpful to document grossly any main duct dilatation and sample its mucosa adequately to establish whether the lesion is branch duct confined or not. However, this may not be feasible in every case. Furthermore, this may not be as pertinent to the pathologic evaluation of the tumor, other than providing feedback to the radiologists. Once the tumor is resected, identification and characterization of in situ or invasive carcinoma supplants the clinical classification, regardless of its location.
Resected main duct IPMNs usually appear as complex fibrosing processes that contain multiple cysts, many of which contain soft, tan, friable, and focally hemorrhagic, granular nodules, intervened by sclerotic stroma. Oncocytic examples are especially prone to appear as multilocular cystic masses with florid papillary nodules. The main duct is typically extensively involved in this process and may be difficult to distinguish and track. The cysts are often filled with mucus (39,236,266,441,444–446,456,459–467).
If present, the areas of invasion may be represented as firm gelatinous tissue in the case of carcinoma (342), or as scirrhous areas, in the case of tubular-type invasive carcinoma. Often, however, it is difficult to recognize an invasive carcinoma amid the fibrosis; therefore, stepwise extensive sampling is warranted, and if carcinoma is not documented in the first attempts, total submission of the fibrotic areas is recommended (1).
Microscopic Features
On microscopic examination, the neoplasm consists of dilated ducts within which there are variable amounts of papillae (Figs. 35.62 to 35.64). The papillae vary greatly in size. Many cells contain apical mucus, in addition to intraluminal mucin (producing cystic dilatation of the duct). Intraluminal mucin, however, is washed off easily during tissue processing, and therefore, any stromal mucin ought to be regarded suspect for colloid carcinoma (1).
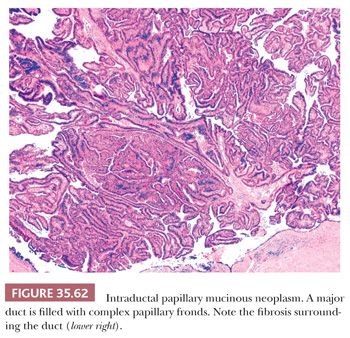
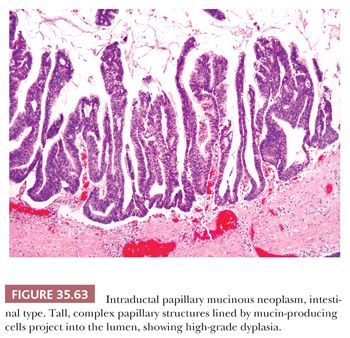
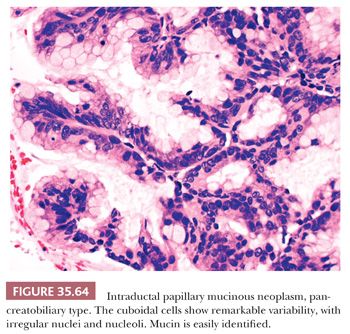
Because pancreatic ducts do not have myoepithelial or basal cells, the intraductal nature of the process is recognized by the smooth contours of the expanded ducts and the proliferation filling the ducts. The papillae are variable in complexity depending on the cell type. Intestinal-type IPMNs typically have a more villous architecture than a branching papillary configuration, whereas pancreatobiliary and oncocytic ones typically have complex arborizing and interconnecting papillary configurations. Those of gastric-type are relatively simple and typically short and often have pyloric-like glandular elements at their base, on the cyst wall. Some examples, especially oncocytic, have a band of edematous stroma around the ducts. Edema may also be seen in the tip of the cores in some cases (346,409).
Based on the degree of cytoarchitectural atypia, the noninvasive components of IPMNs are subclassified as low-grade dysplasia (what used to be called adenoma), intermediate-grade dysplasia (borderline), and high-grade dysplasia (1), which is also synonymous with carcinoma in situ (CIS), although the WHO advocates avoiding the term carcinoma in situ (409). The criteria employed in this grading are those discussed for PanINs earlier.
Scattered neuroendocrine cells can be seen in IPMNs. Presence of acinar cells is rather unusual (514). Osseous metaplasia, squamous metaplasia, and Paneth cells can also be seen.
The changes in the uninvolved pancreas are highly variable. Branch duct and gastric phenotype of IPMNs are often situated in relatively intact pancreatic tissue. Main duct cases (intestinal, pancreatobiliary, or oncocytic) often have significant chronic changes of the adjacent and upstream pancreatic tissue, presumably due to the obstruction. Not surprisingly, incidental, independent foci of PanINs can be encountered (1).
Cellular Phenotypes and Their Significance
The epithelium is extremely variable (345,346,466,515–517). Gastric-type, which most branch duct IPMNs prove to be, is essentially identical to gastric foveolar epithelium (345,515). It is this type that may be virtually indistinguishable from PanINs, by morphology, if taken out of context. When high-grade dysplasia is present in gastric-type tumors, it typically starts to show more complex architecture and cuboidal cells with enlarged nuclei and less mucinous cytoplasm, which are characteristics also of the pancreatobiliary-type of IPMN (Fig. 35.64) (346). For this reason, some authors believe the pancreatobiliary-type is a high-grade version of gastric-type (515). Overlap in immunophenotype may also further support this impression (see the following discussion); however, many examples of pancreatobiliary appear to be pure. The intestinal-type (Fig. 35.63) is characterized by villous architecture and the cytology and growth pattern seen in colonic villous adenomas (345,346), showing pseudostratified columnar cells with basophilic appearance and apical goblet-like mucin. The oncocytic-type, originally called intraductal oncocytic papillary neoplasm (466,517), show complex arborizing papillae with delicate fibrovascular cores (Fig. 35.65). The cells have abundant acidophilic cytoplasm and round, uniform nuclei with single prominent, eccentric nucleoli. Intraepithelial lumina formation is a distinctive feature of this type as well (466,516,517). The oncocytic-type typically has florid papilla formation and is clinically generally interpreted as a complex “cystadenocarcinoma.”
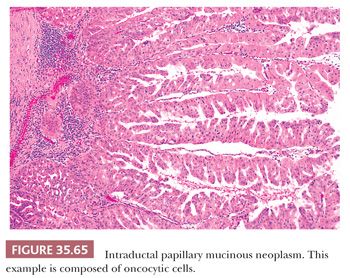
Gastric IPMN seems to be the null type on which other types may develop (518). The frequency of invasive carcinoma is reported to be 15% in the gastric-type (443,455,456,486–489). However, when carcinoma develops, it is typically of the tubular (pancreatobiliary)-type and has aggressive behavior. In other words, the gastric-type appears to be closer to PanINs in its cancer progression pathway. In contrast, intestinal-type IPMNs have a significantly higher incidence of invasive carcinoma, with more than a third of resected cases harboring invasion (409). However, this invasion is typically of the colloid-type and has a protracted clinical course, even when it is large (339,342). Pancreatobiliary examples, which are less common and less well characterized, appear to have tubular-type invasion and display aggressive behavior (488,519–522). Oncocytic tumors are typically very complex and proliferative; however, invasive carcinoma, if present, is typically limited in amount and appears to be indolent, although it has tubular pattern (with oncocytic cytology) (517).
Immunohistochemical and Molecular Features
Immunohistochemically, IPMNs reveal evidence of ductal differentiation and express the ductal markers described for PDACs, including cytokeratins (CK7, AE1/AE3), B72.3, CA19-9, CEA (variable degrees), and others (523). SMAD4 (DPC4) is retained in the vast majority of IPMNs (287,344,524–528), distinctly different from the loss seen in more than 50% of PDACs. Cyclooxygenase-2 (COX-2) is highly expressed (529).
The immunoprofile of different cell types parallels their lineage markers fairly consistently. MUC5AC is detected in most IPMNs, regardless of the type. Intestinal-type IPMNs expresses intestinal lineage markers CDX2 and MUC2 consistently and fairly specifically: These two markers are seldom, if ever, expressed in the normal pancreas or other IPMN types (274,344–346,530,531). In contrast, MUC1 expression, especially luminal membranous, is very common in pancreatobiliary-type but seldom identified in gastric or intestinal (344–346,515,530,532,533). MUC6 is detected in the cystic components and at the base of the papillae in any type. However, in the papillary nodules, its expression is mostly seen in oncocytic and, to a lesser degree, in pancreatobiliary-types and seldom in intestinal-types (344,517,518). MUC4 expression is reported to be significantly higher in the intestinal-type IPMNs than gastric-type IPMNs (534). The correlation of these markers with clinical outcome also appears to be through their correlation with the cell lineages.
K-ras oncogene mutations are detected, although the frequency is not as high as it is in PDACs (40,467,535,536). Oncocytic examples seldom show K-ras mutation (537). Recently, mutation of GNAS was detected in about half of IPMNs (538,539). GNAS appears to be involved in mucin-related pathways and is commonly altered in intestinal-type lesions, and its abnormalities in IPMNs also seem to correlate strongly with the intestinal pathway. It is seldom detected in oncocytic cases (538,539). RNF43, a gene coding for a protein with intrinsic E3 ubiquitin ligase activity, was also reported to be mutated in 75% of IPMNs (540). Neither GNAS nor RNF43 is altered in conventional ductal adenocarcinomas, although RNF43 mutations do occur in MCNs (540).
Invasive Carcinoma Associated with Intraductal Papillary Mucinous Neoplasm
When all comers, including the incidentalomas in elderly patients are considered, the overall incidence of invasive carcinoma is relatively low. For branch duct IPMNs, the frequency is predicted to be about 15% and, for main duct, over 30% (443,455,456,486–489). However, these figures are in flux based on improved imaging technology, earlier detection, and better selection criteria for resection.
Invasive carcinoma arising in IPMNs is of two types, tubular or colloid. Those arising from oncocytic cases tend to have more oncocytoid cytology (395), and those from intestinal types can have intestinal-like features.
Tubular carcinomas are virtually indistinguishable from ordinary PDACs (409,456) and seem to be very similar to PDACs in immunophenotype and prognosis (266,409,455–457,541,542). Colloid carcinomas are invariably associated with intestinal-type IPMNs. The characteristics of colloid carcinoma and the significantly better prognosis than ordinary PDACs is discussed in detail earlier (342,346,519,543).
Emerging evidence suggests that invasive carcinomas not only arise from IPMNs but also sometimes elsewhere in the gland, seemingly independently (544). Studies from Japan suggest that the latter may be different biologically (545). Invasive carcinomas ought to be evaluated separately and their size and extent reported separate from the IPMN component, as in any other cancer.
Differential Diagnosis
By virtue of being intraductal preinvasive lesions with ductal differentiation (mucin and papilla formation), IPMNs have several overlapping features with PanINs (Tables 35.7 and 35.8). Intestinal and oncocytic cytology is exceedingly uncommon in PanINs, but in cases with gastric-type IPMN, it can be impossible to distinguish IPMN extending to smaller ducts from independent, incidental PanINs (39,232,274). If this is encountered in a margin section, the most important issue is to determine and report the degree of dysplasia. If there are no high-grade changes, it is recommended to report the margin as “No invasive or in situ carcinoma. Low- or intermediate-grade mucinous epithelium with the differential of PanIN-1/2 versus a low- or intermediate-grade IPMN.”
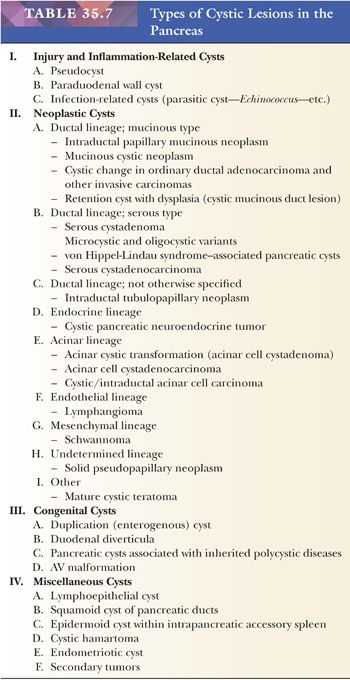
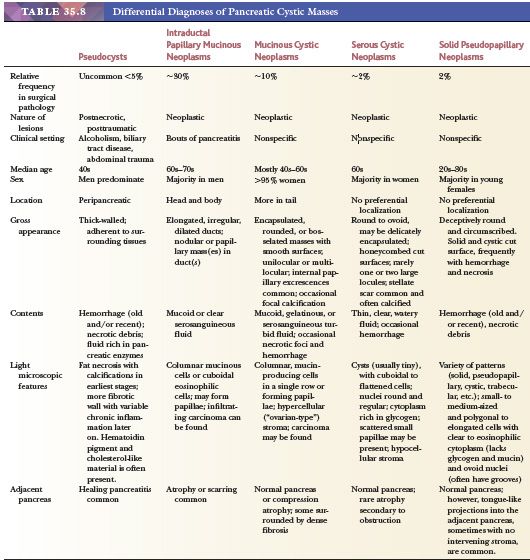
There is another cyst type with mucinous lining but without ovarian-type stroma and overt papillary configuration (Tables 35.7 and 35.8). This lesion type often gets categorized as IPMN for the lack of a better diagnosis and to justify the major operation performed to resect these relatively simple cysts. These are believed to be retention cysts or secondary ectasias that happen to acquire mucinous lining. They have also been termed mucinous nonneoplastic cyst; however, this is not favored, considering that mucinous epithelium in the pancreatic ducts is considered to be the earliest form of neoplastic change (such as PanIN-1A). Recently, cystic mucinous duct lesion (cystic version of PanIN-1A, which is also termed mucinous duct lesion) has been proposed for such cysts. They can harbor various degrees of dysplasia, and therefore careful examination is warranted (304).
Additionally, obstructive lesions, including subtle PDACs or small PanNETs of especially serotonin type, can lead to a dilatation of the main pancreatic duct that can be mistaken as main duct IPMN clinically. If a resection for “main duct IPMN” fails to show florid papillary nodules of intestinal, oncocytic, or pancreatobiliary-type, and especially if the main duct epithelium is devoid of mucinous lining, then the pancreas needs to be examined very carefully. The PanNETs that lead to this picture, also called “pseudo-IPMN,” can be very small, sclerotic, and often serotonin positive; they should not be dismissed as hyperplasia (546).
IPMNs that form multilocular cystic lesions can be indistinguishable from MCNs, and in fact, these IPMNs had been referred to as ductectatic mucinous cystic neoplasms in the past (Tables 35.7 and 35.8). Ovarian-type stroma is now regarded a requirement for the diagnosis of MCN, its presence diagnostic of MCN. If a multilocular cystic tumor is in a perimenopausal woman and in the tail of the pancreas, it is warranted to search for ovarian stroma even more carefully. It should also be kept in mind that frank intestinal phenotype (villous adenoma pattern) is virtually not seen in MCNs, and neither is colloid carcinoma (232,410,444).
IPMNs share several characteristics with ITPNs of the pancreas (412,413,547). In fact, ITPNs can be regarded as tubule-forming versions of IPMNs with minimal or no mucin production.
In complex IPMN cases, it may be difficult to distinguish invasion from pseudoinvasion created by the extension of the IPMN cells into smaller ductules, which, in the setting of acinar atrophy, may appear as spreading ductular elements. Additionally, entrapped ductules on the walls of IPMNs may become atypical due to inflammation and injury. The features described earlier in the PDAC section for the differential diagnosis of invasive versus noninvasive ducts, are also applicable in this setting (409).
A problematic differential that is specific to IPMNs of especially intestinal-type is the presence of stromal mucin. The mucin in the intraductal component of IPMNs is typically washed off during processing. Therefore, the presence of significant amount of mucin in the stroma ought to be regarded highly suspect for colloid-type invasive carcinoma. Occasionally, localized obstruction may lead to mucin retention in a branch, associated with focal rupture. Typically, levels or deeper sections reveal that the mucin in the stroma is in continuum with the rupturing duct. However, additional sections are warranted to rule out colloid-type invasion (409).
Clinical Outcome
As discussed previously, branch duct IPMNs that are small and noncomplex can be managed by watchful waiting; however, a percentage of these develop invasive cancer in follow-up (410,441,446,485,490,491). Main duct IPMNs are typically resected. For a main duct IPMN that involves the pancreas extensively, total pancreatectomy may have to be considered (441,446,455,457,485,490,491,548). This is a challenging balance between the risk of disease progression versus the long-term prognosis of having brittle diabetes.
Resected noninvasive IPMNs have a very good prognosis (457,549). Recurrent/metastatic behavior reported in earlier literature is most likely attributable to missed invasion or field defect phenomenon (or general affinity of the patient) that renders the remainder of organ at risk to develop other carcinomas (544,545). In some studies, margin positivity has been found to increase the risk of malignant behavior (482).
IPMNs with invasive tubular-type adenocarcinoma pursue an aggressive course; however, they seem to do better than conventional ductal carcinoma that arises without a preexisting IPMN (266,520,550). Whether this is due to early stage at detection and/or a reflection of a different biology (although they look morphologically identical to ordinary PDACs) remains to be determined. IPMNs with colloid carcinoma appear to have a much better prognosis than IPMNs with invasive tubular-type adenocarcinoma (342,346,519,543) and significantly better than ordinary PDACs. In fact, many patients with large colloid-type invasive carcinomas showing lymph node metastasis and/or perineurial invasion are alive beyond 10 years. In some studies, cases with “minimal invasion” have been found to have a very good prognosis (551), but the definition of minimal invasion is controversial. For this reason, a different staging protocol has been proposed (408).
Surgical Pathology Reporting
In reporting of IPMNs, the findings of invasive (if present) and noninvasive components such as the size and extent ought to be provided separately. Invasive component ought to be documented and staged as any other cancer. Because invasion is often small in these patients, substaging of AJCC’s pT1 category as T1a 0.5 cm or less, T1b for 0.5 to 1 cm or less, and T1c for 1 to 2 cm has been proposed (408,488). In some cases, it is difficult to determine the size of invasive carcinoma because it manifests as patchy foci in sections. For such cases, an estimate needs to be provided “as measured microscopically” (408).
INTRADUCTAL TUBULOPAPILLARY NEOPLASMS
This is a recently recognized entity (411–413,547,552–554), also endorsed by WHO 2010 as a separate category (409). Essentially, it is the nonmucinous and tubule-forming version of IPMN. For detailed characteristics of IPMN, please refer to the “Intraductal Papillary Mucinous Neoplasms” section, with the discussion here limited to this specific entity.
First reported by Tajiri et al. (554) under the heading of intraductal tubular carcinoma in 2004, the entity is now being named intraductal tubulopapillary neoplasm (409), although papilla formation is seen only in a minority and in a very limited fashion in our experience. Therefore, those that have more papillary configuration ought to be classified as pancreatobiliary variant of IPMN. ITPN is a rare tumor seen at an average age of 53 years which presents with nonspecific symptoms (409,547). The clinical findings are often indistinguishable from those of IPMNs. Most cases have cystic ducts filled with nodular tumor (Fig. 35.66), although the cystic nature may be less evident than for IPMNs. It occurs predominantly in the head but may involve any part of the gland. It is often large (mean: 7 cm; range: up to 15 cm) (412,547,555).
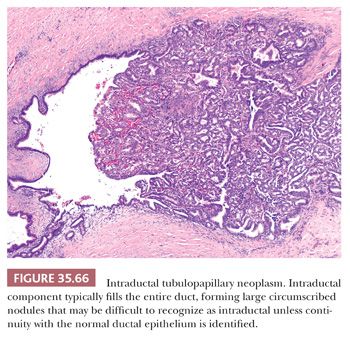
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


