Epithelial tumors have traditionally been subclassified based on the histologic type of the epithelial cells. Recent studies have confirmed the validity of this approach, as the different types of borderline and malignant epithelial tumors, diagnosed based on cell type, differ with respect to underlying molecular abnormalities, precursor lesions, genetic risk factors, patterns of spread, and response to chemotherapy (Table 54.2)(6). Correct cell type diagnosis is clinically relevant for both assessment of genetic risk (high-grade serous carcinomas are associated with BRCA mutations, whereas endometrioid and clear cell carcinomas are associated with Lynch syndrome—see the section “Hereditary Ovarian Cancer”) and treatment. In 2006, a National Cancer Institute consensus conference called for clinical trials for patients with mucinous and clear cell carcinomas, given their poor response rates to conventional chemotherapy (7), and such trials have opened. With the emergence of type-specific treatment for ovarian carcinomas, correct diagnosis of cell type for malignant epithelial tumors has become critically important; fortunately, most diagnoses can be made with a high degree of reproducibility between pathologists and in most cases can be done based on careful examination of routine hematoxylin and eosin–stained sections only, without resorting to ancillary studies (8).
The traditional view that most epithelial tumors are derived from the ovarian surface epithelium that develops from the coelomic epithelium (mesothelium) covering the embryonic gonad has recently changed with the recognition that most high-grade serous carcinomas associated with BRCA mutations arise from the epithelium of the fimbriated end of the fallopian tube (9–11). Thus, there is now no accepted overarching theory of histogenesis of ovarian epithelial tumors, and histogenesis for each type will be discussed in the following sections. Some subtypes that are included in the epithelial category, particularly mucinous and squamous tumors, may be of germ cell derivation in at least some cases (12). Despite these histogenetic problems, these tumors have been retained in the epithelial category to maintain the simplicity of the classification.
The most recent update to the FIGO staging guidelines for ovarian cancer reflects these changes in our understanding of the histogenesis of high-grade serous carcinomas (13). Carcinomas of ovary, fallopian tube, and peritoneum are now staged using identical criteria (Table 54.3). In some advanced-stage high-grade serous carcinomas, it is impossible to assign a primary site with certainty (i.e., primary ovarian, tubal, or peritoneal carcinoma), but with the updated staging criteria, this will not impact on stage assignment nor is treatment changed based on primary site.

Careful study of epithelial tumors may uncommonly reveal two or even three cell types. Malignant tumors most often consist of admixtures of those tumor types associated with endometriosis, that is, clear cell carcinoma, endometrioid carcinoma, and seromucinous borderline tumor. When the additional cell types account for less than 10% of the neoplasm, it is classified on the basis of its predominant cellular element; otherwise, the tumor is designated a mixed epithelial tumor. The 10% figure was selected arbitrarily for classification purposes, but the presence of a smaller component should be recorded in an accompanying note, especially when it is more malignant than the pure or almost pure predominant component. Historically, mixed carcinomas were often diagnosed based on the presence of combinations of high-grade serous and “undifferentiated” carcinoma, high-grade serous and high-grade “endometrioid” carcinoma, high-grade serous and “clear cell” carcinoma, and high-grade serous and “transitional cell” carcinoma. Molecular studies indicate that the vast majority of these cases are variants of high-grade serous carcinoma, with solid growth, glandular growth, cytoplasmic clearing, and transitional-like growth, respectively (14–17).
In addition to being subdivided according to cell type, epithelial tumors are subclassified according to four other criteria, two of them architectural and the remainder related to the extent of tumor cell proliferation, the degree of nuclear atypia, and the presence or absence of stromal invasion. Some tumors, particularly those in the serous category, may be exophytic, endophytic, or both. Except for the Brenner tumor, which almost always has a predominant stromal component, most epithelial–stromal neoplasms of other cell types are predominantly epithelial, with only a minor component derived from the ovarian stroma. When benign gland-forming tumors have a predominant stromal component, the terms adenofibroma and cystadenofibroma (if grossly visible cysts are present) are used.
One group of epithelial tumors are intermediate between clearly benign and obviously malignant in both their histologic features and their clinical behavior. FIGO initially adopted the descriptive designation low malignant potential for such neoplasms, but the term borderline malignancy was later selected as a preferable synonym and was subsequently maintained as such by the World Health Organization (WHO) committees on classification and histologic typing of ovarian tumors (4,5). Tumors in this category are characterized by a degree of epithelial proliferation greater than that seen in benign tumors of the same cell type, nuclear atypia (but to a degree less than that of carcinoma), and an absence of “obvious” or “destructive” stromal invasion. Borderline tumors of different cell types show profound clinical and biologic differences. Because of the differences between the various types of borderline tumor, they will be considered in detail separately under their respective headings.
Grading of ovarian epithelial cancers varies depending on cell type and is accordingly considered within each histologic type. It is noteworthy that for advanced-stage high-grade tumors, more nuanced grading (i.e., grade 2 vs. grade 3) is not of prognostic significance (18). Although grade has long been considered an important prognosticator for localized (FIGO stage I) ovarian carcinomas, these earlier studies did not take into account tumor cell type (19,20). It is uncertain whether grade carries any prognostic significance independent of cell type, even for low-stage tumors.
HEREDITARY OVARIAN CANCER
More than 15% of epithelial ovarian cancers are hereditary (10,21–26). Two autosomal dominant cancer susceptibility syndromes account for a large majority of these cases: hereditary breast and ovarian cancer syndrome, secondary to germline BRCA1 and/or BRCA2 mutations; and Lynch syndrome, or hereditary nonpolyposis colorectal cancer (HNPCC) syndrome, secondary to mutations in DNA mismatch repair enzymes, with MLH1, MSH2, MSH6, and PMS2 being the most commonly mutated genes in this syndrome.
Hereditary breast and ovarian cancer syndrome accounts for 90% of hereditary ovarian cancer; BRCA1 mutation carriers are associated with a lifetime risk of 40% to 50%; the corresponding figure for BRCA2 carriers is 20% to 30%. Almost all of these ovarian carcinomas in this syndrome are high-stage, high-grade serous carcinomas. Overall, they have a better survival than similar sporadic cancers (27–28), although this varies with the specific mutation in BRCA1 or BRCA2, as some are associated with a relatively worse outcome (27), compared to sporadic cases. The frequency of germline mutations in BRCA1 or BRCA2 in patients presenting with high-grade serous carcinoma is sufficiently high that some recommend that all such patients should undergo genetic counselling and BRCA mutation testing (24). Many BRCA1 and BRCA2 carriers are treated by risk-reducing salpingo-oophorectomy (RRSO), which dramatically reduces their risk of developing high-grade serous carcinoma. In toto, histologic examination of these specimens should be performed. Finch et al. (22) found that RRSO specimens in 6.4% of BRCA1 carriers and 1.5% of BRCA2 carriers harbor a carcinoma. The tumors are often microscopic, and most arise in the fallopian tube. Despite their small size, some of the tumors have been associated with metastases. With risk-reducing surgery now being performed at a younger age, compared to when these earlier studies were done, the number of early cancers detected in these specimens has decreased.
Ovarian carcinoma is less common than either colorectal or endometrial carcinoma in patients with Lynch syndrome, and only 10% of patients with the syndrome will develop an ovarian carcinoma. The type of carcinomas associated with Lynch syndrome is less well established than that for hereditary breast and ovarian cancer syndrome, but based on available evidence, it appears that endometrioid and clear cell carcinomas predominate (25,26).
SEROUS TUMORS
Serous neoplasms account for 20% to 50% of ovarian tumors, depending on whether the frequency figures emanate from the Far East or from Western countries, respectively. Approximately 70% of serous tumors are benign, 5% to 10% are borderline, and 20% to 25% are carcinomas. Invasive serous carcinomas account for 70% to 75% of all ovarian carcinomas in North America, but this varies considerably worldwide, being higher in Israel and lower in Asia. Benign serous tumors may occur at any age but are most common during the fifth decade. Borderline tumors are encountered most often between the ages of 30 and 60 years, and carcinomas are most common between the ages of 40 and 70 years.
GROSS FEATURES
Serous tumors, whether benign, borderline, or malignant, tend to be uniform throughout a given specimen, although admixtures of borderline tumor and low-grade serous carcinoma are not uncommon. They usually lack the admixtures of all three subtypes that are often seen in mucinous tumors and are found less often in endometrioid and clear cell tumors.
Benign Tumors
Benign serous tumors are usually endophytic (cystadenomas), but they may be exophytic (surface papillomas) or both. The serous cystadenoma is usually unilocular or paucilocular, but it may be multilocular; it is characterized by thin-walled cysts filled with watery or, occasionally, thin mucinous or hemorrhagic fluid. The cysts may have a smooth, flat lining or one with polypoid excrescences, which are firm if their stroma is dense and fibrous and soft if it is edematous. Serous cystadenomas, which are bilateral in approximately 10% of cases, can be large but only occasionally attain the huge dimensions that are more often reached by mucinous cystadenomas.
Serous surface papillomas appear as polypoid excrescences on the outer surface of one or both ovaries, which have features similar to those seen within cysts. Rarely, edematous excrescences break off and float or become adherent to the pelvic peritoneum. The surface tumors are often accompanied by underlying cystic components. Serous adenofibromas are hard, white, predominantly solid fibromatous tumors, within which are small glands or cysts containing clear fluid and sometimes having polypoid excrescences. When these tumors are bilateral, their hard consistency may lead to a gross misdiagnosis of cancer.
Borderline Tumors
Serous borderline tumors (SBTs) (29–34) may occupy part of an otherwise benign serous tumor (Figs. 54.1 and 54.2). They have gross features similar to those of benign serous tumors, except that their papillae are generally more extensive, confluent, and softer. Microscopic examination is necessary to distinguish these tumors reliably. Cystic SBTs may secrete thick, mucinous fluid, which should not lead to a misdiagnosis of a mucinous tumor on gross examination. SBTs are obviously bilateral in approximately 25% of FIGO stage I cases. In approximately 10% of cases in which a unilateral oophorectomy is being considered a treatment option, tumor is detected in a biopsy specimen of a normal-appearing contralateral ovary. Omental involvement by implants of SBT is often not appreciable macroscopically; in contrast, invasive implants/low-grade serous carcinoma involving the omentum often produces grossly evident omental lesions.
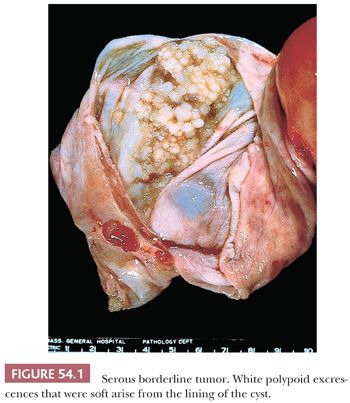
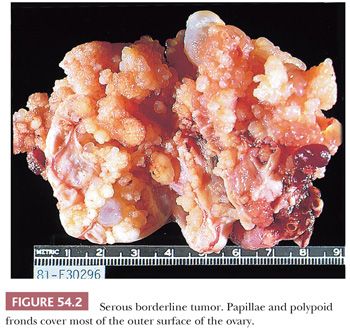
Malignant Tumors
Serous carcinomas encompass high-grade serous carcinomas (HGSCs) and low-grade serous carcinomas (LGSCs). It is now appreciated that these tumor types differ significantly with respect to clinical, molecular, and pathologic characteristics (Table 54.2), and they will therefore be considered separately.
HGSCs may be predominantly cystic and papillary, entirely solid and soft or firm, or both cystic and solid. Friability, necrosis, and hemorrhage are common. HGSCs are bilateral in about two-thirds of advanced cases, but this is seen in only about one-fourth of FIGO stage I cases. Presentation with disease confined to the ovary is very uncommon.
LGSCs can appear as closely packed, soft to firm, sometimes hemorrhagic surface papillae or nodules, requiring microscopic examination for their distinction from SBTs. Some LGSCs are very heavily calcified, with innumerable psammoma bodies, resulting in a gritty sensation on palpation or sectioning. Necrosis is uncommon in LGSCs.
MICROSCOPIC FEATURES
Benign Tumors
The cysts, as well as the polypoid excrescences of benign serous tumors (which may be exophytic, endophytic, or both), are typically lined by epithelium similar to that of the fallopian tube, including focal or diffuse ciliation. Tumors lined entirely by nonciliated cuboidal or columnar epithelium that resembles ovarian surface epithelium are also generally included in the serous category, despite their indifferent appearance. The epithelial cells of benign serous tumors may secrete mucin but, when present, is confined to the lumina of cysts and the apical cytoplasm of the lining cells. Psammoma bodies are usually inconspicuous or absent. Papillae, if present, are composed almost entirely of stroma, which may be dense and collagenous or markedly edematous. In adenofibromas and cystadenofibromas, glands and cysts are scattered within a predominantly fibromatous stroma. Otherwise typical benign cystic serous tumors containing very minor foci (<10% of the cyst lining) of borderline neoplasia (nuclear stratification and atypia) are retained in the benign category (referred to as “focally proliferative” serous cystadenoma or serous cystadenoma with “focal atypia”) by some investigators (29). Although such tumors typically behave in a benign fashion, studies of large numbers of such cases with long-term follow-up have not been performed. Borderline foci are likely more important in cases of serous surface neoplasia because of a possibly greater association with extraovarian peritoneal implants (35). Additionally, Longacre et al. (33) have found that some of these tumors are followed by an ipsilateral or contralateral typical SBT. Whatever terminology is used for these tumors, the proportion of the tumor that consists of borderline neoplasia and its location (intracystic or surface) should be specified in the pathology report.
Borderline Tumors
The polypoid excrescences of typical SBTs gradually lose their stromal support as they branch, terminating in tiny, stroma-free clusters of neoplastic cells that appear to be detached from their moorings, floating into cyst lumina or off the outer surface of the ovary (hierarchical pattern of branching) (Fig. 54.3). The neoplastic cells typically have scanty cytoplasm, but some of them may contain abundant eosinophilic cytoplasm, and occasionally such cells predominate, especially in pregnant patients. Varying degrees of nuclear atypicality, usually mild to moderate, may be present, and mitotic figures range from rare to occasional. Ciliated cells are characteristically present, and psammoma bodies are found in approximately 25% of tumors. In SBTs that secrete thick mucin, the mucin is extracellular and confined to the lumen and the superficial cytoplasm of the cells. Most SBTs are completely or predominantly cystic, but a minority are associated with a background adenofibroma or cystadenofibroma. The stroma of SBTs is often edematous and occasionally hyalinized. A few SBTs involve the glandular or cystic components of adenofibromas or cystadenofibromas. The presence of a surface (serosal) component is associated with an increased frequency of peritoneal implants (35).
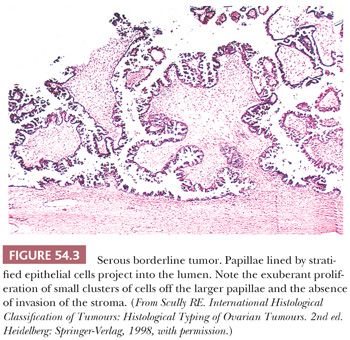
Several changes within SBTs may result in an overdiagnosis of carcinoma. Often, glandular proliferations with or without papillae invaginate into the stromal component of the tumor in an orderly fashion. The stroma in these areas of pseudoinvasion, however, does not differ in its appearance from the stroma in other areas that lack epithelial elements. By contrast, the destructive stromal invasion of an LGSC is characterized by a disorderly disposition of neoplastic glands in stroma that is desmoplastic or, less often, myxoid or hyaline. In some SBTs, areas in which glands and nests of tumor cells lie within a desmoplastic stroma are found between papillae within intracystic tumors or on their serosal surfaces (36). These “autoimplants” resemble desmoplastic noninvasive peritoneal implants (see “Peritoneal Implants”). A similar appearance may occur secondary to necrosis of papillae with organization by granulation and fibrous tissue, with entrapment of residual surviving tumor cells. The location of these findings, the predominance of stroma over epithelial cells, and the presence of epithelial cells resembling those of typical SBTs facilitate distinction from the destructive invasion of a serous carcinoma. Autoimplants are often associated with high-stage disease but are not, per se, an adverse prognostic factor (36). Finally, SBTs are sometimes overlain focally by fibrous adhesions associated with mesothelial cell hyperplasia, which may be misinterpreted as surface borderline tumor or even low-grade carcinoma. The mesothelial cells, however, generally lack atypia; are characteristically aligned in linear fashion, sometimes in parallel, thin, solid, or pseudotubular rows within the fibrous tissue (37); and lack reactivity for epithelial markers (MOC-31, Ber-EP4).
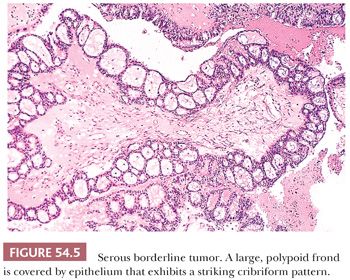
Micropapillary and Cribriform Patterns. Five percent to 10% of SBTs have a micropapillary (and/or a cribriform) pattern (Figs. 54.4 and 54.5) that is associated with a higher frequency of bilaterality, surface involvement, and implants than typical SBTs lacking these micropapillary patterns (38–45); in some (but not all) studies, the implants associated with SBTs with micropapillary features are more frequently invasive than in typical SBTs. The micropapillary pattern is characterized by a nonhierarchical pattern of branching, in which filiform papillae with little or no stromal support emanate from the inner surface of a cyst; from the ovarian surface; or directly from large, nonbranching, polypoid excrescences on either or both surfaces over a continuous extent of at least 5 mm. It has been suggested that the micropapillae should be five times as long as wide (39). The neoplastic cells forming the micropapillary and cribriform patterns are round, nonciliated, and have scanty cytoplasm, mild to moderate nuclear atypia, and often a small nucleolus. The cells are more monomorphic than those in typical SBTs. High-grade atypia other than in rare cells is definitionally absent.
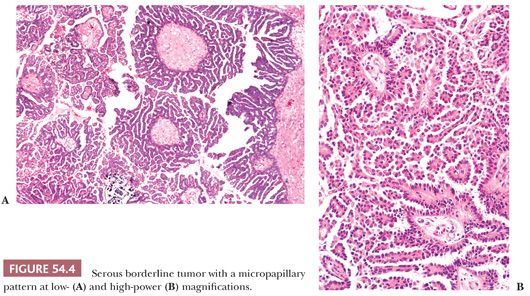
Those who initially described the micropapillary pattern proposed that SBTs with micropapillary and/or cribriform patterns be designated micropapillary carcinomas, even in the absence of stromal invasion, claiming that such tumors are associated with a much worse prognosis than typical SBTs (38). Other investigators studying typical and micropapillary SBTs (MSBTs) agreed to variable extents with some of these findings, although others were unable to confirm any of their major findings, and none concluded that a striking enough difference in prognosis between micropapillary and typical SBTs justified adoption of the term micropapillary carcinoma for this variant of noninvasive serous tumor. The issue is whether the presence of micropapillarity per se is a prognostic factor independent of advanced stage and invasive implants/LGSC, features with which it covaries. In the absence of such evidence, it is not appropriate to classify these tumors as micropapillary carcinoma. A consensus conference (29) recommended that SBTs with micropapillary or cribriform patterns be referred to as micropapillary SBTs rather than micropapillary carcinomas. These tumors, however, should be thoroughly sampled to exclude foci of invasion, that is, LGSC (43).
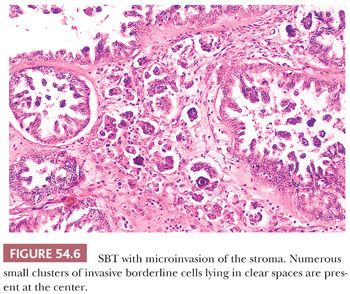
Microinvasion. Microinvasion of the stroma is encountered in 10% to 15% of SBTs (33,42,47–51). One to often multiple discrete foci of invasion are composed of cells that resemble those of a typical SBT. Upper size limits for each focus that have been suggested in the literature have varied from 3 mm or less in diameter, 5 mm or less in diameter, and 10 mm2 or less in area (Fig. 54.6). Single neoplastic cells or small nests of cells with slightly atypical nuclei and often with abundant eosinophilic cytoplasm, sometimes accompanied by psammoma bodies, lie in the stroma in tiny spaces, with or without an endothelial lining. Many patients with these tumors have been pregnant at the time of diagnosis (52). Although microinvasion was not an adverse prognostic factor in early studies, Longacre et al. (33) have found that microinvasion is strongly associated with decreased survival on multivariate analysis independent of stage, micropapillarity, and implant type. Several other recent studies have also noted an adverse prognostic effect associated with microinvasion (42,47). McKenney et al. (51) found that SBTs with areas of invasion similar in appearance to, but more extensive than, typical microinvasion have a behavior similar to that associated with typical microinvasion and should be distinguished from SBTs with foci of destructive stromal invasion in which sizable areas of the tumor are completely replaced by LGSC.
Peritoneal Implants. SBTs are accompanied by foci of proliferation of serous-type cells in the peritoneal cavity in 16% to 59% of cases (33,45,53–62). In some cases, the peritoneal foci are composed of benign-appearing serous cells, which are not considered neoplastic, but possibly preneoplastic, and have been designated endosalpingiosis (see Chapter 58). Peritoneal lesions composed of cells with features of borderline malignancy are present in about 30% of SBTs and are generally referred to as implants. That they represent spread from the ovary is suggested by their much more common occurrence in ovarian SBTs with an exophytic component (33,35). However, some regard such lesions as independent primary peritoneal SBTs. Genetic studies (60,61) suggest that both points of view may be correct. The diagnosis of primary peritoneal SBT should be confined to those cases with extensive peritoneal tumor with little or no involvement of the ovary (see Chapter 58).
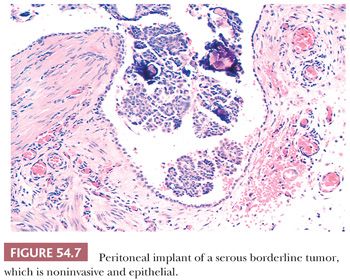
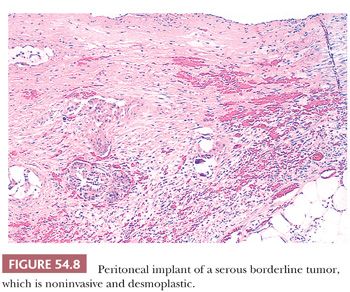
Peritoneal implants of SBTs may be grossly visible as small nodules or plaques but are often microscopic. The implants have been subclassified as noninvasive and invasive, with the former subdivided into epithelial and desmoplastic subtypes (Figs. 54.7 through 54.9) (53). In epithelial noninvasive implants, papillary proliferations of atypical serous cells with a minor or absent stromal component are present on the surface of the peritoneum (Fig. 54.7) in smoothly contoured peritoneal invaginations or in invaginations between lobules of omental fat. The cytologic atypia of these implants usually approximates that in the primary ovarian tumor. Desmoplastic noninvasive implants (Fig. 54.8) are characterized by a predominance of reactive stroma and are layered onto the peritoneal surfaces, including those lining invaginations between omental fat lobules. Within these implants, small glands and papillae lined by atypical serous cells, as well as single cells and psammoma bodies, are entrapped in granulation and scar tissue (Fig. 54.8), which is often infiltrated by acute and chronic inflammatory cells. The glands and papillae may lie in clear spaces, possibly caused by secretory activity of the neoplastic cells. Necrosis, fibrin deposition, and hemorrhage are occasionally seen and may play a role in inducing the desmoplasia.
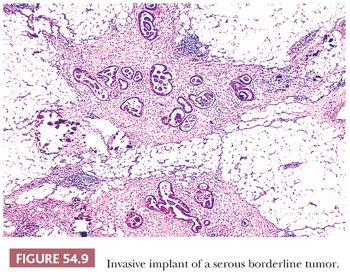
Invasive implants are foci of extraovarian disease in patients with ovarian SBTs that meet the criteria for a diagnosis of LGSC. These are best considered foci of LGSC and diagnosed as such; thus, invasive implant is synonymous with LGSC. However, the designation of “invasive implant” is well established in the literature and will be used in the following discussion. Invasive implants are much less common than noninvasive implants. Although they have been found in as many as 12% of consultation cases of ovarian SBTs, they are much less common or even absent in hospital-based series (44). Invasive implants are characterized by an irregular infiltration and destruction of underlying tissue (Fig. 54.9), identical to LGSC. In one of the largest series of consultation cases of SBT with peritoneal implants (50 cases) (53), separation into invasive and noninvasive categories had important prognostic implications. Ninety-four percent of the patients with noninvasive implants followed up for 4 or more years or until death had no progression of disease, regardless of whether they had received chemotherapy, radiation therapy, or both, or no such therapy, in contrast to only 17% of the patients with invasive implants. Severe nuclear atypicality in the implants was also independently correlated with a poor prognosis in that study (53). In a more recent study, Longacre et al. (33) found that 50% of patients with invasive implants died of disease or had progressive disease; the corresponding figures for patients with implants of indeterminate type and noninvasive implants were 21% and 10%, respectively. Implants that are noninvasive by traditional criteria (53) but that are micropapillary (exophytic, resembling the appearance seen in the primary tumor; or endophytic, in which the micropapillae fuse into a complex network of irregular spaces) or with small nests surrounded by clefts may also be associated with an adverse prognosis (55). Silva et al. (58) found that 57% of patients with this type of implant were alive with or dead of tumor, whereas it was associated with decreased disease-free survival but not overall survival in the study by McKenney et al. (59). The true recurrence rate in patients with noninvasive implants requires long-term follow-up; recurrences often develop during 5 or even 10 years of follow-up (62). It is important to note that assessment of implants as noninvasive versus invasive is only relevant in the context of SBT and is not applicable for HGSC. There are cases of ovarian LGSC associated with extraovarian disease that is indistinguishable from noninvasive implants of SBT. The significance of this rare finding is at present uncertain but should be documented in the pathology report. Because small implants have occasionally been found on microscopic examination in grossly negative omental specimens, the latter should be generously sampled by the pathologist. Implants within peritoneal biopsy specimens that lack underlying tissue are generally considered “consistent with noninvasive implants” on the premise that they were easily scraped off the peritoneal surface and did not require dissection of the underlying tissue to remove them. Adoption of this approach is justified by the excellent correlation of only obviously invasive implants with a poor prognosis. In cases of high-stage SBTs, the presence of invasive implants is the most significant prognostic indicator (33,42,45,53).
Lymph Node Lesions. About 20% to 30% of ovarian SBTs are associated with similar synchronous or metachronous tumor within pelvic and/or para-aortic lymph nodes; rarely, extra-abdominal lymph nodes may be involved (61,63–69). In such cases, the intranodal tumor may be metastatic (an interpretation favored by the presence of tumor within intranodal subcapsular lymphatics), whereas in other cases, it may arise within the lymph node from müllerian inclusion glands (endosalpingiosis) (67) (see Chapter 58). Emerson et al. (61), using X chromosome inactivation patterns, found that in most cases, lymph node inclusions (and implants) shared a common clonal origin with the associated ovarian SBT, although in a minority of cases, the extraovarian lesions appeared to have an independent origin. In rare cases, the serous tumor in the lymph nodes has the appearance of serous carcinoma (63,66,69).
The differential diagnosis of metastatic serous tumor in lymph nodes includes the presence of mesothelial cells floating in lymph node sinuses (70). Involvement of the peritoneum by a malignant tumor of any type, including an SBT, can elicit marked proliferation of mesothelial cells, which can become transported by the lymphatics to the pelvic and para-aortic lymph nodes. IHC investigation may be necessary to distinguish these cells with certainty from serous borderline neoplastic cells.
Currently available but limited evidence suggests that involvement of pelvic and, less often, para-aortic lymph nodes sampled during staging of an SBT may not per se affect the prognosis, with one possible exception. McKenny et al. (64) found that a specific pattern of nodal involvement—confluent tumor greater than 1 mm without intervening lymphoid tissue, often with a micropapillary pattern and/or desmoplastic stroma—may be an adverse prognostic finding. It should be noted that most pathologists would diagnose this pattern of nodal involvement as LGSC (69). Additionally, nodal involvement detected later in the course of the disease, particularly when extra-abdominal, is a worrisome finding in terms of prognosis, especially if it has transformed to an HGSC (63,66,71).
Transformation to Low-Grade Serous Carcinoma. SBTs associated with clinically progressive disease have frequently transformed to an LGSC and this finding is a strong adverse factor (33,40). This complication may occur more commonly with MSBTs. In some cases, the LGSC may represent a new primary peritoneal LGSC, particularly in those occurring after a long interval (71,72). In a study of high-stage SBTs, Deavers et al. (40) found that 75% of the cases that recurred had transformed to LGSC; LGSC was found in 16 of 17 patients dying from tumor. In the study by Longacre et al. (33) (all stages included), transformation to LGSC occurred in 5.5% of those with low-stage and 8% of those with high-stage disease, often after 5 or more years of follow-up. Transformation correlated positively with the presence of microinvasion and/or invasive implants. In this study, LGSC was diagnosed in the presence of both florid epithelial proliferation and destructive stromal invasion of normal tissues. The tumor had marked epithelial predominance, often with confluent, irregular, and haphazardly arranged nests, cords, and micropapillae composed of cells with mild to moderate nuclear atypia and mitotic activity. Almost half of the patients died of disease after a mean interval of 21.5 months following diagnosis of LGSC (33). Rare instances of SBT transforming to high-grade carcinoma are documented (73–75), although such tumors appear to be distinct from conventional HGSC in terms of their molecular abnormalities. Such high-grade transformation is associated with a poor prognosis.
Malignant Tumors
Serous carcinomas are classified as high-grade or low-grade based on variability in nuclear size, with HGSC showing greater than threefold variation in nuclear size. In cases that are problematic to classify based on nuclear features, mitotic activity can be used as a secondary feature, with HGSC showing greater than 12 mitotic figures/10 high-power microscopic fields (76,77).
High-Grade Serous Carcinoma. It is now appreciated that almost all HGSCs arise de novo from the fallopian tube epithelium or ovarian surface epithelium or its inclusion cysts rather than through progression of an LGSC. HGSCs spread rapidly early in their course, such that stage I tumors are rare (78,79). HGSC almost all have TP53 mutations (80), resulting in either high-level expression of p53 protein or complete loss of p53 immunoreactivity (“all or nothing” staining pattern) indicative of abnormal p53 (81,82). HGSCs are chromosomally unstable, with complex karyotypes, aneuploidy, and considerable intratumoral genetic heterogeneity (83,84).
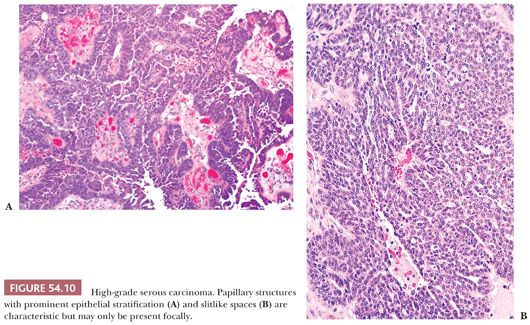
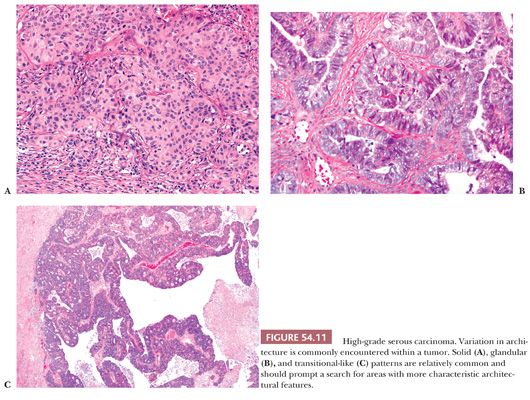
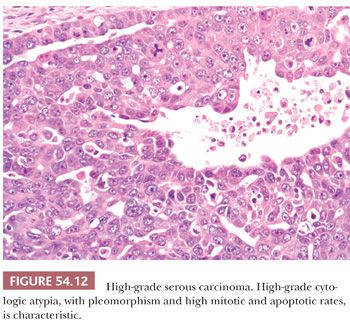
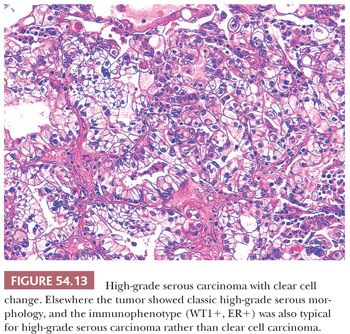
HGSCs show a wide range of architectural patterns, with multiple patterns frequently coexisting in a single tumor. The most characteristic pattern is that of fine, disorderly papillae with little or no stromal support and irregular, often slitlike, rounded, or microcystic glandular lumina (Fig. 54.10). Solid, glandular, and transitional-like patterns are also common and may predominate (Fig. 54.11). The cytologic features are usually high grade throughout, with numerous mitotic figures (Fig. 54.12), but in some cases, the tumor cells may be of relatively uniform size. Signet ring morphology may be present. The immunophenotype is typical of HGSC (including positivity for CK7, WT1, and estrogen receptor [ER]) (85). The different architectural patterns are not of clinical significance. Occasional HGSC contain variable numbers of cells with clear cytoplasm (Fig. 54.13) that have a typical HGSC immunophenotype (WT1+/ER+/HNF-1β−) (14,15) allowing their distinction from clear cell carcinoma.
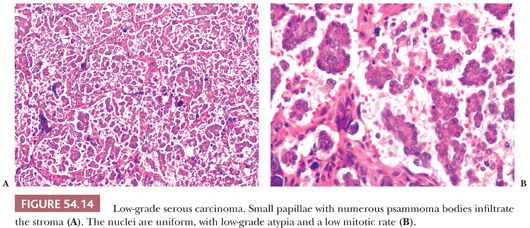
Low-Grade Serous Carcinomas. LGSCs are much less common than HGSC and tend to occur in younger women (61). Many, and perhaps most, LGSCs arise within SBTs. LGSCs are usually characterized by irregular infiltration of small, tight nests of tumor cells within variable amounts of desmoplastic or hyaline stroma, which often contains psammoma bodies or larger calcific deposits (Fig. 54.14). Some tumors have a micropapillary pattern that may be exophytic (like that seen in MSBTs) or endophytic, in which the micropapillae fuse into a complex network of irregular spaces (33). Rare LGSCs are characterized by the presence within a fibrous stroma of stromal papillae lined by tumor cells lying in spaces that lack an endothelial lining (macropapillary pattern) (86); the spaces are probably created by secretion of serous fluid by the neoplastic cells. The cytologic features are low grade throughout (Fig. 54.14). A rare form of LGSC with a favorable prognosis is the psammocarcinoma (87). This tumor, which is often primary in the peritoneum, appears to behave clinically more like an SBT than a serous carcinoma, but it differs in its microscopic features from the former by infiltrating the ovarian stroma or other peritoneal organs. Four criteria have been recommended for the diagnosis of psammocarcinoma: (a) invasion of the ovarian stroma or its lymphovascular spaces (or, in extraovarian areas of involvement, invasion of any intraperitoneal tissue or its lymphovascular spaces), (b) no more than mild to moderate nuclear atypia, (c) epithelial nests no greater than 15 cells in their largest linear dimension, and (d) the presence of psammoma bodies in at least 75% of papillae or nests. LGSCs differ from HGSCs in being diploid or near diploid, with relatively few mutations (88–92). BRAF and KRAS mutations are common in both SBTs and LGSCs but not HGSCs (90).
DIFFERENTIAL DIAGNOSIS
Cystadenoma
Serous cystadenomas may be confused with rare rete cystadenomas (see Chapter 56), but the latter have a hilar location, are commonly surrounded by a layer of smooth muscle (and occasionally, a band of hyperplastic hilus cells), typically exhibit shallow crevices along their inner surface, and are lined by cells that contain few or no cilia. Ciliated serous cystadenomas, however, arise rarely within the hilus and may also have shallow crevices along their inner surfaces. Rare examples of cystic struma ovarii simulate serous cystadenomas but contain at least minor foci of recognizable thyroid follicles in the outer wall or septa of the cyst. Occasional serous cystadenomas have subepithelial histocytes containing pigment which may be confused with the pseudoxanthoma cells of endometriotic cysts. In the serous cystadenomas, there is usually not the vascularity and definitionally not the endometrial stroma, admittingly sometimes limited, that is present in almost all endometriotic cysts. Serous surface papillary adenofibromas should be distinguished from the relatively common tiny, warty excrescences (surface stromal proliferations) that may be encountered on the surface of the ovary. The latter lesions are typically multifocal; have a simple, cuboidal epithelial covering; and do not form a mass lesion.
Borderline Tumors
SBTs are only occasionally confused with other neoplasms. Their distinction from LGSC has already been discussed. Serous borderline neoplasia may also exist as a component of mixed müllerian borderline tumors (page 2564). An SBT can be confused with a papillary clear cell carcinoma (93). Features favoring the latter include an association with endometriosis, predominance of clear and/or hobnail cells, basement membrane–rich stroma, and an HNF-1β+/WT1−/ER− immunoprofile. Further, hobnail-like cells in otherwise typical SBTs are usually smaller than those seen in clear cell carcinomas and are typically present only in small foci. Retiform SLCTs can have areas that are almost indistinguishable from SBTs, but they occur in a much younger age group and have other distinctive features such as irregular networks of epithelial-lined spaces, long ribbons of sex cord–type cells, glomeruloid formations, other patterns of SLCTs, and immunoreactivity for markers of sex cord differentiation, such as inhibin.
Carcinoma
A primary serous carcinoma of the ovary must be distinguished from an endometrial serous carcinoma metastatic to the ovary. Although endometrial and ovarian/tubal/peritoneal serous carcinomas have different molecular abnormalities (83,94,95), they are morphologically identical, and clinical correlation with evaluation of overall disease distribution is required to establish the primary site. Most advanced-stage serous carcinomas with endometrial involvement, even if the uterine tumor is not myoinvasive, are primary endometrial serous carcinomas (96). With regards to distinction between primary ovarian, tubal, and peritoneal HGSC, it is clear that previous criteria for these distinctions, based on “dominant tumor mass,” do not always indicate the primary site and underestimate the number of tumors originating in the fallopian tube. The propensity of secondary ovarian involvement by carcinoma to result in tumor masses larger than the primary tumor is well known (e.g., ovarian metastases from carcinomas of vermiform appendix, stomach, endocervix, colon). As there are no morphologic or molecular markers that can distinguish between HGSCs originating in ovary, fallopian tube, or peritoneum, it may not be possible to determine the primary site in some cases of advanced-stage HGSC. The treatment is the same for all three primary sites, so that accurate determination of primary site is not necessary for clinical management and it is acceptable to acknowledge that primary site cannot be determined with certainty in a given case.
If the carcinoma is confined to the ovarian surface and is the only or almost only intraperitoneal tumor, it is considered to be of ovarian origin; if the ovarian tumor is invasive, it is considered primary if the depth of invasion of the ovarian parenchyma exceeds 5 mm in maximal dimension, even though the peritoneal tumor masses may be much larger.
Although serous carcinoma is the epithelial cancer most often associated with papillae, other carcinomas (particularly endometrioid and clear cell) may be papillary as well and enter the differential diagnosis. The papillae in endometrioid carcinomas are typically villous, as seen in their uterine counterparts, but occasionally, they resemble endometrial endometrioid carcinomas with small, nonvillous papillae. In the typical example of the latter tumor subtype, small, solid papillary tufts of well-differentiated cells with abundant eosinophilic cytoplasm and often focal squamous differentiation extend into endometrioid glands or from villous projections. The papillae in clear cell carcinomas are lined by hobnail or clear cells and typically have hyalinized cores; other distinctive patterns of clear cell carcinoma are almost always present. HGSC with predominantly glandular architecture can raise the differential diagnosis of endometrioid carcinoma. Such tumors lack other features of endometrioid carcinoma, such as squamous differentiation, and show high-grade nuclear features throughout. In contrast, more than 90% of ovarian endometrioid carcinomas are grade 1 or 2 (13). WT1 immunostaining can be used in problematic cases and if strongly positive favors a diagnosis of HGSC (96,97). An “all or nothing” pattern of p53 staining also strongly favors HGSC (98).
Rare serous carcinomas containing cells with abundant eosinophilic cytoplasm simulate malignant mesotheliomas, but they are usually distinguished by their intraovarian location, their distinctive pattern of glandlike formation, and their nuclear characteristics. Very rarely, however, true mesotheliomas appear to originate within the ovary rather than on its peritoneal surface (99). Malignant mesothelioma cells are typically less pleomorphic than those of serous carcinoma, and their papillae are less cellular. The tubulopapillary variant of malignant mesothelioma does not typically have the irregular, slitlike glandular spaces of serous carcinoma. Psammoma bodies are much less common in mesotheliomas than in serous carcinomas. IHC staining may be necessary to make the correct diagnosis in rare cases (see Chapter 58).
Women with breast cancer are at increased risk of primary ovarian carcinoma; indeed, one study (100) showed that when a patient with breast cancer also has an adnexal cancer, the latter is more likely to be primary in the ovary or fallopian tube than metastatic. Making the distinction between serous carcinoma and metastatic breast carcinoma can rarely be a problem when the latter closely simulates serous carcinoma both microscopically and in its pattern of spread within the abdomen. In such cases, both tumors should be compared in terms of their grades, patterns, and cell types. IHC staining may be helpful in difficult cases; staining for gross cystic disease fluid protein-15 and/or mammoglobin strongly favors metastatic breast carcinoma, whereas PAX8 and/or WT1 staining strongly favors primary ovarian carcinoma.
MUCINOUS TUMORS
Mucinous ovarian tumors are characterized by glands and cysts, which may have papillae, lined to variable extents by mucin-containing epithelial cells. These tumors account for 12% to 15% of ovarian tumors in Western countries and a higher percentage in Far Eastern countries, at least partly because of a decreased prevalence of other tumors, such as serous tumors, in Asia. Approximately 5% of ovarian mucinous neoplasms are invasive carcinomas, 20% are borderline, and 75% are benign.
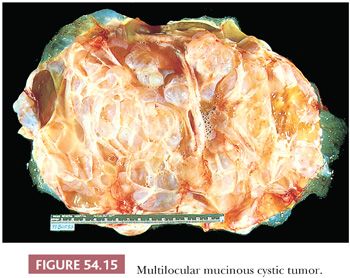
Mucinous tumors tend to be the largest of all ovarian tumors. Many are 15 or more cm in diameter (Fig. 54.15); some exceed 30 cm and may weigh up to 4000 g or more. Borderline tumors of intestinal type typically do not differ grossly from cystadenomas. Mucinous cystadenocarcinomas may have conventional features that are grossly suggestive of malignancy, such as solid nodules, necrosis, and hemorrhage, but many of them are grossly indistinguishable from benign and borderline mucinous tumors. It should be noted that occasional benign tumors have a focal adenofibromatous component which can produce a solid nodule that on gross evaluation may be concerning for malignancy. In addition, occasional benign tumors may exhibit infarct-type necrosis that may impart a deceptively worrisome appearance on gross examination.
The origin of individual mucinous tumors is obscure in most cases. There is evidence, however, that mucinous tumors in the müllerian/endocervical-like mucinous and mixed müllerian borderline group are associated with and probably arise from endometriotic cysts. Although only about 5% of mucinous tumors arise in the wall of dermoid cysts, this association is somewhat higher than it is for other tumors in the surface epithelial category. The mucinous tumors associated with teratoma show genetic features of germ cells, whereas mucinous tumors without a teratoma have normal somatic cell genetic makeup, indicating that the former but not the latter tumors are of germ cell origin (12). Most mucinous borderline tumors are characterized by intestinal-type epithelium, in which goblet cells are almost always found, argentaffin cells are seen in them occasionally, and Paneth cells are encountered much less often. Neuroendocrine cells can be demonstrated in a high percentage of mucinous tumors of the ovary by special staining (101). IHC staining and electron microscopic examination have also shown that even the epithelium lining typical mucinous cystadenomas has features of gastric pylorus rather than endocervical epithelium (102). A search for early mucinous ovarian tumors has suggested that some of them arise from mucinous inclusions in the medullary-hilar area of the ovary or from transitional cell nests similar to those in the Brenner tumor in the same locations (103).
Instead of presenting with symptoms or physical evidence of ovarian masses in the pelvis or abdomen, mucinous tumors present rarely with endocrine manifestations. The presence of neuroendocrine cells accounts for the exceptional occurrence of the carcinoid syndrome related to its content of serotonin-secreting cells and, somewhat more often, production of the Zollinger-Ellison syndrome caused by gastrin-secreting cells (104). Mucinous cystic tumors are among the most common that stimulate the ovarian stroma to luteinize and to produce steroid hormone effects that simulate those of typical functioning tumors of the ovary. These tumors are among the most common of those causing virilization in young women during pregnancy.
BENIGN TUMORS
Mucinous cystadenomas are lined by a single layer of mucin-containing columnar cells, almost always resembling those of the endocervix; the rare mucinous adenofibromas contain glands lined by similar cells embedded in fibromatous stroma (105). Cystadenomas are bilateral in 2% to 5% of cases and are associated with dermoid cysts in up to 5% of cases.
BORDERLINE TUMORS
Mucinous cystadenomas of borderline malignancy (low malignant potential) (106–111), as conventionally diagnosed and classified in the WHO classification, are almost as common as SBTs; they account for about half of all mucinous malignant tumors and for about 70% of those that are stage I. These tumors were first subdivided in 1988 into müllerian/endocervical-like and intestinal-type tumors based on the appearance of their neoplastic epithelium (112). These two subtypes of mucinous borderline tumor show consistent clinical and pathologic differences, justifying considering them as separate entities.
Endocervical-Like (Müllerian-Type) Mucinous Borderline Tumors (Seromucinous Borderline Tumors)
Endocervical-like papillary cystic tumors of borderline malignancy (EMBTs) accounted for approximately 15% of mucinous borderline tumors in a consultation series, 5% in a tumor registry series, and 20% in a mixed consultation and hospital-based series (108,112–119). These tumors differ in many respects from intestinal-type mucinous borderline tumors (IMBTs). Most of the patients are in the early reproductive age group (mean ages 35 to 39 years). Eighty percent to 95% of tumors are stage I. They are not associated with pseudomyxoma peritonei, but 30% to 50% of the tumors are associated with pelvic endometriosis, some of which arise within endometriotic cysts; in such cases, a transition between the endometriosis and the tumor may be seen. EMBTs are synchronously or metachronously bilateral in 20% to 40% of cases. They tend to be smaller than IMBTs, being typically 8 to 10 cm in diameter, and are usually unilocular or contain three or fewer locules. Most tumors have grossly visible intracystic papillae like those in SBTs.
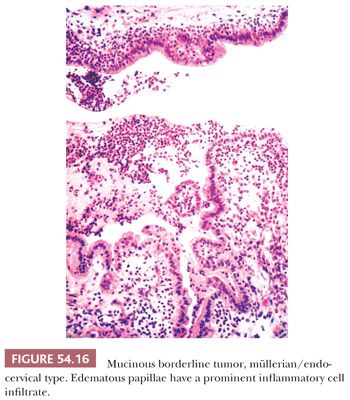
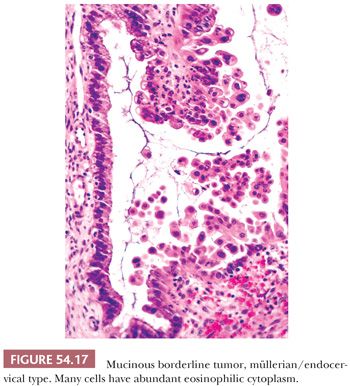
Microscopic examination reveals papillae that mimic architecturally those of SBTs. The epithelial lining is composed of columnar mucin-containing cells (Fig. 54.16), as well as polygonal cells with abundant eosinophilic cytoplasm (Fig. 54.17), which are usually located at the tips of papillae. The nuclei exhibit mild to focally severe atypia. Nuclear stratification of the polygonal eosinophilic cells in EMBTs can be much greater than that of the cells in IMBTs. Areas of intestinal differentiation are absent. A prominent acute inflammatory cell infiltrate in the stroma (Fig. 54.16), within the neoplastic epithelium, and in the cyst lumina is found in most cases. Foci of microinvasion (with size limits as described for SBTs) may occur. Typically, the microinvasive foci are composed of cells with mild to moderate atypia (microinvasive EMBT), but occasionally, the invasive cells are severely atypical (EMBT with microinvasive carcinoma); rarely, frankly invasive mucinous adenocarcinoma has been encountered (117,118). In contrast to IMBTs, the tumors are typically immunoreactive for ER, progesterone receptor, and CA-125 but are typically negative for CK20 and CDX2 (119). In common with IMBTs, they frequently have mutations in KRAS and lack PTEN mutations (120). They also frequently have mutations in ARID1A, a mutation commonly encountered in endometrioid carcinomas (121).
Patients with stage II or III tumors have peritoneal implants (which, when classified, have been noninvasive) or lymph node metastases, findings that appear to have no adverse prognostic significance. Tumor-related deaths are rare and have usually been due to a component of frankly invasive adenocarcinoma (118) or, less commonly, microinvasive carcinoma (122) or intraepithelial carcinoma (116).
Intestinal-Type Mucinous Borderline Tumors
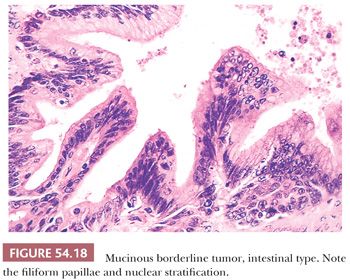
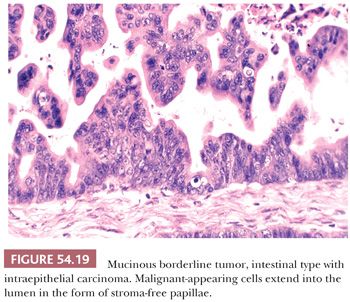
IMBTs account for the remaining majority of mucinous borderline tumors (106–111). They are encountered most often in women in the fourth to seventh decades, with an average age of 52 years; they are bilateral in only about 5% of cases, are more than twice as large (19 cm in average diameter) as EMBTs, and are usually multiloculated. Microscopic examination reveals cysts and glands lined in significant part by atypical epithelium of intestinal type (Figs. 54.18 and 54.19). In some cases, the cysts contain papillae, which, when present, are usually filiform and often branching. The epithelial lining almost always contains goblet cells, neuroendocrine cells (on special staining) in most cases, and occasionally Paneth cells. The lining cells are often stratified, generally to two or three layers, and exhibit nuclear atypia that is usually only mild or moderate. The presence of severely atypical lining cells warrants a diagnosis of IMBT with intraepithelial carcinoma (see “Malignant Tumors”).
IMBTs often contain small, circumscribed pools of extravasated mucin in the stroma, sometimes associated with a histiocytic and foreign body giant cell reaction (mucin granuloma) (123). This finding should be distinguished from extensive dissection of mucin into the ovarian stroma, usually without an inflammatory cell response, a finding referred to as pseudomyxoma ovarii that may be accompanied by pseudomyxoma peritonei. Ovarian tumors with the appearance of IMBTs associated with pseudomyxoma ovarii and pseudomyxoma peritonei almost always represent ovarian metastases from an extraovarian primary mucinous tumor that is usually in the appendix but occasionally elsewhere (see Chapter 56). An exception to the foregoing are rare IMBTs that arise is association with a dermoid cyst. Such tumors appear to have an immunoprofile (CK7−/CK20+) and a behavior similar that of low-grade mucinous tumors of the appendix and share with them the occasional presence of pseudomyxoma ovarii and pseudomyxoma peritonei (124).
Rare, otherwise typical IMBTs may contain foci of microinvasion (each focus is <10 mm2) in which the invasive cells resemble the noninvasive cells, exhibiting no more than mild or moderate atypia (110,113,120). Based on a limited number of reported cases, these microinvasive IMBTs have a benign clinical course and should be distinguished from microinvasive mucinous carcinomas in which the invasive cells show moderate to high-grade atypia and usually an origin from foci of intraepithelial carcinoma (see “Malignant Tumors”). Typical and microinvasive IMBTs are almost invariably stage I and behave in a benign fashion, although only small numbers of microinvasive IMBTs have been reported. However, rare otherwise typical IMBTs may recur, especially if treated by cystectomy (126,127). Recurrent tumor can retain a borderline appearance and involve the ipsilateral or contralateral ovary or the peritoneum as implants that may invade underlying tissues such as bowel or vagina. Some of the implants may contain residual ovarian stroma.
MALIGNANT TUMORS
Mucinous tumors that contain overtly carcinomatous epithelium (107,109–111,128–134) can be subdivided into those with (a) intraepithelial carcinoma (also referred to as noninvasive or intraglandular carcinoma [128]), such foci usually arising within a background of IMBT (“IMBT with intraepithelial carcinoma”) (Fig. 54.19); (b) microinvasive carcinoma (i.e., tumors with foci of invasive carcinoma that are <10 mm2) (the invasive foci usually arise from intraepithelial carcinoma); and (c) invasive carcinoma in which the invasive focus exceeds that allowable for microinvasive carcinoma (the invasion can be of expansile or infiltrative type). Almost all invasive mucinous carcinomas are of intestinal type and usually arise from foci of intraepithelial carcinoma, often within a background of IMBT, findings suggesting an origin of these carcinomas from less malignant precursors. As noted earlier, very rare mucinous carcinomas of the endocervical-like or mixed müllerian type have been encountered; three of the four cases were associated with endometriosis and one with an EMBT of the contralateral ovary (117).
We diagnose intraepithelial carcinoma in an IMBT if the nuclear atypia is marked. In such cases, the carcinomatous cells usually (but not invariably) exhibit marked cellular stratification, stroma-free papillae, a cribriform pattern, or combinations thereof. IMBTs with intraepithelial carcinoma warrant more extensive sampling than usual to rule out foci of invasion. These tumors are almost invariably stage I and associated with a favorable prognosis, although rare tumors have recurred, especially if stage IC (135).
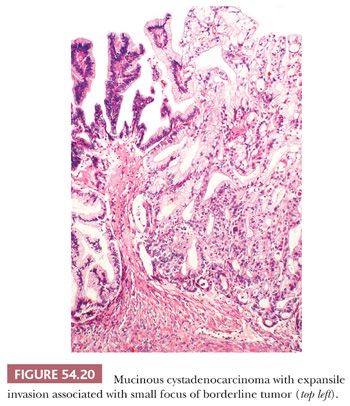
Expansile invasion (Fig. 54.20) in mucinous carcinomas is characterized by a confluent growth of back-to-back or almost back-to-back glands or cysts lined by malignant epithelium that occupy an area greater than 10 mm2. In some cases, the expansile pattern is intracystic. Infiltrative invasion (Fig. 54.21) is diagnosed when there is irregular penetration of the stroma by often-misshapen glands, clusters, cords, or nests of cells, typically evoking a stromal response. Mucinous carcinomas with expansile invasion are almost invariably stage I and recur in only about 5% of cases, a recurrence risk similar to that of IMBTs with intraepithelial carcinoma and/or microinvasive carcinoma (131). In contrast, about half of mucinous carcinomas with infiltrative invasion are stage II or higher; such tumors are usually fatal. Even stage I mucinous carcinomas with infiltrative invasion are associated with a risk of recurrence of approximately 20% (131).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


