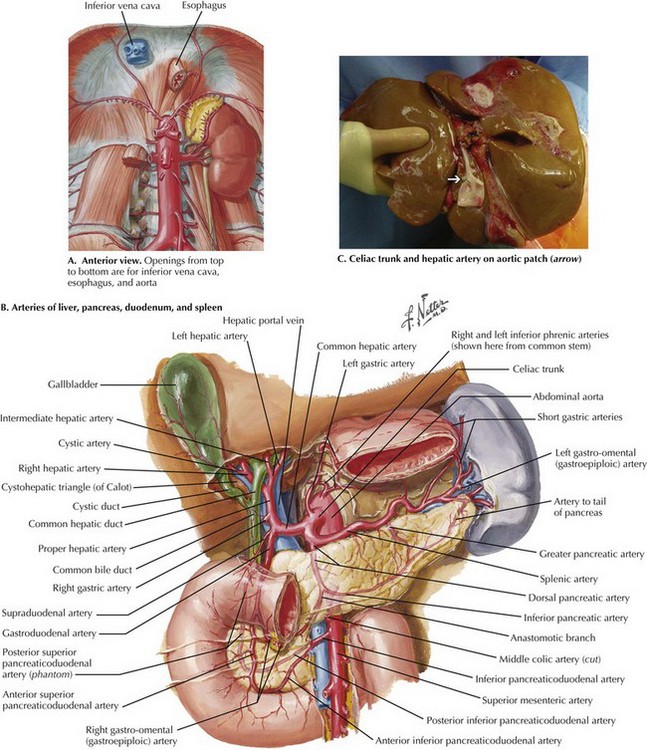
Chapter 18 The goals of the donor operation are to isolate the necessary anatomic structures and to prevent any damage to the structures to allow successful transplantation. This procedure involves the preservation of arterial and venous supplies to the relatively solid organs. Also, the cannulas must be placed for organ flush and perfusion with preservative solution. Typically, retrograde flush of the organs is accomplished through the distal aorta, with proximal clamping above the celiac axis. The venous effluent is released through an incision in the right atrium or cannulation of the distal cava (Fig. 18-1, A). FIGURE 18–1 Abdominal anatomy and organ procurement exposures. The incision for the donor surgery extends from the sternal notch to the pubis of the donor (Fig. 18-1, B and C). A sternotomy is performed, and most often, a sternal retractor and large Balfour retractor are used to provide exposure. When more abdominal exposure is needed, an abdominal cruciate incision can be utilized. Mobilization of the right hemicolon with a right medial visceral rotation (Cattell-Braasch maneuver) is performed to expose the distal abdominal aorta and inferior vena cava (IVC) (Fig 18-1, D). The left renal vein can be exposed through this maneuver, and further dissection cephalad to the left renal vein will allow the surgeon to control the superior mesenteric artery (SMA). The distal aorta is encircled and cannulated, and the aorta is ligated just proximal to the iliac bifurcation after full heparinization before cross-clamping. Additionally, the inferior mesenteric vein (IMV) also can be isolated and encircled, which can provide access for portal system flush, if elected (Fig. 18-1, E). To obtain proximal intraabdominal aortic control for retrograde flush, the surgeon exposes the supraceliac aorta by dividing the crus of the diaphragm while retracting the left lobe of the liver (Fig. 18-2, A). The supraceliac aorta is encircled at this time with a vessel loop or umbilical tape. The gastrohepatic ligament is inspected for accessory left hepatic artery, which arises from the left gastric artery (see Chapter 16, Fig. 16-3). If present, the accessory left hepatic artery is preserved by careful dissection along the lesser curvature of the stomach. Also, the lateral portion of the portal triad is palpated for the presence of a replaced right hepatic artery; if present, it is preserved down to its origin arising from the SMA. The portal triad structures are dissected to expose more of the hepatic artery, portal vein, and common bile duct (CBD) (Fig. 18-2, B). The peritoneum is divided at the cephalad border of the duodenum to expose the CBD laterally, and the gastroduodenal artery and common hepatic artery (CHA) medially. A full Kocher maneuver can also be performed at this time to trace a replaced right hepatic artery to the SMA origin. This is not necessary if there is no replaced right hepatic artery, or if the pancreas will not be procured. The gastroduodenal artery is identified and followed up to the CHA, which is dissected out to determine whether there are any other anatomic variations. Further dissection to the origin of the splenic artery is often performed “in the warm” (before cross-clamp and perfusion), whereas the rest of the dissection down to the celiac trunk is usually performed “in the cold” (after cross-clamp and perfusion). The donor liver must have a celiac trunk in continuity with the CHA for the intended recipient (Fig. 18-2, C). When the pancreas is also being recovered for transplant, the entire pancreaticoduodenal allograft is taken in continuity. The spleen is then mobilized in continuity with the tail of the pancreas. The maneuvers are identical to performing a distal pancreatectomy (see Chapter 15). Once completed, the jejunum just distal to the ligament of Treitz is divided. Some surgeons perform gut decontamination of the stomach and duodenum by flushing iodine solution down the nasogastric tube. The proximal duodenum is then divided distal to the pylorus. The vascular supply of the whole pancreaticoduodenal allograft consists of the portal vein, the SMA stump, and the splenic artery. These structures are left intact for engraftment (see Pancreas Transplantation). The reconstruction of the arterial system is performed at the backtable (backbench) by anastomosing the iliac artery conduit (from the same donor) limbs to the SMA and the splenic artery by the recipient surgeon. After cross-clamping the aorta and retrograde flushing of the organs, the surgeon completes dissection of the vessels for all the allografts being retrieved. The IVC is mobilized from the intrapericardial IVC to the infrahepatic IVC proximal to the renal veins. The CHA is dissected to the celiac trunk with a Carrel patch from the aorta. A stump of splenic artery is left with the pancreas, and the gastroduodenal artery is divided. The portal vein can be mobilized to the confluence of the superior mesenteric vein (SMV) and IMV. and splenic vein if the pancreas is not being taken (Fig. 18-3, A). In cases of pancreas recovery, approximately 1 cm of portal vein is left with the pancreas allograft.
Organ Transplantation
Abdominal Organ Donation
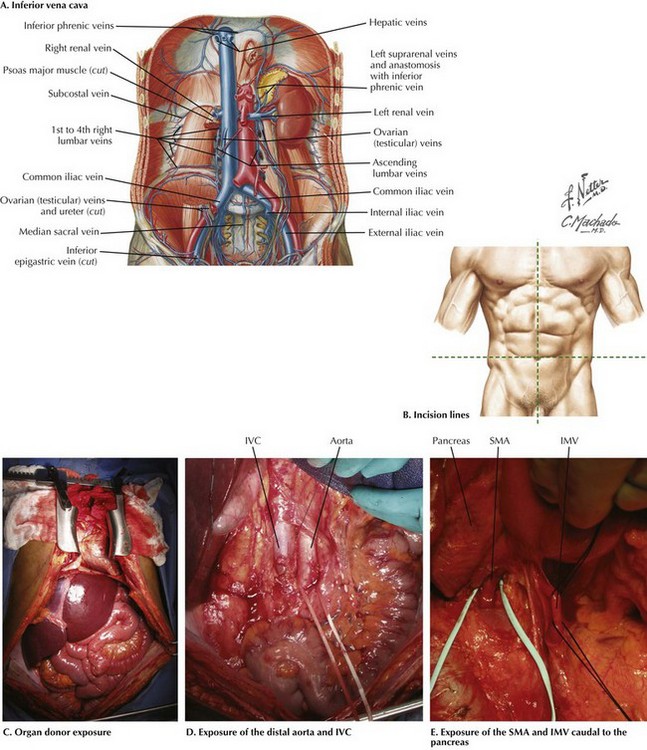
IVC, Inferior vena cava; SMA, superior mesenteric artery; IMV, inferior mesenteric vein.
Abdominal Surgical Approach
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Organ Transplantation